Abstract
Intercellular adhesion molecule-1 (ICAM-1) is a target for drug delivery to endothelial cells (ECs), which internalize multivalent anti-ICAM nanocarriers (anti-ICAM/NCs) within 15 to 30 minutes. The concomitant ICAM-1 disappearance from the EC surface transiently inhibited subsequent binding and uptake of anti-ICAM/NCs. Within 1 hour, internalized ICAM-1 diverged from anti-ICAM/NCs into prelysosomal vesicles, resurfaced, and enabled uptake of a subsequent anti-ICAM/NC dose. Thus, internalized ICAM-1 was able to recycle back to the plasma membrane. In vivo pulmonary targeting of a second anti-ICAM/NC dose injected 15 minutes after the first dose was decreased by 50% but recovered between 30 minutes and 2.5 hours, comparable to cultured ECs. Anti-ICAM/NCs affected neither EC viability nor fluid-phase endocytosis and traffic to lysosomes. However, lysosomal trafficking of the second dose of anti-ICAM/NCs was decelerated at least 2-fold versus the first dose; hence the major fraction of anti-ICAM/NCs resided in prelysosomal vesicles for at least 5 hours without degradation. Two successive doses of anti-ICAM/NC/catalase protected ECs against H2O2 for at least 8 hours versus 2 hours afforded by a single dose, suggesting that recurrent targeting to ICAM-1 affords longer effects. ICAM-1 recycling and inhibited lysosomal traffic/degradation of subsequent doses may help to prolong activity of therapeutic agents delivered into ECs by anti-ICAM/NCs.
Introduction
Intercellular adhesion molecule-1 (ICAM-1) is an Ig family transmembrane glycoprotein constitutively exposed on the luminal surface of endothelial cells (ECs).1-3 ICAM-1 represents an attractive target for drug delivery to ECs, since it is up-regulated and functionally involved in vascular inflammation, oxidant stress, and thrombosis.4-7 Antibodies to ICAM-1 are being explored as therapeutics and affinity carriers in cell cultures, animal models, and early clinical studies.8-13 In addition to acting as delivery vehicles, antibody blocking of ICAM-1 suppresses leukocyte adhesion to ECs, providing an anti-inflammatory benefit to the effects of drugs.14,15
Targeting nanocarriers (NCs) to EC determinants decreases the clearance of drugs from the bloodstream and permits site-specific delivery, increasing therapeutic capacity and reducing side effects (Muzykantov16 ). Internalization and proper subcellular processing of drugs also are critical in the rational design of drug delivery systems (Muro et al17 ). For instance, intracellular targeting of antioxidants in ECs may help to detoxify oxidants produced within the cell body and decrease elimination of drugs that otherwise shed from the EC surface.18-22
ICAM-1 targeting offers the possibility of intracellular drug delivery, given that ECs internalize multimeric anti-ICAM conjugates and anti-ICAM/NCs via a unique, newly defined pathway, cell adhesion molecule (CAM)–mediated endocytosis.23 ICAM-1 engagement by multimeric ligands triggers signaling via protein kinase C, Src family kinases, and Rho-dependent kinase, also involves dynamin and amiloride-sensitive Na+/H+ exchangers, leading to rapid reorganization of the actin cytoskeleton and formation of endocytic compartments.23
Intracellular delivery of an antioxidant enzyme, catalase, to ECs via CAM-mediated endocytosis may help contain vascular oxidant stress by minimizing catalase shedding from the cell surface. Endocytosed catalase does not escape endosomes but retains enzymatic activity within these organelles. Due to the high diffusion rate of H2O2 across cellular membranes, catalase within endocytic vesicles intercepts intracellular oxidants and provides antioxidant protection. Decay of this antioxidant effect occurs approximately 2-3 hours after internalization, due to pH-dependent proteolytic degradation following delivery to lysosomes.13 This time frame is sufficient to protect lung vasculature from acute oxidant stress in animal models.11,24,25
By analogy with classical endocytic receptors, internalized ICAM-1 could follow nanoparticle trafficking to lysosomes or dissociate from anti-ICAM in a sorting prelysosomal compartment. The latter scenario, including recycling of internalized ICAM-1 molecules to the cell surface, could provide a pathway for recurrent drug delivery permitting sustained effects. However, the fate of ICAM-1 molecules involved in endocytosis is not known. The only pathway for endothelial ICAM-1 turnover identified to date is shedding from the plasma membrane, a negative feedback mechanism reducing leukocyte adhesion.21,22
In the present study we characterized intracellular trafficking of ICAM-1 after CAM-mediated anti-ICAM/NC endocytosis in ECs and ICAM-1 availability for retargeting. The effects of 2 consecutive doses of anti-ICAM/NCs on the internalization capacity, intracellular trafficking, and the fate of anti-ICAM/NCs were examined. Results from cell culture and in vivo animal model studies showed that a large fraction of ICAM-1 molecules dissociated from internalized anti-ICAM/NCs, recycled to EC surface, and permitted recurrent, sustained targeting of anti-ICAM/NCs, providing a prolonged therapeutic effect.
Materials and methods
Antibodies and reagents
Monoclonal antibodies recognizing the extracellular domain of human or murine ICAM-1 were mAb R6.526 and mAb YN1,27 respectively. An antibody to the cytoplasmic domain of human ICAM-1 (LB-2) was from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were from Molecular Probes (Eugene, OR). Polystyrene latex microspheres, 100 nm in diameter, were from Polysciences (Warrington, PA). Unless otherwise stated, all other reagents were from Sigma (St Louis, MO).
Preparation of anti-ICAM nanocarriers
For fluorescence microscopy in cell cultures, latex nanospheres were coated with anti-ICAM alone (anti-ICAM/NC) or anti-ICAM and catalase (anti-ICAM/NC/catalase), as described previously.13 Radiolabeled nanocarriers for in vivo studies were prepared using anti-ICAM and 125I-IgG (95:5) or a mix of IgG and 125I-IgG (95:5). The effective diameter of coated nanocarriers ranged from 200 to 300 nm, as determined by dynamic light scattering.28
Cell culture
Pooled human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA) and cultured in supplemented M199 medium as described.13 For experiments, HUVECs (passage 4 to 5) were seeded onto 12-mm2 gelatin-coated coverslips in 24-well plates and then activated by overnight incubation with tumor necrosis factor–α (TNF-α), unless otherwise stated.
Detection of surface ICAM-1 during nanocarrier internalization
TNF-α–activated HUVECs were treated with 50 μg/mL cyclohexamide for 30 minutes to inhibit protein synthesis, then all further incubations were done in medium containing 10 μg/mL cyclohexamide. The cells were incubated at 4°C for 10 minutes with anti-ICAM/NCs to enable binding, then washed and warmed to 37°C to permit internalization. Surface ICAM-1 was determined by incubation with 125I-labeled anti-ICAM at 4°C, followed by elution with acid glycine solution and quantification in a gamma counter.29 The results were normalized by constitutive ICAM-1 turnover, determined in HUVECs treated with cyclohexamide but not anti-ICAM/NCs, and total cell protein in the samples. In parallel experiments, nanocarrier uptake by either resting or TNF-α–activated cells was quantified by fluorescence microscopy as described previously.23 Cells incubated at 4°C were used as controls.
Recycling experiments
TNF-α–activated HUVECs were incubated for a 10-minute pulse at 37°C in the presence of fluorescein isothiocyanate (FITC)–labeled anti-ICAM/NCs and 2 mg/mL amine-fixable Texas Red dextran (10 000 molecular weight [MW]) to permit internalization of both counterparts within common endocytic vesicles. The cells were then washed, incubated at 37°C to enable intracellular trafficking, and fixed and incubated with goat anti–mouse IgG conjugated to blue Alexa Fluor 350 (Excitation: 350 nm, Emission: 450 nm) to label surface-bound nanocarriers. Alternatively, HUVECs were incubated with only FITC-labeled anti-ICAM/NCs, and ICAM-1 intracellular trafficking was followed by permeabilization and immunolabeling of ICAM-1 cytoplasmic tail in red. The samples were analyzed by fluorescence microscopy (Eclipse TE2000-U, Nikon, Melville, NY) using filters optimized for Alexa Fluor 350, FITC, and Texas Red, and a 60×/NA1.4 PlanApo objective (Nikon, Melville, NY). Images were obtained with Orca-1CCD camera (Hamamatsu, Bridgewater, NJ) and analyzed using ImagePro 3.0 software (Media Cybernetics, Silver Spring, MD).
Uptake and intracellular trafficking of anti-ICAM nanocarriers
HUVECs, either resting or activated with TNF-α, were incubated first with a dose of anti-ICAM/NCs, followed by a second dose of FITC-labeled anti-ICAM/NCs, varying the amount of time between the application of the first and the second dose of nanocarriers. Finally, ECs were washed, fixed, and treated with Texas Red goat anti–mouse IgG to label surface-bound anti-ICAM/NCs. Merged micrographs were analyzed automatically to determine the percentage of anti-ICAM/NCs internalized by the cells with respect to the total number of nanocarriers associated to these.23
To follow intracellular trafficking of anti-ICAM/NCs, ECs were incubated for 1 hour at 37°C with Texas Red dextran to label lysosomes,13 washed, and incubated with a first and a second dose of anti-ICAM/NCs as described above for uptake studies. At varying periods of time after internalization, the cells were fixed and the colocalization of anti-ICAM/NCs within dextran-labeled compartments was determined. The results were confirmed by labeling lysosomes with phycoerythrin-conjugated rabbit anti–human LAMP-1.
Effects of internalized nanocarriers on endocytic trafficking and cell viability
To evaluate the effect of nanocarriers on the uptake of Texas Red dextran, HUVECs were untreated or pretreated with FITC anti-ICAM/NCs and then incubated with the fluid phase marker for 15 minutes at 37°C, washed, and fixed. To identify the endocytic pathways employed by HUVECs in this study, the cells were treated with inhibitors of clathrin-coated pits (monodansyl cadaverine [MDC])), caveoli (filipin), or macropinocytosis (amiloride), as previously described.23
The number of dextran-labeled vesicles and the percentage of these that trafficked to lysosomal compartments preloaded with nanocarriers were determined from fluorescent micrographs. Trafficking of Texas Red dextran to FITC-labeled anti–LAMP-1–positive compartments by unloaded cells was used as a control. Also, HUVECs were incubated for 48 hours after internalization of anti-ICAM/NCs, then cells were stained using the Live/Dead kit described in “Antioxidant protection of anti-ICAM/NC/catalase” to determine the fraction of cells retaining intracellular nanocarriers, morphological appearance of the cell monolayer, total number of cells per sample, and cell viability.
Antioxidant protection of anti-ICAM/NC/catalase
HUVECs were treated with a single dose or 2 sequential doses of control anti-ICAM/NCs or anti-ICAM/NC/catalase. At varying periods of time after internalization, the cells were incubated with a 5 mM H2O2 solution to induce oxidative injury. The cells were then washed, incubated with 0.1 μM calcein AM and 1 μM ethidium (Live/Dead kit, Molecular Probes) and finally scored as percentage of surviving cells (calcein positive/ethidium negative).13
Recurrent targeting to pulmonary vasculature in mice
A single dose of 125I-labeled anti-ICAM/NCs or control 125I-IgG/NCs was injected intravenously to anesthetized C57BL/6 mice, and lungs were collected 30 minutes after injection to determine the uptake (percent of injected dose ID per gram of lung).12 In the next series, 125I-labeled anti-ICAM/NCs (second dose) were injected either 15 minutes, 30 minutes, or 150 minutes after injection of nonlabeled anti-ICAM/NCs or nonlabeled IgG/NCs (first dose). A second dose of 125I-labeled IgG/NCs was injected into a separate group to control for mechanical retention of second-dose nanocarriers in the pulmonary vasculature.
Statistics
Unless otherwise stated, the data were calculated as the mean ± standard deviation, where statistical significance was determined by Student t test.
Results
Recycling of internalized ICAM-1 by endothelial cells
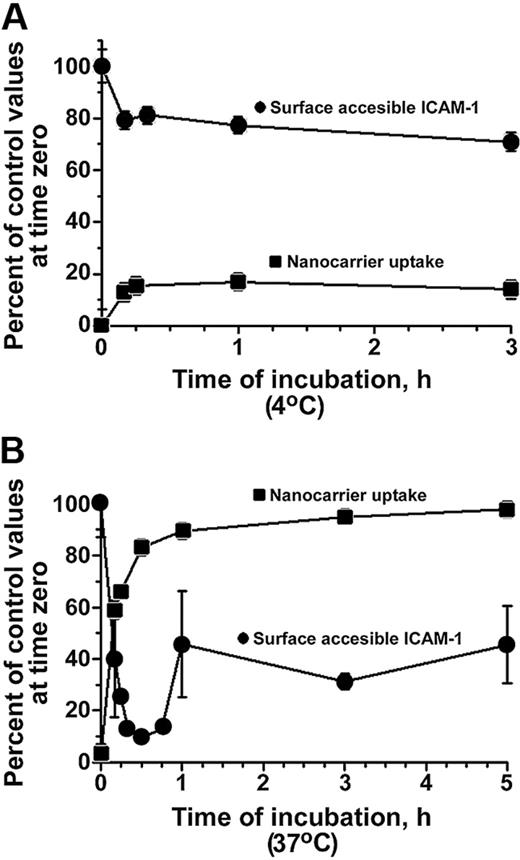
Binding of anti-ICAM/NCs (hereafter referred to as “nanocarriers”) to ICAM-1 on the surface of activated HUVECs at 4°C partially inhibited the subsequent binding of 125I–anti-ICAM (Figure 1A). Warming ECs to 37°C caused nanocarriers to be rapidly internalized, which reached a maximum level at 30 minutes (Figure 1B). Anti-ICAM/NCs also bound to quiescent ECs, although at a lower extent than to TNF-α–activated cells (3.9 ± 1.4-fold less) due to lower ICAM-1 expression. Given lower nanocarrier binding, the absolute amount of nanocarriers internalized by resting HUVECs also was lower than in activated cells. However, the rate and relative extent of nanocarrier internalization was comparable for quiescent and TNF-α–activated cells (82.1% ± 4.1% vs 82.8% ± 2.0% [30 minutes], 92.9% ± 3.6% vs 89.5% ± 1.7% [1 hour], and 90.1% ± 5.5% vs 97.9% ± 0.6% [3 hours]).
ICAM-1 reappears on HUVEC surface after internalization of anti-ICAM nanocarriers. Kinetics of FITC-labeled anti-ICAM/NC internalization (uptake, squares) and accessibility of ICAM-1 on EC surface to 125I-labeled anti-ICAM (circles) were evaluated in cyclohexamide-treated HUVECs at 4°C (A) or at 37°C (B). Cells were activated with TNF-α in all experiments shown in the figures. Data represent M ± SEM for n = 9 wells from 3 independent experiments.
ICAM-1 reappears on HUVEC surface after internalization of anti-ICAM nanocarriers. Kinetics of FITC-labeled anti-ICAM/NC internalization (uptake, squares) and accessibility of ICAM-1 on EC surface to 125I-labeled anti-ICAM (circles) were evaluated in cyclohexamide-treated HUVECs at 4°C (A) or at 37°C (B). Cells were activated with TNF-α in all experiments shown in the figures. Data represent M ± SEM for n = 9 wells from 3 independent experiments.
Probing with 125I-anti-ICAM revealed a rapid disappearance of ICAM-1 from the EC surface, coinciding with the internalization of anti-ICAM/NCs at 37°C (Figure 1B). However, in contrast with the plateau value of 20% to 25% blockage for 125I–anti-ICAM by anti-ICAM/NCs at 4°C, CAM-mediated endocytosis was followed by a rapid reappearance of ICAM-1 on the endothelial surface (Figure 1B). In this experiment, cells were pretreated with cyclohexamide to inhibit protein synthesis and rule out the appearance of newly synthesized ICAM-1. The reappearance of ICAM-1 on the cell surface implies that approximately 50% of internalized ICAM-1 recycled to the plasma membrane in a relatively intact form, within less than one hour after anti-ICAM/NC uptake.
We examined the intracellular itinerary of FITC-labeled anti-ICAM/NCs and the target ICAM-1 using monoclonal antibodies to its cytosolic domain, which is not blocked by nanocarriers. Multilabel fluorescence microscopy revealed that ICAM-1 rapidly clustered in the vicinity of nanocarriers bound to the cell surface, where the white “ICAM-1” color in Figure 2A shows its colocalization with surface-bound nanocarriers. Texas Red dextran did not bind directly to anti-ICAM/NCs, since it did not label surface-bound nanocarriers. However, as a fluid phase marker, Texas Red dextran entered cells concomitantly to nanocarrier internalization (Figure 2A). Both ICAM-1 and Texas Red dextran colocalized with nanocarriers in nascent vesicles negative for EEA-1 and LAMP-1 (Figure 2A). However, 1 hour after internalization, when practically all nanocarriers reside in EEA-1–positive endosomal compartments, about 50% of the dextran-labeled vesicles and 40% of ICAM-1–positive vesicles did not colocalize with the nanocarriers (Figure 2B,D), likely representing the fraction of vesicles containing ICAM-1 that will recycle to the plasma membrane. At 3 hours after internalization, nanocarriers reside almost exclusively in lysosomes that are partially positive for the ICAM-1 cytosolic domain and contain the fluid phase tracer; hence, a fraction of the target ICAM-1 antigen cannot escape lysosomal traffic driven by the nanocarriers.
Dissociation of ICAM-1 from internalized anti-ICAM nanocarriers. (A) Fluorescence microscopy of green FITC-labeled anti-ICAM/NCs in HUVECs at indicated time at 37°C. In 4 columns, red labeling depicts markers of early endosomes (anti–EEA-1), lysosomes (anti–LAMP-1), Texas Red dextran, or ICAM-1 cytosolic domain. Yellow color, vesicles in which anti-ICAM/NC particles colocalize with indicated markers (arrowhead); green color, anti-ICAM/NC–containing vesicles negative for indicated markers (arrow); red color, anti-ICAM/NC–free vesicles positive for indicated markers; blue color, noninternalized anti-ICAM/NCs (asterisk); white color, sites of ICAM-1 clustering by anti-ICAM/NC at the cell surface. Bar = 1 μm. (B) Percentage of localization of the markers (EEA-1, LAMP-1, Texas Red [TR] dextran, or cytosolic ICAM-1) to anti-ICAM/NC–containing vesicles, quantified by image analysis and plotted as a function of the time after anti-ICAM/NC internalization. (C) Schema of ICAM-1 recycling. ICAM-1 enters ECs via nascent vesicles along with anti-ICAM/NCs. Anti-ICAM/NCs traffic to lysosomes via early endosomes, whereas ICAM-1 partially escapes lysosomal pathway and recycles to the plasma membrane. The fluid phase TR dextran labels both the lysosomal and the recycling route. (D) Single-labeled dextran-containing vesicles, diverging from anti-ICAM/NC–containing vesicles, can be detected 1 hour after internalization and disappear by 3 hours, likely due to release from recycling compartments. Data in panels B and D are shown as M ± SD for n > 10 cells from 2 independent experiments.
Dissociation of ICAM-1 from internalized anti-ICAM nanocarriers. (A) Fluorescence microscopy of green FITC-labeled anti-ICAM/NCs in HUVECs at indicated time at 37°C. In 4 columns, red labeling depicts markers of early endosomes (anti–EEA-1), lysosomes (anti–LAMP-1), Texas Red dextran, or ICAM-1 cytosolic domain. Yellow color, vesicles in which anti-ICAM/NC particles colocalize with indicated markers (arrowhead); green color, anti-ICAM/NC–containing vesicles negative for indicated markers (arrow); red color, anti-ICAM/NC–free vesicles positive for indicated markers; blue color, noninternalized anti-ICAM/NCs (asterisk); white color, sites of ICAM-1 clustering by anti-ICAM/NC at the cell surface. Bar = 1 μm. (B) Percentage of localization of the markers (EEA-1, LAMP-1, Texas Red [TR] dextran, or cytosolic ICAM-1) to anti-ICAM/NC–containing vesicles, quantified by image analysis and plotted as a function of the time after anti-ICAM/NC internalization. (C) Schema of ICAM-1 recycling. ICAM-1 enters ECs via nascent vesicles along with anti-ICAM/NCs. Anti-ICAM/NCs traffic to lysosomes via early endosomes, whereas ICAM-1 partially escapes lysosomal pathway and recycles to the plasma membrane. The fluid phase TR dextran labels both the lysosomal and the recycling route. (D) Single-labeled dextran-containing vesicles, diverging from anti-ICAM/NC–containing vesicles, can be detected 1 hour after internalization and disappear by 3 hours, likely due to release from recycling compartments. Data in panels B and D are shown as M ± SD for n > 10 cells from 2 independent experiments.
Recurrent targeting of anti-ICAM nanocarriers to endothelium in vitro and in vivo
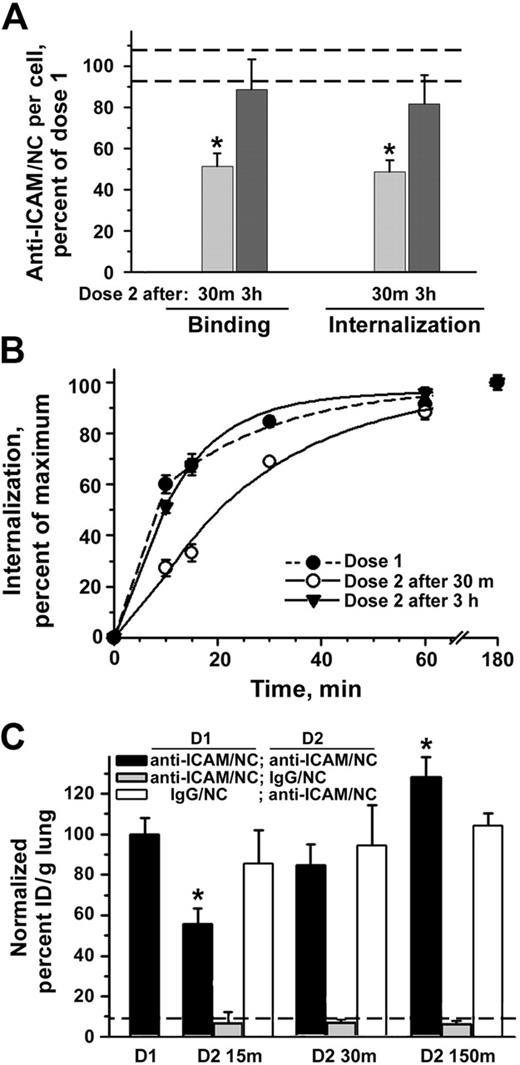
In theory, the reappearance of ICAM-1 on the endothelial surface could be used for sustained or recurrent intracellular delivery of nanocarriers, thus, exceeding initial saturating dose and duration of a drug effect. To test this possibility, we treated cells with 2 subsequent doses of nanocarriers, imitating recurrent injections. Indeed, binding of second-dose anti-ICAM/NCs to TNF-α–activated cells was reduced by 50% when applied 30 minutes after internalization of the first dose, but recovered to the control level when applied 3 hours after the first dose (Figure 3A). Also, the amount of internalized second-dose anti-ICAM/NCs decreased 30 minutes after the first dose, but recovered to the control value by 3 hours, both in the case of TNF-α–activated HUVECs (Figure 3A) and quiescent cells (68.2% ± 3.9% and 101.2% ± 4.2% of the control value 30 minutes and 3 hours after the first dose, respectively). Furthermore, in the case of quiescent cells, the binding capacity of anti-ICAM/NCs even increased to 134.2% ± 12.5% of the control level 3 hours after the first dose. Perhaps because of maximal surface expression of ICAM-1 already induced by cytokine treatment, this “overshoot” effect was not seen in TNF-α–activated cells. Anti-ICAM/NC internalization kinetics, which were significantly decreased when the second dose was added 30 minutes following the first dose, recovered to a markedly more rapid rate of internalization when the second dose was applied 3 hours after the initial dose (Figure 3B).
Recurrent targeting of anti-ICAM nanocarriers to endothelium in vitro and in vivo. (A) Sequential targeting of 2 doses of anti-ICAM/NCs into TNF-α–activated HUVECs. The number of surface-bound and internalized anti-ICAM/NCs in the first dose is taken as 100% (dashed lines represent the standard deviation of this control value). Binding and internalization of the second dose anti-ICAM/NCs were inhibited at 30 minutes after the internalization of the first dose, yet recovered to 100% by 3 hours. (B) Internalization kinetics for the first dose and second dose at 30 minutes versus 3 hours, determined as percent of internalized nanocarriers. Data are means ± SEM for n > 20 cells from 2 independent experiments. (C) Control mice received a single injection intravenously of either 125I-labeled anti-ICAM/NCs (first dose, D1) or IgG/NCs (dashed line) to test targeting to the lungs. In other groups, mice were injected with nonlabeled anti-ICAM/NCs followed by a similar dose of 125I-labeled anti-ICAM/NCs (black bars) or 125I-IgG/NCs (gray bars) either 15, 30, or 150 minutes later (second dose, D2). In a separate group, mice were injected with a first dose of nonlabeled IgG/NCs and a second dose of 125I-labeled anti-ICAM/NCs (white bars). Lung uptake was calculated as percent of injected dose per gram and plotted as percent of the level obtained with a single dose of anti-ICAM/NCs (D1) as M ± SEM, n = 4-5.
Recurrent targeting of anti-ICAM nanocarriers to endothelium in vitro and in vivo. (A) Sequential targeting of 2 doses of anti-ICAM/NCs into TNF-α–activated HUVECs. The number of surface-bound and internalized anti-ICAM/NCs in the first dose is taken as 100% (dashed lines represent the standard deviation of this control value). Binding and internalization of the second dose anti-ICAM/NCs were inhibited at 30 minutes after the internalization of the first dose, yet recovered to 100% by 3 hours. (B) Internalization kinetics for the first dose and second dose at 30 minutes versus 3 hours, determined as percent of internalized nanocarriers. Data are means ± SEM for n > 20 cells from 2 independent experiments. (C) Control mice received a single injection intravenously of either 125I-labeled anti-ICAM/NCs (first dose, D1) or IgG/NCs (dashed line) to test targeting to the lungs. In other groups, mice were injected with nonlabeled anti-ICAM/NCs followed by a similar dose of 125I-labeled anti-ICAM/NCs (black bars) or 125I-IgG/NCs (gray bars) either 15, 30, or 150 minutes later (second dose, D2). In a separate group, mice were injected with a first dose of nonlabeled IgG/NCs and a second dose of 125I-labeled anti-ICAM/NCs (white bars). Lung uptake was calculated as percent of injected dose per gram and plotted as percent of the level obtained with a single dose of anti-ICAM/NCs (D1) as M ± SEM, n = 4-5.
We tested EC targeting in vivo by a single versus repetitive dose of intravenously injected 125I-labeled anti-ICAM/NCs, which bind to pulmonary ECs and preferentially accumulate in the pulmonary vasculature.11,12,30,31 We observed a highly specific pulmonary uptake of anti-ICAM/NCs after intravenous injection in mice, 137% ± 10.7% versus 12.9% ± 4% ID/g of control IgG/NC counterpart (Figure 3C). Similar to the increased targeting of anti-ICAM/NCs to cytokine-activated HUVECs in culture, the pulmonary uptake of anti-ICAM/NCs was further increased in mice pre-injected with lipopolysaccharide prior to nanocarriers (173.6% ± 21% of the value obtained in control mice). Also, in agreement with cell culture data, pulmonary targeting of 125I-labeled anti-ICAM/NCs injected 15 minutes after nonlabeled anti-ICAM/NCs was markedly inhibited (55.6% ± 7.8% of control level), yet gradually recovered (84.5% ± 10% and 128.2% ± 10% of control level) when administered 30 minutes and 150 minutes after injection of the first dose, respectively. In contrast, the level of pulmonary accumulation of 125I-anti-ICAM/NCs did not differ from control values when animals had received a first dose of nonspecific IgG/NCs regardless of the time interval between the nanocarrier injection (Figure 3C). Pulmonary targeting of second-dose of anti-ICAM/NCs was not due to mechanical uptake or other nonspecific effects, since second doses of control IgG/NCs did not accumulate in lungs after the administration of nonlabeled anti-ICAM/NCs (Figure 3C).
The second dose of anti-ICAM nanocarriers persists in a prelysosomal compartment
Nanocarriers internalized at 2 subsequent doses trafficked as 2 separate pools within the cell, and only a minor fraction (24.9% ± 13.2%) colocalized 3 hours after internalization of the second dose, regardless of the time interval between the first and second doses (not shown). The second dose, applied either 30 minutes or 1 hour after the first one, did not affect delivery of the first dose to lysosomes, which occurred between 2 and 3 hours after internalization (Figure 4A). However, lysosomal trafficking of the second-dose nanocarriers was markedly decreased: 3 hours after internalization, only 21.4% ± 14.4% of the second-dose nanocarriers could be detected in lysosomes versus 70.7% ± 7% of the first-dose nanocarriers (Figure 4B-C). Thus, loading of lysosomes by nanocarriers inhibits lysosomal delivery of the second dose (Figure 4D).
Decelerated intracellular traffic of second dose of anti-ICAM nanocarriers. Cells prelabeled with Texas Red dextran were incubated with FITC-labeled anti-ICAM/NCs (A) or nonlabeled anti-ICAM/NCs (B) for 1 hour at 4°C, warmed to 37°C after washing of nonbound materials, incubated for indicated time at 37°C, and counterstained with secondary labeled antibodies, which would stain surface-bound nanocarriers in blue. The first dose was followed by the same dose of FITC-labeled anti-ICAM/NCs at the indicated time (B). The absence of blue staining in panels A and B indicates that both first and second doses are internalized. Yellow color shows colocalization (arrowhead) of anti-ICAM/NCs with red-labeled lysosomes. Bar = 10 μm. (C) Anti-ICAM/NC colocalization with lysosomes is plotted as a function of time after internalization of the corresponding dose. Data are M ± SEM for n > 10 cells from 2 independent experiments. (D) Schema of recurrent intracellular traffic and deceleration of lysosomal delivery of the second dose of anti-ICAM/NCs.
Decelerated intracellular traffic of second dose of anti-ICAM nanocarriers. Cells prelabeled with Texas Red dextran were incubated with FITC-labeled anti-ICAM/NCs (A) or nonlabeled anti-ICAM/NCs (B) for 1 hour at 4°C, warmed to 37°C after washing of nonbound materials, incubated for indicated time at 37°C, and counterstained with secondary labeled antibodies, which would stain surface-bound nanocarriers in blue. The first dose was followed by the same dose of FITC-labeled anti-ICAM/NCs at the indicated time (B). The absence of blue staining in panels A and B indicates that both first and second doses are internalized. Yellow color shows colocalization (arrowhead) of anti-ICAM/NCs with red-labeled lysosomes. Bar = 10 μm. (C) Anti-ICAM/NC colocalization with lysosomes is plotted as a function of time after internalization of the corresponding dose. Data are M ± SEM for n > 10 cells from 2 independent experiments. (D) Schema of recurrent intracellular traffic and deceleration of lysosomal delivery of the second dose of anti-ICAM/NCs.
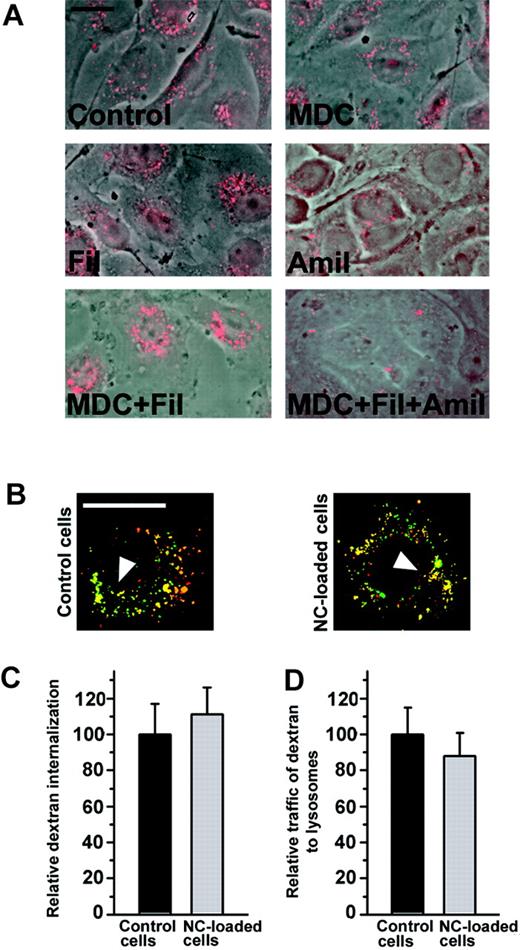
To test whether anti-ICAM/NCs affect constitutive endocytosis and lysosomal traffic, we used fluorescent dextran as a fluid phase marker. HUVECs treated with either single or double combinations of pharmacological inhibitors of internalization via clathrin-coated pits (MDC), caveoli (filipin), or macropinocytosis (amiloride) were still able to internalize dextran (Figure 5A). The fact that dextran uptake could only be inhibited by simultaneous treatment with drugs affecting all 3 pathways confirms that it enters ECs through all these classical endocytic mechanisms. Furthermore, dextran was similarly internalized and delivered to lysosomes by control cells and cells that had internalized a saturating dose of anti-ICAM/NCs (Figure 5B-C). Therefore, anti-ICAM/NC internalization via CAM-mediated endocytosis does not affect other endocytic pathways in ECs.
Loading cells with anti-ICAM nanocarriers does not affect endocytosis and trafficking of dextran. (A) Internalization of a fluid phase marker, fluorescent Texas Red dextran, by either control HUVECs or cells treated with pharmacological inhibitors of internalization by clathrin-coated pits (MDC), caveoli (Filipin = Fil), or macropinocytosis (Amiloride = Amil). Note that TR dextran enters cells via diverse endocytic pathways. (B) HUVECs, either control or preloaded with a saturating dose anti-ICAM/NCs, were incubated with TR dextran and traffic to lysosomes was tested. Yellow color (arrowhead): colocalization of TR dextran with lysosomes labeled in green by FITC–anti–LAMP-1 (control cells) or FITC–anti-ICAM/NCs (particle-loaded cells). Bar = 10 μm. (C) The number of TR dextran-labeled endocytic vesicles per cell and (D) percent of these localizing to lysosomal compartments was determined by fluorescence microscopy. Data are M ± SEM from n > 10 cells.
Loading cells with anti-ICAM nanocarriers does not affect endocytosis and trafficking of dextran. (A) Internalization of a fluid phase marker, fluorescent Texas Red dextran, by either control HUVECs or cells treated with pharmacological inhibitors of internalization by clathrin-coated pits (MDC), caveoli (Filipin = Fil), or macropinocytosis (Amiloride = Amil). Note that TR dextran enters cells via diverse endocytic pathways. (B) HUVECs, either control or preloaded with a saturating dose anti-ICAM/NCs, were incubated with TR dextran and traffic to lysosomes was tested. Yellow color (arrowhead): colocalization of TR dextran with lysosomes labeled in green by FITC–anti–LAMP-1 (control cells) or FITC–anti-ICAM/NCs (particle-loaded cells). Bar = 10 μm. (C) The number of TR dextran-labeled endocytic vesicles per cell and (D) percent of these localizing to lysosomal compartments was determined by fluorescence microscopy. Data are M ± SEM from n > 10 cells.
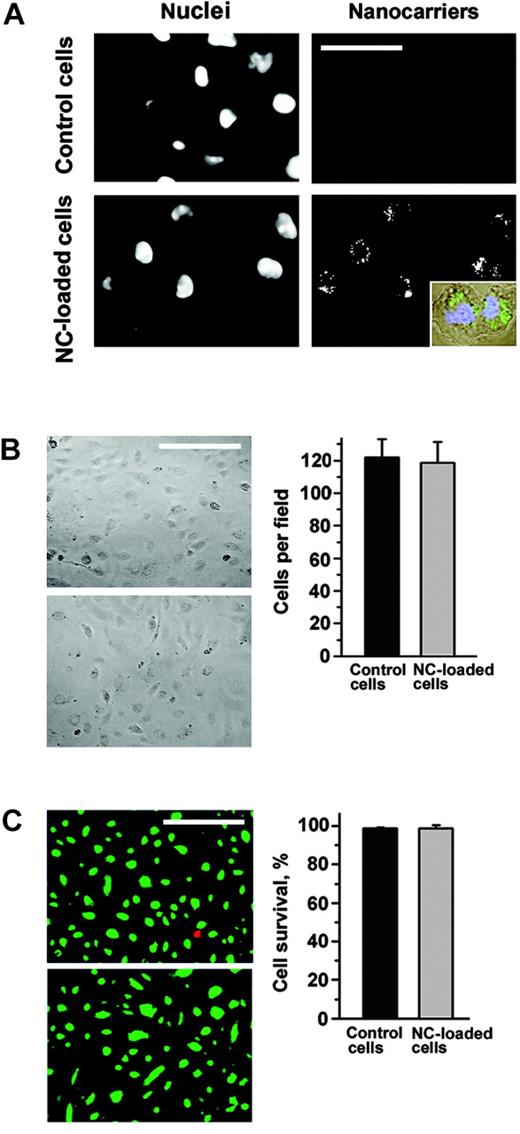
Furthermore, anti-ICAM/NCs still resided in intracellular vesicular compartments 48 hours after uptake by HUVECs (Figure 6A); 97% ± 12% of the cells still contained nanocarriers at this time (Figure 6B). Neither cell number nor morphology of the endothelial monolayer nor cellular viability was affected by the prolonged intracellular retention of nanocarriers (Figure 6B-C). Interestingly, an almost equal share of intracellular nanocarriers could be identified in dividing endothelial cells (Figure 6A, insert).
Anti-ICAM nanocarriers do not compromise endothelial cell viability. Both control and FITC–anti-ICAM/NC–treated HUVECs were maintained in culture for 48 hours. (A) Cells, visualized by nuclear staining with DAPI (4′6-diamidino-2-phenylindole 2HCl), retain intracellular anti-ICAM/NCs, which distribute between dividing cells (inset). Bar = 30 μm. (B) The morphology of the HUVEC monolayer and cell number is not affected by anti-ICAM/NC retention in cells. Magnification bar = 50 μm. (C) Intracellular anti-ICAM/NCs do not affect HUVEC survival revealed by fluorescent staining of alive (green) and dead (red) cells. Bar = 50 μm. Data are M ± SEM from at least 500 cells per condition.
Anti-ICAM nanocarriers do not compromise endothelial cell viability. Both control and FITC–anti-ICAM/NC–treated HUVECs were maintained in culture for 48 hours. (A) Cells, visualized by nuclear staining with DAPI (4′6-diamidino-2-phenylindole 2HCl), retain intracellular anti-ICAM/NCs, which distribute between dividing cells (inset). Bar = 30 μm. (B) The morphology of the HUVEC monolayer and cell number is not affected by anti-ICAM/NC retention in cells. Magnification bar = 50 μm. (C) Intracellular anti-ICAM/NCs do not affect HUVEC survival revealed by fluorescent staining of alive (green) and dead (red) cells. Bar = 50 μm. Data are M ± SEM from at least 500 cells per condition.
Delivery of 2 sequential doses of anti-ICAM/NC/catalase provides sustained protection against oxidant stress
To evaluate the potential therapeutic implications of our findings, we employed anti-ICAM/NC/catalase in a model of H2O2-induced oxidative injury in HUVECs, as previously described.13 Cells were transiently protected against H2O2 injury by anti-ICAM/NC/catalase, but protection was markedly diminished after 2 hours (Figure 7). However, if a second dose of anti-ICAM/NC/catalase was delivered by this time, the cells were protected from oxidative injury for at least 6 more hours; hence continuous protection by 2 sequential doses of anti-ICAM/NC/catalase lasted for at least 8 hours versus 2 hours afforded by a single dose.
Recurrent catalase targeting by anti-ICAM nanocarriers provides prolonged antioxidant protection of EC. HUVECs were treated with first-dose “empty” anti-ICAM/NCs (gray bars) or anti-ICAM/NC/catalase (hatched and black bars) and 170 minutes later with a second-dose anti-ICAM/NC/catalase (black and gray bars). As determined by a cell viability assay, the first-dose anti-ICAM/NC/catalase protected cells from H2O2-induced injury for 2 hours, while double-dose treated cells remained protected for at least 8 hours. Data were quantified by fluorescence microscopy from at least 500 cells per condition and represent M ± SD as percent of cell survival.
Recurrent catalase targeting by anti-ICAM nanocarriers provides prolonged antioxidant protection of EC. HUVECs were treated with first-dose “empty” anti-ICAM/NCs (gray bars) or anti-ICAM/NC/catalase (hatched and black bars) and 170 minutes later with a second-dose anti-ICAM/NC/catalase (black and gray bars). As determined by a cell viability assay, the first-dose anti-ICAM/NC/catalase protected cells from H2O2-induced injury for 2 hours, while double-dose treated cells remained protected for at least 8 hours. Data were quantified by fluorescence microscopy from at least 500 cells per condition and represent M ± SD as percent of cell survival.
Protection by the second dose of anti-ICAM/NC/catalase applied after catalase-free anti-ICAM/NCs also lasted for at least 5 hours after internalization, indicating that, in good agreement with data shown in Figure 4, the synergistic character of the increased duration of protection was not simply due to delivery of twice the amount of catalase, but rather, due to the inhibited lysosomal trafficking and degradation of the second dose of anti-ICAM/NC/catalase.
Discussion
We found that ICAM-1 trafficking in ECs has 2 key features that increase the efficacy of recurrent drug targeting using anti-ICAM/ NCs. First, after mediating internalization of nanocarriers, ICAM-1 recycles to the cell surface, indicating that a single ICAM-1 molecule can participate in multiple rounds of nanocarrier binding and internalization. Second, the capacity of lysosomal trafficking of anti-ICAM/NCs in ECs is limited, for example, delivery of a second dose of nanocarriers to lysosomes is markedly inhibited. These new aspects of the recently described CAM-mediated endocytic pathway23 further enhance its potential use for drug targeting into ECs.
Most studies of uptake and trafficking in ECs have been focused on known receptors for endocytosis and transcytosis, for example, transferrin receptor and albondin endocytosed via clathrin-coated pits and caveoli, respectively.32-34 Surface cell adhesion molecules are less characterized in this context, since their initially identified natural ligands (white blood cells) appear too large to be internalized by ECs.28,35 However, recent work has examined the internalization and trafficking of an inducible cell adhesion molecule, E-selectin, which has been suggested to be a potential receptor for endothelial drug targeting strategies since it is internalized by clathrin-mediated endocytosis.36-38
ICAM-1 belongs to a different family of EC adhesion molecules, Ig-superfamily CAM. Neither ICAM-1 nor another member of this superfamily, PECAM-1, is constitutively internalized or significantly internalize monomolecular ligands.12,28,39 However, binding of multimeric ICAM-1 or PECAM-1 ligands (eg, anti-ICAM or anti-PECAM nanocarriers) induces internalization by ECs via a mechanism that differs from all previously described endocytic pathways and requires CAM cross-linking (CAM-mediated endocytosis23 ). Thus, CAM-endocytosis may represent a specific case of activating EC signaling by multivalent ICAM-1 ligands (Hubbard and Rothlein40 ). In contrast, pre-activation of ECs (eg, by TNF-α) is not required to induce CAM-mediated endocytosis.23 In fact, we found that internalization induced by anti-ICAM/NCs occurs with a similar kinetics by both activated and quiescent ECs.
At 37°C, ICAM-1 rapidly disappears from the plasma membrane concomitantly to anti-ICAM/NC internalization. The extent of ICAM-1 disappearance (∼ 90%) in this process exceeds the extent of ICAM-1 blocking by anti-ICAM/NCs at 4°C in the absence of internalization (∼ 20%). One possibility is that ICAM-1 is clustered by anti-ICAM/NCs at 37°C (Figure 2C), similar to the “zipperlike” mechanism observed when phagocytic receptors are clustered by particulate ligands.41 A quantitative analysis correlating anti-ICAM/NC concentration and surface density of anti-ICAM on nanocarriers to ICAM-1 internalization remains to be determined. However, a rough estimation of these parameters under the conditions used in this study (∼ 300 anti-ICAM molecules per particle × 2 ICAM-1 molecules engaged per antibody × ∼ 2 × 107 particles/well = 1.2 × 1010 anti-ICAM molecules/well28,42 ) implies that anti-ICAM/NCs have the theoretical capacity to cluster most of ICAM-1 molecules expressed on the EC surface (∼ 105 cells/well × ∼ 1-2 × 105 ICAM-1 molecules/cell = 1-2 × 1010 ICAM-1 molecules/well17 ). Noteworthy, a significant reduction (∼ 20%) of anti-ICAM/NC binding also was observed in HUVECs pretreated with anti-PECAM/NC, which is targeted to an unrelated ligand (S.M., M.K., V.M. unpublished data, June, 2004), implying that a fraction of ICAM-1 not engaged by its ligands may be passively internalized from the plasmalemma into the numerous induced endocytic vesicles. Free ICAM-1, associated directly or via adaptor or cytoskeleton molecules with ICAM-1 engaged by anti-ICAM/NCs, also may be involved in nanocarrier-masked membrane domains and endocytic vesicles. These factors may account for the massive disappearance of ICAM-1 from the EC surface concomitant to anti-ICAM/NC internalization at 37°C.
The notion that multivalent ligands induce CAM-mediated endocytosis and trafficking is of interest in the context of vascular pathophysiology, since ICAM-1 mediates internalization of multivalent pathogens (Hopkins et al43 ), including HIV,44 rhinoviruses, poliovirus,45,46 and plasmodium-infected erythrocytes.47 ICAM-1 and VCAM-1 clustering induced by cells too large to be internalized also helps promote endothelial cell signaling and actin remodeling in response to leukocyte adhesion and transmigration.35,48
We provide the first evidence that internalized ICAM-1 has the capacity to be recycled to the plasma membrane in ECs. Recently, it has been shown that there is a PECAM-1 pool that recycles from an endothelial submembrane storage compartment to the plasma membrane to support leukocyte transmigration at the cell borders.49 However, this PECAM-1 storage compartment is accessible to small tracer molecules in the extracellular milieu, which suggests that it is distinct from a bona fide endocytic compartment, as opposed to internalized ICAM-1 that moves via endocytic vesicles. Recycling of internalized ICAM-1 might be more analogous to the recycling pathway taken by endocytosed receptors, such as transferrin receptor.50 However, transferrin receptor recycling is rapid (eg, 5-15 minutes),51 while the kinetics for recycling of ICAM-1 internalized by multivalent nanocarriers (1 hour) is more comparable to the slower recycling kinetics for multivalent, cross-linked transferrin.52 Recruitment of ICAM-1 from the secretory pathway seems unlikely, since ICAM-1 reappeared at the plasma membrane in the absence of new protein synthesis. Also, to date, EC secretory vesicles containing ICAM-1 have not been described, as opposed to vesicles such as Weibel-Palade bodies, which mediate stimulated secretion of other cell adhesion molecules, P-selectin, within 2 minutes.53
ICAM-1 recycling provides a pathway for recurrent drug targeting, for example, continuous or subsequent doses of nanocarriers can be efficiently delivered to the same cell. ECs in the lung vessels represent a privileged vascular target, because lungs (1) contain approximately 30% of total endothelial surface in the body; (2) represent the first pass extended vascular bed after intravenous injections; (3) receive 100% cardiac venous blood output at each systole; and, (4) experience a relatively slow perfusion rate via high-capacity, low-resistance vascular bed, which favors binding of EC ligands. These factors explain why anti-ICAM/NCs, as well as other carriers directed against pan-endothelial surface determinants, accumulate preferentially in the pulmonary vasculature after intravenous injections in animals and humans.16 We employed anti-ICAM pulmonary targeting to verify our findings in vivo.
Rapid and effective restoration of the pulmonary uptake of a second dose of anti-ICAM/NCs injected into mice (Figure 3C) strongly supports physiological and potential therapeutic significance of cell culture findings (Figures 1, 2 and 3A-B). It appears that nanocarriers provide an important advantage in targeting, since amplitude of the targeting exceeds that of anti-ICAM itself by an order of magnitude (> 30 vs < 5% ID in lungs, Murciano et al12 ). Reappearance of ICAM-1 on the luminal surface of pulmonary ECs in mice occurs relatively quickly, which would argue against the reappearance of ICAM-1 due to de novo synthesis, as this is expected to require a lag of 2 to 4 hours.54 Interestingly, we observed that at later time points (eg, 150 minutes after the first administration of nanocarriers), pulmonary uptake of second-dose anti-ICAM/NCs overshoots the basal level of uptake (Figure 3C). The nature of this enhancement remains to be determined. The absence of this effect in animals injected with a first dose of control IgG/NCs rules out the possibility that ICAM-1 expression is up-regulated as a consequence of a systemic release of cytokines in response to FcR-mediated uptake of IgG/NCs and activation of reticulo endothelial system (RES) macrophages. It is possible, however, that ICAM-1 engagement by first-dose anti-ICAM/NCs results in a positive feedback loop, leading to up-regulation of ICAM-1 expression.40 This hypothesis would agree with the finding that there was enhanced binding of anti-ICAM/NCs applied 3 hours after the first dose in quiescent cells in culture. However, a comparable overshoot was not observed in TNF-α–activated cells, most likely due to the maximal level of ICAM-1 expression by these cells. Also, lack of pulmonary uptake of IgG/NCs injected after anti-ICAM/NC counterpart suggests that anti-ICAM/NCs rapidly disappear from the lumen and rules out the possibility that lung uptake of the second dose is merely due to vessel occlusion and mechanical entrapment.
In addition to opportunities of sustained or recurrent targeting provided by ICAM-1 recycling, lysosomal trafficking of internalized nanocarriers was retarded due to loading of the first dose (Figure 5). Under normal conditions, lysosomes freely intermix and exchange contents.55 However, accumulation of nondegradable material in lysosomes (eg, the latex nanocarriers in this study) can inhibit the delivery of subsequent doses of nanocarriers to these compartments and, as a secondary consequence, decelerate degradation of the nanocarrier protein cargo. A similar effect has been previously observed in macrophage lysosomes and has been largely considered to be a pathological condition that diminishes macrophage defensive function and is related to lysosomal storage diseases.56 Nevertheless, we have found that macromolecules (eg, 10 000 MW dextran) that enter ECs by classical endocytic mechanisms can still traffic to lysosomes containing nondegraded nanocarriers (Figure 5). The difference in trafficking of fluid phase markers versus multimeric anti-ICAM/NCs may reflect that multimeric ligands traffic more slowly to lysosomal compartments. This is the case for oligomerized transferrin, which is retained in an early endocytic compartment resistant to degradation.52 However, the slow degradation of a second dose of anti-ICAM/NCs, due to delayed lysosomal trafficking, had a therapeutic benefit since it had the capacity to prolong the efficacy of catalase nanocarriers for protection against oxidative injury from 2 hours to at least 8 hours (Figure 7), providing an alternative to pharmacological means that block lysosomal traffic and degradation of anti-ICAM/NCs.13
The goal of prolonging the therapeutic effects of drugs delivered to target cells (eg, endothelial cells) is critical. Gene therapy strategies that, in theory, can afford prolonged therapeutic interventions are risky and cannot afford an immediate effect that is desirable in acute settings, such as oxidant stress in acute lung injury, ischemia reperfusion, or organ transplantation. On the other hand, the duration of the effect for the short time period afforded by a single bolus of nanocarriers targeted to ICAM-1 or PECAM-1 may be insufficient for successful containment of the stress.
Multiple injections of anti-ICAM/NCs, as with any repetitive protein therapy (eg, enzyme replacement therapies), require a rigorous safety study to avoid potential immune responses. It is unlikely, however, that injection of the second and perhaps even third dose within a 5- to 50-hour time period would elicit more severe immune reactions than a single injection (in most cases, such immunization requires a second boost by the sixth or seventh day). Use of “stealth” nanocarriers coated with polyethylene glycol, which provides prolonged circulation and reduced immune recognition, may offer even safer interventions.57-60 In theory, a long-circulating pool of stealth anti-ICAM/NCs could serve as a source of sustained intake by ECs via recycling ICAM-1, providing prolonged therapeutic effects.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-05-1714.
Supported by National Institutes of Health grants HL/GM 71175-01 (V.R.M), GM61012 (M.K.), and P01 HL019737-26; Project 3 (M.K.); Department of Defense grant PR 012262 (V.R.M.); and American Heart Association grant 0435481N (S.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors express their deep gratitude to Ms Tanya Krasik and Mr John Leferovich for invaluable help in animal experiments.

![Figure 2. Dissociation of ICAM-1 from internalized anti-ICAM nanocarriers. (A) Fluorescence microscopy of green FITC-labeled anti-ICAM/NCs in HUVECs at indicated time at 37°C. In 4 columns, red labeling depicts markers of early endosomes (anti–EEA-1), lysosomes (anti–LAMP-1), Texas Red dextran, or ICAM-1 cytosolic domain. Yellow color, vesicles in which anti-ICAM/NC particles colocalize with indicated markers (arrowhead); green color, anti-ICAM/NC–containing vesicles negative for indicated markers (arrow); red color, anti-ICAM/NC–free vesicles positive for indicated markers; blue color, noninternalized anti-ICAM/NCs (asterisk); white color, sites of ICAM-1 clustering by anti-ICAM/NC at the cell surface. Bar = 1 μm. (B) Percentage of localization of the markers (EEA-1, LAMP-1, Texas Red [TR] dextran, or cytosolic ICAM-1) to anti-ICAM/NC–containing vesicles, quantified by image analysis and plotted as a function of the time after anti-ICAM/NC internalization. (C) Schema of ICAM-1 recycling. ICAM-1 enters ECs via nascent vesicles along with anti-ICAM/NCs. Anti-ICAM/NCs traffic to lysosomes via early endosomes, whereas ICAM-1 partially escapes lysosomal pathway and recycles to the plasma membrane. The fluid phase TR dextran labels both the lysosomal and the recycling route. (D) Single-labeled dextran-containing vesicles, diverging from anti-ICAM/NC–containing vesicles, can be detected 1 hour after internalization and disappear by 3 hours, likely due to release from recycling compartments. Data in panels B and D are shown as M ± SD for n > 10 cells from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-05-1714/6/m_zh80020572590002.jpeg?Expires=1769082105&Signature=VTViefFVVVwEkl9XWYkGhKkd8TeIYlhM~wrmjKoyktGpJ7969vM0ZkZ6dOD0I~fVPksHl4FZ6T015GmXT~s7TQKflAe2bK6VQztn1CbFIyGAdRP8fZSaZBl49aUiqCx-X2ATET-Gpd6X-imNHZzKPr9jFet5oczj9hm~woznpxox5n8N7Dk511YgYnrkL~~vdPlcU5hNu0CY5LkhE1-MQnsSIFupkgIzbw58v1CY~Bh3fxOjuBB0it-J5c2VAiyE-e15rDOiO3USYpEZeANDSyrxvLLZWVpx-2MUvH4PFWYK3G5RvyMXZ~4iatEy6OeL94NQ~CYDS3kK3vu9Fo8ZIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal