In order to establish a causal link between RPS19 gene mutations and DBA physiopathology, 2 very elegant studies report that reducing RPS19 levels by siRNA in normal CD34+ cells leads to a major impairment in the hematopoietic process.
Almost every available strategy in experimental hematology has been tried in attempts to unravel the molecular defect or defects underlying Diamond-Blackfan anemia (DBA).1 With the identification of heterozygous mutations in the ribosomal protein S19 (RPS19) gene, which encodes a 16-kDa ribosomal protein,2 in 25% of DBA patients, the challenge is now to prove the causal link between RPS19 haploinsufficiency and erythroid alterations characterizing DBA. Unfortunately, C57BL/6J mice heterozygous for the RPS19 deletion are not anemic,3 limiting experimental approaches to the use of human primary cells. The good news is that in vitro, human erythroid (erythroid burst-forming unit [BFU-E] and erythroid colony-forming unit [CFU-E]) progenitors will proliferate and complete differentiation up to the reticulocyte stage, and granulocytic (granulocyte macro-phage-colony-forming unit [CFU-GM]) progenitors will do so to the polymorphonuclear stage with specific cytokines. The bad news is that proliferation and differentiation are coupled in hematopoiesis, making it very tricky to tell which pathway is guilty and to evaluate differentiation if proliferation is inhibited. We know that in patients with DBA, the progression of CFU-E into hemoglobinized erythroblasts is preferentially affected, independently of the status of the RPS19 gene.4 FIG1
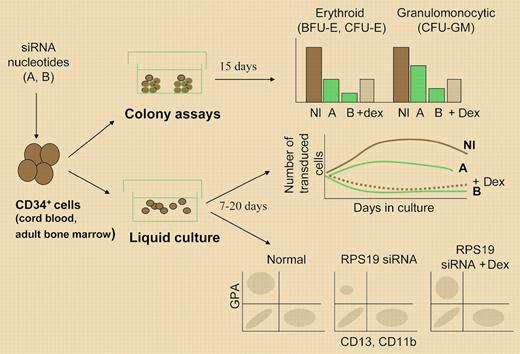
Presentation of the experimental strategy used to analyze the consequences of reducing RPS19 levels by introducing siRNA in human CD34+ cells. Erythroid and granulocytic colonies were scored in semisolid assays. Cell proliferation was determined by counting cells in liquid culture, and differentiation by analyzing cell phenotype by flow cytometry or cytologic analysis. A indicates 50% RPS19 levels; B, less than 50% RPS19 levels; and Dex, dexamethasone. Results are adapted schematically from the original figures in the papers by Flygare et al, page 4627 and Ebert et al, page 4620.
Presentation of the experimental strategy used to analyze the consequences of reducing RPS19 levels by introducing siRNA in human CD34+ cells. Erythroid and granulocytic colonies were scored in semisolid assays. Cell proliferation was determined by counting cells in liquid culture, and differentiation by analyzing cell phenotype by flow cytometry or cytologic analysis. A indicates 50% RPS19 levels; B, less than 50% RPS19 levels; and Dex, dexamethasone. Results are adapted schematically from the original figures in the papers by Flygare et al, page 4627 and Ebert et al, page 4620.
Direct loss- and gain-of-function experiments now yield important information on the cellular functions of the RPS19 gene: Hamaguchi and colleagues5 recently found that forced expression of RPS19 in cells from patients with DBA partially rescued the erythroid defect, and in this issue of Blood, Flygare and colleagues and Ebert and colleagues report a major impairment in the development of CD34+ cells in both erythroid and granulocytic lineages after reducing RPS19 levels by siRNA. Different oligonucleotides targeting endogenous RPS19 RNA were expressed in primary fetal or adult CD34+ cells via retroviral or lentiviral vectors, decreasing the RPS19 protein to various levels. In both studies, this led to a dramatic drop not only in the number of erythroid colonies generated by transduced CD34+ cells, but also in the output of marrow-derived CFU-GMs, which was of the same order of magnitude. This drop in both CFU-GMs and BFU-Es was explained by a major defect in progenitor cell proliferation, proportional to the level of the RPS19 protein; this proliferation defect was also observed in leukemic cell lines, and the nonhematopoietic 293 cells when expressing the RPS19 siRNAs. Reintroducing the RPS19 cDNA in siRNA-treated cells rescued the defect. If CD34+ cells do not proliferate, a putative alteration along their erythroid differentiation process would be masked. Flygare and colleagues tried to address this point by comparing expression of erythroid (glycophorin A)- and granulocytic-specific antigens in cells that escaped the blockade, and showed that terminal erythroid differentiation is more severely compromised than granulocytic maturation. Interestingly, as shown by Ebert and colleagues, adding dexamethasone (to which 65% of the patients respond in vivo) to siRNA-treated cells did not rescue the proliferation defect, but boosted erythroid differentiation through an RPS19-independent mechanism.
The in vitro defects fit with the broad action expected from a ribosomal gene in which deletion is embryonically lethal,2 but their severity and extension exceed what is seen in patients, even though compensatory mechanisms may exist in vivo. Of note, Ebert and colleagues used a large-scale micro array analysis of CD34+ erythroid differentiation to show that the expression of ribosomal genes (including RPS19) and erythroid-specific genes varies independently. Is there a specific crossroad between the RPS19 and the erythroid differentiation pathway, and, if so, when in the hematopoietic hierarchy does it occur? Does it involve an extraribosomal role for RPS19 or RPS19 partners encoded by the additional genetic loci recently involved in DBA?6 The invariant clinical picture and in vitro defects in patients with DBA with an intact RPS19 gene strongly argues that RPS19 should not be the only one to blame. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal