A number of mechanisms may explain the clinical and biologic differences in behavior between monoclonal gammopathy of undetermined significance (MGUS) and myeloma plasma cells (PCs). These include inherent differences in PC programming, immortalization of PCs at different stages of differentiation, different cells of origin, and potentially the influence of the normal/neoplastic bone marrow (BM) microenvironment. Chemokines and their receptors are known to be paramount to normal cell differentiation and homing. As B cells differentiate into PCs they undergo a coordinated change in chemokine receptor expression and chemokine responsiveness. We therefore hypothesized that the expression of chemokine receptors on myeloma PCs would be altered compared with normal PCs and may explain some of the differences in behavior between normal and malignant populations. As our previous studies have demonstrated that 2 populations of PCs (normal and neoplastic) can be identified in myeloma and MGUS cases based on expression of CD19 expression1-3 and that MGUS cases with a predominantly neoplastic phenotype have a higher chance of progressing to myeloma than those with a mixed normal and malignant phenotype,3 we also hypothesized that by examining both normal and neoplastic PCs from the same BM in MGUS patients we could determine whether any variation in chemokine receptor profile was due to the marrow microenvironment or to neoplastic transformation.
Normal (CD38+CD138+CD19+CD56-) and neoplastic PCs (CD38+CD138+CD19-CD56+/-) were distinguished from other leukocytes using 4-color flow cytometry with CELLQuest version 3.1 on a FACSort (BD Biosciences, Oxford, United Kingdom) as previously described.1,4 Cells were incubated with CD38 allophycocyanin; CD19 phycoerythrin-cyanine 5.5; CD45 fluorescein isothiocyanate (in-house conjugates) combined with phycoerythrin-conjugated CCR1, CCR2, CCR3, CXCR-5 (R&D, Minneapolis, MN), CCR5, CCR6, CXCR1, CXCR2, CXCR3, or CXCR4 (BD Biosciences, Mountain View, CA); and a minimum of 3000 PCs were studied. Expression levels were recorded as the geometric mean fluorescence intensity and compared using the Student t test.
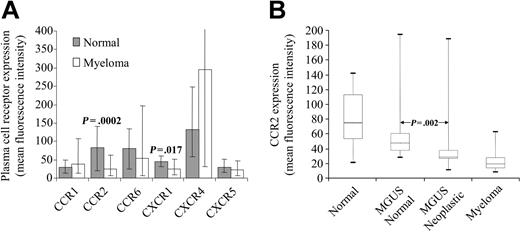
Flow-cytometric analysis of chemokine receptor expression. (A) Chemokine receptor expression on normal and myeloma PCs. The mean expression level and range is shown. (B) CCR2 expression is significantly lower on CD19- PCs from MGUS patients than that on CD19+ PCs from the same bone marrow. The figure shows 5th, 25th, 50th, 75th, and 95th percentiles for expression, measured as mean fluorescence intensity.
Flow-cytometric analysis of chemokine receptor expression. (A) Chemokine receptor expression on normal and myeloma PCs. The mean expression level and range is shown. (B) CCR2 expression is significantly lower on CD19- PCs from MGUS patients than that on CD19+ PCs from the same bone marrow. The figure shows 5th, 25th, 50th, 75th, and 95th percentiles for expression, measured as mean fluorescence intensity.
Chemokine receptor expression was assessed on PCs from 20 normal and 19 myeloma BMs. CCR3, CCR5, CXCR2, and CXCR3 were not expressed on PCs (< 3-fold difference in expression with control). In contrast, CCR1, CCR2, CCR6, CXCR1, CXCR4, and CXCR5 were expressed on both types of PCs, in keeping with data from other groups.5-9 The differences in chemokine receptor expression between normal and myeloma PCs were small (Figure 1A). CCR1 and CXCR5 showed no difference in expression between the 2 cell types. CCR2, CCR6, and CXCR1 showed decreased expression on myeloma PCs compared with normal PCs (average 3.3, 1.5, and 1.8 fold, respectively). In contrast, CXCR4 was up-regulated on myeloma PCs in comparison to normal PCs (average 2.2 fold). This suggests that PCs have a specific chemokine receptor expression profile; however, differences in the level of expression of some receptors, particularly CCR2 and CXCR1, may explain the abnormal localization of myeloma PCs.
These differences were studied further by assessing normal and neoplastic PCs from the same BM in 20 MGUS patients. CCR6, CXCR1, and CXCR4 showed no significant difference in expression between the 2 cell types, suggesting that feedback loops between neoplastic PCs and BM stroma also influence normal PC chemokine receptor expression. In contrast, CCR2 expression on CD19- PCs was on average 1.6-fold lower than on their CD19+ counterparts (range, 1.1-6.8 fold), suggesting that a key feature of neoplastic PCs is the down-regulation of CCR2 (Figure 1B). This reduced expression is therefore not a consequence of differences in BM microenvironment but rather a function of the neoplastic process or of the stage of differentiation of the originating neoplastic cell. Interfering with chemokines and their receptors that are related to malignant transformation may prove useful as adjunct to chemotherapy approaches.
Supported by the British Society of Haematology (A.C.R., F.E.D.), Leukaemia Research Fund UK (F.E.D., G.J.M.), Department of Health (F.E.D.), International Myeloma Foundation UK (A.C.R.), and The Egyptian Missions Department (M.H.A.R.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal