Abstract
The Akt kinases promote hematopoietic cell growth and accumulation through phosphorylation of apoptotic effectors and stimulation of mTOR-dependent translation. In Akt-transformed leukemic cells, tumor growth can be inhibited by the mTOR inhibitor rapamycin, and clinical trials of rapamycin analogs for the treatment of leukemia are under way. Surprisingly, nontransformed hematopoietic cells can grow and proliferate in the presence of rapamycin. Here, we show that Pim-2 is required to confer rapamycin resistance. Primary hematopoietic cells from Pim-2– and Pim-1/Pim-2–deficient animals failed to accumulate and underwent apoptosis in the presence of rapamycin. Although animals deficient in Akt-1 or Pim-1/Pim-2 are viable, few animals with a compound deletion survived development, and those that were born had severe anemia. Primary hematopoietic cells from Akt-1/Pim-1/Pim-2–deficient animals displayed marked impairments in cell growth and survival. Conversely, ectopic expression of either Pim-2 or Akt-1 induced increased cell size and apoptotic resistance. However, though the effects of ectopic Akt-1 were reversed by rapamycin or a nonphosphorylatable form of 4EBP-1, those of Pim-2 were not. Coexpression of the transgenes in mice led to additive increases in cell size and survival and predisposed animals to rapid tumor formation. Together, these data indicate that Pim-2 and Akt-1 are critical components of overlapping but independent pathways, either of which is sufficient to promote the growth and survival of nontransformed hematopoietic cells.
Introduction
Growth factors regulate hematopoietic cell growth and survival through the modulation of intracellular signaling cascades in which oncogenic kinases are important effectors. In nontransformed cells, the activity of these kinases is tightly controlled by growth factor availability, whereas their sustained activation can lead to apoptotic resistance and uncontrolled cell proliferation. One pathway commonly activated in leukemia/lymphoma is the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway, which is activated downstream of a variety of transforming oncogenes, including breakpoint-cluster region/Abelson leukemia (BCR/ABL), activated Ras, and platelet-derived growth factor receptor β (PDGFRβ) fusion proteins.1-3 In addition, deletion of the tumor suppressor PTEN leads to sustained activation of Akt, and deletion of TSC1/2 leads to sustained activation of mTOR.4,5 This has led to the speculation that inhibitors of these kinases would be effective chemotherapeutic agents either alone or in combination with other oncogene-specific inhibition, such as tyrosine-kinase specific inhibitors. However, since neither PI3K nor Akt inhibitors are available for clinical use, pharmacologic inhibition of this pathway has focused on rapamycin, a Food and Drug Administration (FDA)–approved macrolide. Rapamycin, when bound to its target FKBP12, is a potent inhibitor of mTOR.6,7
Inhibition of the serine/threonine kinase mTOR is an attractive target for leukemic therapy because mTOR functions downstream of Akt to stimulate cell growth. In addition to its effects on cell growth, rapamycin can reverse Akt-induced apoptotic resistance.8 As a result of these observations, rapamycin is being investigated as a chemotherapeutic agent and has demonstrated efficacy in the treatment of tumors with known activation of the Akt/mTOR pathway, such as those containing germline or spontaneous loss of the PTEN or TSC tumor suppressors.9,10 In addition, rapamycin has been effective in the treatment of more diverse cancers, such as leukemia, non-Hodgkin lymphoma, and multiple myeloma, and has demonstrated synergy with known chemotherapeutic agents, such as STI-571 and 5-fluorouracil.11-17 Despite the efficacy of rapamycin as a chemotherapeutic agent, rapamycin and its analogs have favorable side effect profiles, suggesting that though mTOR is a critical component of transformation downstream of Akt activation, it is not absolutely required to maintain the growth and survival of nontransformed hematopoietic cells. This suggests that hematopoietic cell growth can be maintained independently of the activities of Akt and mTOR. Furthermore, if such alternative pathways to maintain cell growth can be defined, they may lead to targeted therapies to suppress the growth of tumors resistant to rapamycin therapy.
Overexpression of the Pim-2 oncogene promotes growth factor–independent survival of hematopoietic cell lines. This resistance to apoptosis is distinct from that conferred by the overexpression of activated Akt because Pim-2–induced survival cannot be suppressed by rapamycin.18 Endogenous levels of expression of the Pim serine/threonine kinases, Pim-1 and Pim-2, have been shown to be induced by a variety of growth factors and cytokines that regulate blood cell growth and differentiation.19 Despite this, animals deficient in Pim-1 and Pim-2 are viable and, apart from a mild decrease in red cell size, display minimal defects in hematopoiesis.20 These results suggest that if Pim kinases contribute to hematopoietic cell growth and survival, their roles are secondary to the primary regulation of hematopoietic cell growth by PI3K/Akt/mTOR.
Here, we present evidence that the resistance of primary hematopoietic cells to rapamycin depends on the endogenous activity of the Pim-2 kinase. In animals deficient in the mTOR activator Akt-1, Pim kinases were required to maintain normal hematopoiesis. Combined deficiency of Akt-1 and Pim-1/Pim-2 led to the sub-Mendelian generation of anemic progeny, and the progeny that were born displayed severe impairments in hematopoietic cell function with respect to cell growth, survival, and proliferation. Although ectopic Pim-2 and Akt-1 expression promoted protein synthesis, only Akt promotion of protein synthesis was repressed by rapamycin treatment. In addition to inducing the phosphorylation of 4EBP-1, Pim-2 expression led to increased eIF4E expression and phosphorylation. This correlated with the ability of Pim-2 to stimulate cell growth and survival even in cells in which 4EBP-1 phosphorylation was inhibited by the introduction of a constitutively active form of the 4EBP-1 translational repressor. Akt-1 and Pim-2 promoted increases in cell size and survival in transgenic mice, and these increases were additive in double-transgenic mice. In addition, the coexpression of Akt-1 and Pim-2 in transgenic mice cooperatively induced transformation, with 100% of progeny developing lymphoma by 12 weeks of age. Thus, Akt-1 and Pim-2 represent critical components of 2 independent pathways that contribute to hematopoietic cell growth and survival. These results help explain the safety profile of rapamycin as a chemotherapeutic drug and suggest that Pim kinase inhibitors should be developed and tested in rapamycin-insensitive forms of leukemia/lymphoma.
Materials and methods
Plasmids and cell lines
A 4EBP-1 expression construct in which threonines 37/46 had been mutated to alanines was generously provided by N. Sonenberg (McGill University, Montreal, Canada).21 An shRNA vector targeting eIF4E (004-C-2) was purchased from Open Biosystems (Huntsville, AL). Transfections were performed using the Nucleofector system (Amaxa, Gaithersburg, MD), and stable transfectants were created with the addition of 1 μg/mL puromycin (Sigma-Aldrich, St Louis, MO) to the culture medium. FL5.12 cells stably expressing Bcl-XL, myristolated Akt-1, and Pim-2 were generated as described previously.18 For standard growth and transient transfection experiments, FL5.12 cells were grown in standard medium: RPMI 1640 (Invitrogen, Carlsbad, CA) plus 10% fetal calf serum (FCS; Gemini, Woodland, CA), penicillin-streptomycin (Invitrogen), and 400 pg/mL recombinant interleukin-3 (IL-3; BD Biosciences, San Jose, CA). For IL-3 withdrawal experiments, cells were washed 3 times and then grown in RMPI plus 10% FCS. In transient transfection experiments, cells were allowed to recover in standard medium for 24 hours after transfection before IL-3 was withdrawn. Primary IL-3–dependent bone marrow cultures were generated by harvesting marrow from the tibias and femurs of mice and subsequent incubation in RPMI 1640 plus 10% FCS and 4 ng/mL recombinant IL-3. Suspension cells were transferred to new flasks with fresh media every 3 days for 9 days, at which time homogenous IL-3–dependent cultures had been established. Enrichment for live cells was performed by Ficoll centrifugation, as previously described.18 Rapamycin was purchased from Sigma-Aldrich. Primary murine T cells were purified from homogenized spleens and lymph nodes using the StemStep T Cell Purification kit (Stem Cell Technologies, Vancouver, Canada). Primary T cells were grown in Dulbecco modified Eagle medium (DMEM) plus 10% FCS with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), l-glutamine, nonessential amino acids, and penicillin-streptomycin. Cell viability was assessed by exclusion of 1 μg/mL propidium iodide (Molecular Probes, Eugene, OR) using a FACScalibur flow cytometer (BD Biosciences). Cell cycle analysis was performed by incubation of cells for 30 minutes with Hoechst 33342 (Molecular Probes) and subsequent analysis using an LSR flow cytometer (BD Biosciences). Cell size was measured by Coulter Counter Z2 Particle Count and Size Analyzer (Beckman-Coulter, Fullerton, CA) or forward scatter using an LSR flow cytometer.
Western blots and antibodies
Whole cell extracts were prepared in radioimmunoprecipitation (RIPA) buffer or phosphate-buffered saline (PBS) containing 1% NP-40 supplemented with protease inhibitors (Roche, Nutley, NJ) and phosphatase inhibitors (Sigma). Protein concentrations were determined using the Dc protein assay (Bio-Rad, Hercules, CA). For Western blots, 50 μg protein was resolved on a 4% to 12% or on a 10% NuPage Bis-Tris polyacrylamide gel and was transferred to nitrocellulose as directed (Invitrogen), then blocked in PBS containing 10% milk and 0.2% Tween-20 and incubated in primary antibody in 5% milk overnight at 4°C with subsequent incubation with a species-appropriate horseradish peroxidase (HRP)–conjugated secondary antibody for 1 hour. Blots were stripped by incubation for 30 minutes at 65°C in 2% sodium dodecyl sulfate (SDS) and 100 mM β-mercaptoethanol followed by 3 washes in PBS plus 0.2% Tween-20. Mouse anti–Pim-2 1D12, mouse anti–Akt-1 B-1, goat anti–actin I-19, and mouse and goat HRP-conjugated immunoglobulin G (IgG) secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti–phospho-473 Akt, rabbit anti–phospho-209 eIF4E, rabbit anti-eIF4E, rabbit anti–phospho-37/46 4EBP-1, rabbit anti–phospho-70 4EBP-1, rabbit anti–4EBP-1 and anti–rabbit HRP-conjugated IgG secondary antibody were purchased from Cell Signaling Technologies (Beverly, MA). Rat anti-hemagglutinin (anti-HA) was purchased from Roche. Bcl-XL was detected with an antibody created previously.18 ECL-Plus (Amersham, Piscataway, NJ) was used to visualize target proteins.
Mouse strains
Lck-Akt mice were obtained22 and bred to Pim-2 transgenic mice,23 which were derived from 2 founder lines. Pim-1– and Pim-2–deficient mice were provided previously by P. Rothman (Columbia University, Bronx, NY). Akt-1–null mice were provided by M. Birnbaum (University of Pennsylvania, Philadelphia). Hemoglobin levels of mice (5 or more) were measured after retro-orbital sinus puncture using a Hemavet 1500 analyzer (CDC Technologies, Oxford, CT). All experiments were performed in accordance with approved animal safety protocols.
Metabolic labeling
35S incorporation was determined as previously described.24 Briefly, FL5.12 cells were grown in the presence or absence of IL-3 and rapamycin for 24 hours before 500 000 cells were plated in media without cysteine/methionine with the continued presence or absence of IL-3 and rapamycin for 30 minutes before the addition of 0.1 mCi/mL (37 MBq) labeled cysteine/methionine (Amersham). Lysates were prepared using RIPA after 4 hours, and proteins were TCA precipitated and then spotted onto filters for scintillation counting.
Colony-forming assays
In vitro differentiation was performed using the Methocult kit (Stem Cell Technologies) according to the manufacturer's instructions. For erythroid differentiation, bone marrow was harvested from the tibias and femurs of mice, and nucleated cells were plated in triplicate 35-mm dishes at a density of 2 × 105/mL in medium containing 1% methylcellulose, 15% fetal bovine serum (FCS), 1% bovine serum albumin (BSA), 10 μg/mL insulin, 200 μg/mL transferrin, and 3 U/mL erythropoietin. Cultures were incubated for 2 days before counting of erythroid colonies.
Results
Pim-2 deficiency sensitizes cells to rapamycin treatment
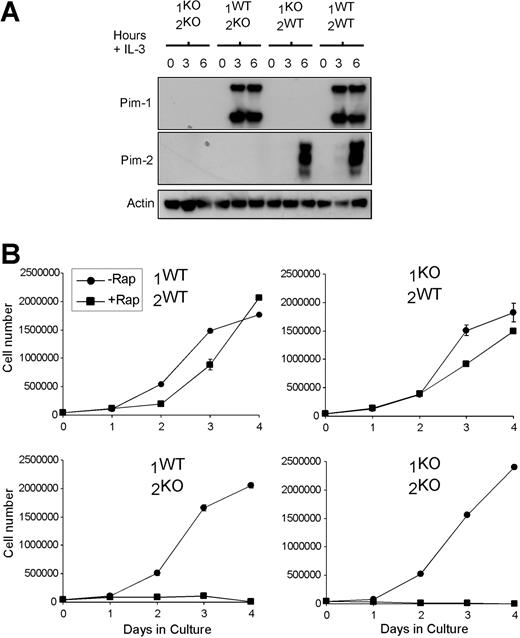
To investigate the possibility that endogenous Pim kinases can confer rapamycin resistance to hematopoietic cells, we generated primary IL-3–dependent bone marrow cultures from Pim-1–deficient (1KO/2WT), Pim-2–deficient (1WT/2KO), Pim-1/Pim-2–double-deficient (1KO/2KO), and littermate control (1WT/2WT) mice. Genotype was confirmed by Western blot (Figure 1A). Loss of Pim-1 or Pim-2 did not affect the expression level or phosphorylation status of Akt-1 (data not shown). The expression of Pim-1 and Pim-2 in hematopoietic progenitors was found to be regulated by IL-3, as indicated by the rapid induction of Pim-1 and Pim-2 when IL-3 was added to the bone marrow cultures after overnight culture in the absence of IL-3 (Figure 1A). Primary bone marrow cells from wild-type, Pim-1KO, Pim-2KO, and Pim-1KO/Pim-2KO mice maintained in IL-3 underwent proliferative expansion, and no genotype-specific differences were observed in the rate of cell growth or proliferation in the presence of IL-3. However, the addition of rapamycin resulted in growth arrest and subsequent apoptosis in the Pim-2– and Pim-1/Pim-2–deficient cell cultures in the presence of IL-3, whereas IL-3–induced accumulation of wild-type or Pim-1–deficient cells was minimally affected (Figure 1B), suggesting that the deletion of Pim-2 was sufficient to confer the phenotype of increased rapamycin sensitivity.
Pim-2 deficiency sensitizes cells to rapamycin treatment. (A) Primary IL-3–dependent bone marrow cultures were established from Pim-1/Pim-2–deficient (1KO/2KO), Pim-2–deficient (1WT/2KO), Pim-1–deficient (1KO/2WT), and wild-type littermate (1WT/2WT) mice. Lysates were probed for the expression of Pim-1, Pim-2, and Actin. Cells were deprived of IL-3 for 12 hours and then stimulated for the number of hours indicated before lysis. (B) Cell number versus days in culture is shown for IL-3–dependent primary bone marrow cultures from the mice described in panel A, grown in the absence (-Rap) or presence (+Rap) of 20 nM rapamycin. Cells were seeded at a concentration of 50 000/mL at the beginning of the experiment. Data indicate the mean ± SD of triplicate samples.
Pim-2 deficiency sensitizes cells to rapamycin treatment. (A) Primary IL-3–dependent bone marrow cultures were established from Pim-1/Pim-2–deficient (1KO/2KO), Pim-2–deficient (1WT/2KO), Pim-1–deficient (1KO/2WT), and wild-type littermate (1WT/2WT) mice. Lysates were probed for the expression of Pim-1, Pim-2, and Actin. Cells were deprived of IL-3 for 12 hours and then stimulated for the number of hours indicated before lysis. (B) Cell number versus days in culture is shown for IL-3–dependent primary bone marrow cultures from the mice described in panel A, grown in the absence (-Rap) or presence (+Rap) of 20 nM rapamycin. Cells were seeded at a concentration of 50 000/mL at the beginning of the experiment. Data indicate the mean ± SD of triplicate samples.
Germline deletion of the Pim kinases and Akt-1 impairs hematopoietic cell function
In hematopoietic cells, the activation of mTOR is regulated by Akt. To explore the possibility that Akt-dependent signaling might be required for normal hematopoietic cell development in the absence of Pim-1 and Pim-2, we examined the F1 progeny of Akt-1-heterozygous/Pim-1/2–null parents. Mendelian genetics predicts that 25% of progeny would be deficient in all 3 genes, yet of 124 generated mice, only 9 triple knockouts (7%) were generated, which is roughly one third to one quarter the predicted frequency (Table 1). In addition, there appeared to be a bias against the generation of Akt-1–heterozygous/Pim-1/2–null mice. Although Mendelian segregation predicts that twice as many Akt-1 heterozygotes as Akt-1 wild-type mice should be generated, we observed nearly equal frequency in the generation of genotypes because the decreased frequency of triple knockout mice was fully accounted for by the increased births of Akt-1–wild-type/Pim-1/2–null animals (Table 1). These data suggested that a genetic interaction exists between the 2 pathways such that fewer mice deficient in all 3 genes are born.
F1 progeny of intercrossed Akt-1Het/Pim-1/2KO mice
F1 progeny . | Predicted . | Observed at weaning (no./no. total) . | Observed at E13.5 . |
|---|---|---|---|
| Akt-1WT/Pim-1/2KO | 0.25 | 0.47 (58/124)* | 0.38 (12/31) |
| Akt-1Het/Pim-1/2KO | 0.5 | 0.46 (57/124) | 0.43 (14/31) |
| Akt-1KO/Pim-1/2KO | 0.25 | 0.07 (9/124)* | 0.15 (5/31)† |
F1 progeny . | Predicted . | Observed at weaning (no./no. total) . | Observed at E13.5 . |
|---|---|---|---|
| Akt-1WT/Pim-1/2KO | 0.25 | 0.47 (58/124)* | 0.38 (12/31) |
| Akt-1Het/Pim-1/2KO | 0.5 | 0.46 (57/124) | 0.43 (14/31) |
| Akt-1KO/Pim-1/2KO | 0.25 | 0.07 (9/124)* | 0.15 (5/31)† |
Frequency of generation of each genotype, as determined by tail-snip PCR, is shown.
E13.5 indicates embryonic day 13.5; WT, wild type; KO, knockout; and Het, heterozygous.
P < .001 by χ2 analysis
3 of 5 mice were dead in utero
Substantial bone marrow erythropoiesis is required for normal embryonic development. Pim-1–deficient mice display a mild impairment in hematopoiesis.20 We report that a combined deficiency of Pim-2 and Akt-1 impairs erythropoiesis because comparison of the hemoglobin levels of wild-type, Pim-1/2KO, Akt-1KO/Pim-2KO, Akt-1Het/Pim-1/2KO, and Akt-1KO/Pim-1/2KO mice revealed a decrease in hemoglobin levels that correlated with the combined loss of Pim-2 and Akt-1 (Table 2). Complete blood analysis revealed no significant differences in the number or distribution of other cell types, suggesting that the combined loss of Pim-2 and Akt-1 selectively impaired erythropoiesis (data not shown).
Reduced hemoglobin levels in blood of Akt-1KO/Pim-1/2KO mice
Genotype . | Hemoglobin, g/dL . |
|---|---|
| Akt-1WT/Pim-1/2WT | 12.2 ± 0.99 |
| Akt-1WT/Pim-1/2KO | 10.9 ± 0.82 |
| Akt-1Het/Pim-1/2KO | 11.2 ± 1.15 |
| Akt-1KO/Pim-2KO | 8.0 ± 2.18* |
| Akt-1KO/Pim-1/2KO | 6.6 ± 2.45* |
Genotype . | Hemoglobin, g/dL . |
|---|---|
| Akt-1WT/Pim-1/2WT | 12.2 ± 0.99 |
| Akt-1WT/Pim-1/2KO | 10.9 ± 0.82 |
| Akt-1Het/Pim-1/2KO | 11.2 ± 1.15 |
| Akt-1KO/Pim-2KO | 8.0 ± 2.18* |
| Akt-1KO/Pim-1/2KO | 6.6 ± 2.45* |
Mean ± SD hemoglobin concentration, as determined from the blood of 5 or more mice of the indicated genotypes.
See Table 1 for abbreviations.
P < .05 by ANOVA compared with WT
To determine whether this phenotype was the result of impaired erythropoiesis, we examined erythroid differentiation of bone marrow cells in vitro. We observed decreased colony formation in the cultures of cells from Pim-1– and Pim-2–deficient mice that was most severe when Pim-1, Pim-2, and Akt-1 were deleted (Table 3). Differentiation of other cell types appeared normal (data not shown). We next sought to determine whether any developmental abnormalities might result from the combined loss of these genes. Of 31 day 13.5 embryos derived from matings of Pim-1/2KO/Akt-1Het mice (Table 1), 5 triple knockouts were observed—a 2-fold increase compared with their frequency of live birth. However, 3 of these triple knockouts did not appear to be viable, demonstrating that a combined deficiency of Pim-1, Pim-2, and Akt-1 leads to embryonic lethality of a significant fraction of pups in the second half of gestation.
Impaired erythropoiesis in Pim-1-, Pim-2-, and Akt-1-deficient animals
Genotype . | CFU-Es . |
|---|---|
| WT | 355 ± 28 |
| Pim-1KO | 154 ± 24 |
| Pim-2KO | 113 ± 11 |
| Pim-1KO/Pim-2KO | 105 ± 13 |
| Pim-1KO/Akt-1KO | 79 ± 11 |
| Pim-2KO/Akt-1KO | 53 ± 19 |
| Pim-1KO/Pim-2KO/Akt-1KO | 18 ± 8 |
Genotype . | CFU-Es . |
|---|---|
| WT | 355 ± 28 |
| Pim-1KO | 154 ± 24 |
| Pim-2KO | 113 ± 11 |
| Pim-1KO/Pim-2KO | 105 ± 13 |
| Pim-1KO/Akt-1KO | 79 ± 11 |
| Pim-2KO/Akt-1KO | 53 ± 19 |
| Pim-1KO/Pim-2KO/Akt-1KO | 18 ± 8 |
Mean ± SD of erythroid colony-forming units (CFU-Es) (n = 3) observed after the differentiation of bone marrow cells from mice of each genotype.
See Table 1 for abbreviations.
The combined deficiency of the Pim and Akt pathways also affected the growth response of hematopoietic cells. Primary IL-3–dependent bone marrow cultures from mice deficient in combinations of Akt-1, Pim-1, and Pim-2 were generated. Cell size measurements revealed that a significant decrease in size was observed with the loss of Akt-1, Pim-1, or Pim-2 compared with wild-type controls and that the greatest impairments were observed in cells deficient in both Akt-1 and Pim-2, with cells 20% smaller than cells with wild-type alleles for Pim-2 and Akt-1 (Figure 2A).
To test the consequences of deficiencies in these genes on cell survival and proliferation, primary IL-3–dependent bone marrow cultures were cultured for 12 hours in the absence of IL-3 to synchronize the cultures in G1, and then live cells were plated at a uniform density in fresh media containing IL-3. Cell accumulation over 48 hours was measured and was nearly eliminated in the combined absence of Pim-2 and Akt-1 (Figure 2B).
Observed differences in cell accumulation could be explained by effects on cell viability, cell cycle entry, or both. Measurements of cell viability, when cell numbers were counted, revealed that cultures of wild-type cells were 76% viable, cultures of Pim-1KO/Pim-2Het/Akt-1Het cells were 66% viable, cultures of Pim-1KO/Pim-2Het/Akt-1KO cells were 45% viable, cultures of Pim-1KO/Pim-2KO/Akt-1Het cells were 59% viable, cultures Pim-1Het/Pim-2KO/Akt-1KO cells were 43% viable, and cultures of Pim-1KO/Pim-2KO/Akt-1KO cells were 41% viable. In addition, cell cycle analysis after 24 hours of IL-3 stimulation revealed impaired cell cycle entry in Akt-1– or Pim-2–deficient cells and greater impairment when both genes were deleted (Figure 2C). Together, these data suggest that Pim-2 and Akt-1 make overlapping contributions to the regulation of IL-3–dependent hematopoietic cell growth, survival, and proliferation and reinforce the idea of genetic interaction between the 2 pathways.
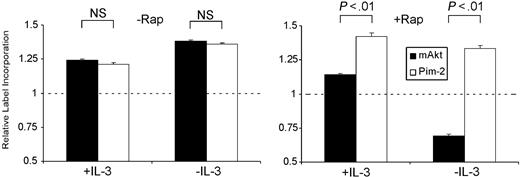
Pim-2 promotes rapamycin-insensitive translation and cell growth. Although the loss of Pim-2 results in fairly minor phenotypes, a coincident impairment in Akt activity by gene deletion or rapamycin treatment results in severe impairments in hematopoietic cell function. Therefore, we investigated whether ectopic expression of Pim-2 and Akt-1, each of which leads to increases in growth factor–independent cell survival, might also make independent phenotypic contributions. Expression of either transgene is associated with phosphorylation and inactivation of the 4EBP-1 translational repressor, suggesting that these kinases play a role in the regulation of translation. To compare the effects of these proteins on translation, myristolated Akt-1 (mAkt) or Pim-2 was stably introduced into the IL-3–dependent cell line FL5.12. Measurements of 35S incorporation from cells expressing mAkt and Pim-2 revealed that each transgene maintained higher rates of label incorporation when standardized to cells transfected with Bcl-XL, which confers an equivalent level of apoptotic resistance but fails to prevent cellular atrophy or decreased label incorporation after growth factor withdrawal (Figure 3).25 However, the effect of the Pim-2 and Akt-1 transgenes was distinguishable because the mAkt-dependent increase in 35S incorporation was suppressed by rapamycin treatment (Figure 3), particularly in the absence of IL-3 when endogenous Pim-2 would not be expressed. Pim-2 stimulation of 35S incorporation was unaffected by rapamycin in the presence or absence of IL-3.
Compound effects of germline deletion of Pim-1, Pim-2, and Akt-1. (A, left) Cell size measurements are depicted from IL-3–dependent bone marrow cultures from wild-type (WT), Pim-1–null/Pim-2–heterozygous/Akt-1–heterozygous (Pim-1KO/Pim-2Het/Akt-1Het), Pim-1–null/Pim-2–heterozygous/Akt-1–null (Pim-1KO/Pim-2Het/Akt-1KO), Pim-1–heterozygous/Pim-2–null/Akt-1–null (Pim-1Het/Pim-2KO/Akt-1KO), Pim-1–null/Pim-2–null/Akt-1–heterozygous (Pim-1KO/Pim-2KO/Akt-1Het), and Pim-1–null/Pim-2–null/Akt-1–null (Pim-1KO/Pim-2KO/Akt-1KO) animals. Data represent mean ± SD of 3 independent samples of live G1 cells. (Right) Lysates generated from these cells were probed for the expression of Pim-1, Pim-2, Akt-1, and Actin. (B) Cell accumulation after 48 hours of culture is shown for the cells described in panel A. Live cells were seeded in fresh IL-3 containing media at 105 cells/mL, as indicated by the dashed line, after synchronization by 12 hours of IL-3 withdrawal. Data represent mean ± SD of triplicate samples. (C) Cell cycle profiles from the cells described in panel B 24 hours after plating. The percentage of cells with greater than G1 DNA content is indicated.
Compound effects of germline deletion of Pim-1, Pim-2, and Akt-1. (A, left) Cell size measurements are depicted from IL-3–dependent bone marrow cultures from wild-type (WT), Pim-1–null/Pim-2–heterozygous/Akt-1–heterozygous (Pim-1KO/Pim-2Het/Akt-1Het), Pim-1–null/Pim-2–heterozygous/Akt-1–null (Pim-1KO/Pim-2Het/Akt-1KO), Pim-1–heterozygous/Pim-2–null/Akt-1–null (Pim-1Het/Pim-2KO/Akt-1KO), Pim-1–null/Pim-2–null/Akt-1–heterozygous (Pim-1KO/Pim-2KO/Akt-1Het), and Pim-1–null/Pim-2–null/Akt-1–null (Pim-1KO/Pim-2KO/Akt-1KO) animals. Data represent mean ± SD of 3 independent samples of live G1 cells. (Right) Lysates generated from these cells were probed for the expression of Pim-1, Pim-2, Akt-1, and Actin. (B) Cell accumulation after 48 hours of culture is shown for the cells described in panel A. Live cells were seeded in fresh IL-3 containing media at 105 cells/mL, as indicated by the dashed line, after synchronization by 12 hours of IL-3 withdrawal. Data represent mean ± SD of triplicate samples. (C) Cell cycle profiles from the cells described in panel B 24 hours after plating. The percentage of cells with greater than G1 DNA content is indicated.
Pim-2 promotes rapamycin-insensitive translation. Four-hour 35S incorporation of FL5.12 cells expressing myristolated Akt-1 (mAkt) or Pim-2 grown in the presence of IL-3 (+IL-3) or in the absence of IL-3 (-IL-3) treated with vehicle (-Rap) or 20 nM rapamycin (+Rap) for 24 hours before and during cysteine/methionine labeling. Data represent mean ± SD of triplicate samples of label incorporation of Akt-1– and Pim-2–expressing cells normalized to counts from similarly treated Bcl-XL–expressing cells, indicated by the dashed line. Significance testing is indicated above the bars.
Pim-2 promotes rapamycin-insensitive translation. Four-hour 35S incorporation of FL5.12 cells expressing myristolated Akt-1 (mAkt) or Pim-2 grown in the presence of IL-3 (+IL-3) or in the absence of IL-3 (-IL-3) treated with vehicle (-Rap) or 20 nM rapamycin (+Rap) for 24 hours before and during cysteine/methionine labeling. Data represent mean ± SD of triplicate samples of label incorporation of Akt-1– and Pim-2–expressing cells normalized to counts from similarly treated Bcl-XL–expressing cells, indicated by the dashed line. Significance testing is indicated above the bars.
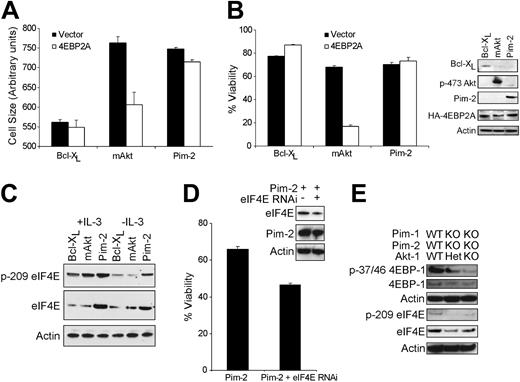
Akt promotes increased protein translation through mTOR-dependent phosphorylation and inactivation of 4EBP-1. Therefore, to examine the differential effects of rapamycin on Akt-1 and Pim-2 stimulation of cell growth, we used a 4EBP-1 mutant in which threonines 37/46 were mutated to alanines (4EBP2A) to prevent its dissociation from eIF4E in a dominant-negative fashion. Although the size of Pim-2– and Bcl-XL–expressing cells was minimally affected by the 4EBP2A transgene, we noted a precipitous decline in the size of Akt-1–expressing cells (Figure 4A). In addition, cell viability measurements indicated that the 4EBP2A construct resulted in a survival decrease in Akt-1– but not in Pim-2–expressing cells, indicating that Pim-2 and Akt-1 differed in their sensitivity to this 4EBP-1 mutant (Figure 4B).
These data suggested that the survival phenotype conferred by Pim-2 did not require 4EBP-1 phosphorylation or that the regulation of translation was dispensable for Pim-2–mediated cell survival. Given that Pim-2 expression promotes cell growth and that Pim-2 cannot promote growth factor–independent cell growth or survival when translation is inhibited by cycloheximide,18 it seems that the antiapoptotic effects of Pim-2 require sustained protein translation. However, it remained unclear how Pim-2 might decrease the sensitivity of a cell to 4EBP2A. To this end, we examined the possibility that Pim-2 might modulate the expression or activity of eIF4E, the proposed limiting component of the cap-dependent translation initiation complex, thereby providing eIF4E levels in excess of what could be titrated by 4EBP2A expression. Western blot analysis of FL5.12 cells indicated that Pim-2–expressing cells maintained higher levels of total and phosphorylated eIF4E in the presence and absence of growth factor than cells expressing Akt-1 or Bcl-XL (Figure 4C). Quantitative polymerase chain reaction (PCR) measurements of eIF4E mRNA showed no significant Pim-2–mediated increase in eIF4E mRNA, suggesting that this effect is posttranscriptional and eIF4E protein levels were not affected by rapamycin treatment (data not shown). In addition, eIF4E participates in Pim-2–dependent survival because its repression through RNAi decreased growth factor–independent Pim-2–induced cell survival (Figure 4D). Furthermore, bone marrow cells deficient in Pim-1, Pim-2, and Akt-1 displayed decreased phosphorylation of 4EBP-1 and decreased expression and phosphorylation of eIF4E (Figure 4E).
Pim-2 mediates 4EBP-1–independent cell growth and survival. (A) Cell size 24 hours after transfection and growth factor withdrawal shown for FL5.12 cells expressing Bcl-XL, myristolated Akt-1 (mAkt), or Pim-2 transiently transfected with empty vector (Vector) or an HA-tagged 4EBP2A expression vector in which threonines 37/46 have been mutated to alanines (4EBP2A). Measurements shown are forward scatter of live cells from each sample from triplicate samples and indicate the mean ± SD. (B, left) Cell viability measurements after 48 hours of IL-3 withdrawal are shown for FL5.12 cells described in panel A. Data shown are the mean ± SD of 3 independent samples. (Right) Lysates prepared from these cells were probed for the expression of Bcl-XL, phospho-serine 473 Akt (p-473 Akt), Pim-2, HA-4EBP2A, and Actin. (C) Lysates generated from FL5.12 cells expressing Bcl-XL, myristolated Akt (mAkt), or Pim-2 grown in the presence (+IL-3) or absence (-IL-3) of IL-3 for 24 hours were probed for the expression of phospho-serine 209 eIF4E (p-209 eIF4E), total eIF4E, and Actin. (D) Viability is shown 24 hours after IL-3 withdrawal for Pim-2–expressing FL5.12 cells stably transfected with a plasmid expressing an shRNA targeting eIF4E and control. (Inset) Lysates were probed for the expression of eIF4E, Pim-2, and Actin. (E) Lysates were generated from IL-3–dependent bone marrow cell lines described in Figure 3. The genotype of each cell line is indicated above the immunoblots. Western blots of lysates were probed for the expression of phospho-threonine 37/46 4EBP-1, 4EBP-1, Actin, phospho-serine 209 eIF4E, eIF4E, and Actin as indicated.
Pim-2 mediates 4EBP-1–independent cell growth and survival. (A) Cell size 24 hours after transfection and growth factor withdrawal shown for FL5.12 cells expressing Bcl-XL, myristolated Akt-1 (mAkt), or Pim-2 transiently transfected with empty vector (Vector) or an HA-tagged 4EBP2A expression vector in which threonines 37/46 have been mutated to alanines (4EBP2A). Measurements shown are forward scatter of live cells from each sample from triplicate samples and indicate the mean ± SD. (B, left) Cell viability measurements after 48 hours of IL-3 withdrawal are shown for FL5.12 cells described in panel A. Data shown are the mean ± SD of 3 independent samples. (Right) Lysates prepared from these cells were probed for the expression of Bcl-XL, phospho-serine 473 Akt (p-473 Akt), Pim-2, HA-4EBP2A, and Actin. (C) Lysates generated from FL5.12 cells expressing Bcl-XL, myristolated Akt (mAkt), or Pim-2 grown in the presence (+IL-3) or absence (-IL-3) of IL-3 for 24 hours were probed for the expression of phospho-serine 209 eIF4E (p-209 eIF4E), total eIF4E, and Actin. (D) Viability is shown 24 hours after IL-3 withdrawal for Pim-2–expressing FL5.12 cells stably transfected with a plasmid expressing an shRNA targeting eIF4E and control. (Inset) Lysates were probed for the expression of eIF4E, Pim-2, and Actin. (E) Lysates were generated from IL-3–dependent bone marrow cell lines described in Figure 3. The genotype of each cell line is indicated above the immunoblots. Western blots of lysates were probed for the expression of phospho-threonine 37/46 4EBP-1, 4EBP-1, Actin, phospho-serine 209 eIF4E, eIF4E, and Actin as indicated.
Pim-2 and Akt-1 cooperate to promote cell growth, survival, and tumor formation in vivo
Given that the deletion of Pim-2 and Akt-1 led to combinatorial phenotypes in cell lines, we sought to determine whether coexpression of these transgenes might cooperate in the regulation of cell growth and survival in vivo. To this end, both mAkt-1 transgenic (Akt TG) and Pim-2 transgenic (Pim-2 TG) animals were bred in which the transgenes were expressed downstream of the T cell–specific Lck promoter. T cells were isolated from these mice at 4 weeks of age and were used to generate lysates for Western blot analysis to confirm transgene expression and to assess the expression and phosphorylation status of 4EBP-1 and eIF4E. We observed increased 4EBP-1 phosphorylation at residues 37/46 and 70 in the Pim-2, Akt-1, and double-transgenic mice compared with littermate controls (Figure 5A), though Akt-1 appeared to induce phosphorylation at residues 37/46 to a greater degree than did Pim-2. Phosphorylated and total eIF4E levels were increased in the Pim-2 and double-transgenic mice compared with Akt-1 transgenic or control mice (Figure 5A).
In the absence of cytokine stimulation, murine T cells undergo progressive atrophy and apoptosis in culture.26 T cells were isolated from wild-type, Pim-2, Akt-1, and double-transgenic mice to determine the effects of transgene expression on cell size and survival. Cell size analysis performed immediately after isolation demonstrated increased T cell size in the Pim-2 and Akt-1 transgenic mice and an additive increase in the size of T cells from double-transgenic mice (Figure 5B). Cell survival, as assayed 24 hours after the beginning of culture in the absence of cytokines, revealed that the expression of either or both transgenes led to increased T cell survival compared with control and that coexpression of the transgenes led to an additive increase in survival (Figure 5C). These data suggested that Akt-1 and Pim-2 could function to independently promote cell growth and survival in primary cells, as indicated by their cooperative effects on these processes.
If Pim-2 and Akt-1 are critical components of overlapping but independent growth and survival pathways, they might show oncogenic cooperativity. Tumor formation should represent the integration of the phenotypes conferred by transgene expression, including the promotion of cell growth, survival, and proliferation. A comparison of littermate (wild type), Pim-2 transgenic, Akt-1 transgenic, and double-transgenic mice revealed that double-transgenic mice developed tumors with a penetrance of 100% by 12 weeks of age, whereas no Pim-2 transgenic animals and only 1 of 10 Akt-1 transgenic mice developed thymic lymphoma by 24 weeks of age (Figure 5D). Thus, Pim-2 and Akt-1 display cooperativity in the regulation of cell growth, survival, and tumor formation.
Pim-2 and Akt cooperate to promote increases in cell size, cell survival, and tumorigenesis in transgenic animals. (A) Lysates prepared from thymuses of littermate control, Akt1 transgenic (Akt TG), Pim-2 transgenic (Pim-2 TG), and double-transgenic animals were probed for the expression of phosphothreonine 37/46 4EBP-1 (p-37/46 4EBP-1), phospho-threonine 70 4EBP-1, 4EBP-1, phospho-serine 209 eIF4E (p-209 eIF4E), eIF4E, Pim-2, phospho-serine 473 Akt (p-473 Akt), and Actin. A nonspecific band is observed above Pim-2 in all lanes of the sixth blot. (B) Cell size measurements are shown from peripheral T cells isolated from wild-type (WT), Pim-2 transgenic (Pim-2), Akt transgenic (Akt), and compound transgenic (Akt + Pim-2) cells. Data represent mean ± SD of 3 independent samples. *Significance (P < .05) compared with wild-type cells (analysis of variance [ANOVA]). (C) Viability is shown for transgenic T cells 24 hours after isolation. Mean ± SD of triplicate measurements are depicted. *Significance (P < .05) compared with wild-type cells (ANOVA). (D) Incidence of lethal lymphoma in transgenic mice of the following genotypes: WT, Pim-2 TG, Akt TG, and Akt TG + Pim-2 TG. Ten mice are included for each genotype.
Pim-2 and Akt cooperate to promote increases in cell size, cell survival, and tumorigenesis in transgenic animals. (A) Lysates prepared from thymuses of littermate control, Akt1 transgenic (Akt TG), Pim-2 transgenic (Pim-2 TG), and double-transgenic animals were probed for the expression of phosphothreonine 37/46 4EBP-1 (p-37/46 4EBP-1), phospho-threonine 70 4EBP-1, 4EBP-1, phospho-serine 209 eIF4E (p-209 eIF4E), eIF4E, Pim-2, phospho-serine 473 Akt (p-473 Akt), and Actin. A nonspecific band is observed above Pim-2 in all lanes of the sixth blot. (B) Cell size measurements are shown from peripheral T cells isolated from wild-type (WT), Pim-2 transgenic (Pim-2), Akt transgenic (Akt), and compound transgenic (Akt + Pim-2) cells. Data represent mean ± SD of 3 independent samples. *Significance (P < .05) compared with wild-type cells (analysis of variance [ANOVA]). (C) Viability is shown for transgenic T cells 24 hours after isolation. Mean ± SD of triplicate measurements are depicted. *Significance (P < .05) compared with wild-type cells (ANOVA). (D) Incidence of lethal lymphoma in transgenic mice of the following genotypes: WT, Pim-2 TG, Akt TG, and Akt TG + Pim-2 TG. Ten mice are included for each genotype.
Discussion
Pim-2 and Akt-1 are growth factor–regulated serine/threonine kinases that function to promote cell growth, survival, and transformation. Endogenous Pim-2 activity was required for cells to resist growth arrest and apoptosis induced by rapamycin treatment. Although deletion of either kinase led to relatively minor phenotypes, deletion of both resulted in the sub-Mendelian generation of anemic progeny that manifested marked defects in hematopoietic cell function. Ectopic expression of Pim-2 and Akt-1 led to mechanistically independent increases in cell growth and survival, and coexpression led to increased cell growth, survival, and tumor formation in transgenic animals.
The present data suggest that Pim-2 and Akt-1 are critical components of distinct pathways that play important roles in the regulation of a variety of cellular functions, including cell growth, proliferation, and survival. Previous studies have indicated that the loss of Pim-1 and Pim-2 leads to modest developmental and cellular phenotypes. However, the ability of endogenous Pim-2 to promote hematopoietic cell growth and survival in the presence of pharmacologic doses of rapamycin suggests that understanding how Pim kinases contribute to cell growth and survival is important. That Pim-1 only partially compensates for the loss of Pim-2 in preventing rapamycin-induced inhibition of hematopoietic cell growth and survival suggests that Pim-1 and Pim-2 have biologic roles that do not completely overlap, possibly because of differences in the kinetics of their induction or of their relative roles in specific cell types. Although only a limited amount of information is known about how Pim-2 functions mechanistically, previous work has shown that ectopic Pim-2 expression appears to share several properties with oncogenic Akt. Both oncogenes have been reported to induce phosphorylation of the proapoptotic protein BAD and to lead to the activation of NF-κB.27-30 The present data demonstrate that endogenous levels of Pim-2 and Akt-1 also provide mechanistically distinct but functionally overlapping control of hematopoietic cell proliferation.
These data may to help explain the remarkable safety profile of rapamycin when it is used as a chemotherapeutic agent because the endogenous activity of Pim kinases appears to be sufficient to support the growth and survival of nontransformed hematopoietic cells treated with rapamycin. Moreover, the function of Pim-2 may account for the maintenance of immune function in cancer patients treated with rapamycin, an initial concern when patients are treated for leukemia with this potentially immunosuppressive agent. The data also suggest that the activation of Pim-2 may play an important role in leukemic cell growth resistant to rapamycin chemotherapy. For example, FMS-like tyrosine kinase 3 (FLT3) transformation is associated with increased Pim-2 expression; we have recently observed that RNAi inhibition of Pim-2 blocks the survival of cells transformed with FLT3 but not with BCR/ABL (manuscript submitted, September 2004). These data suggest that the development of Pim kinase inhibitors may prove useful in the treatment of specific hematologic malignancies. Given the mild phenotype of Pim knockout mice, it is anticipated that these inhibitors would have mild adverse effect profiles.
Although Pim-2 and Akt-1 have been shown to regulate cell survival by increasing nutrient uptake, activating NF-κB, and restraining the proapoptotic molecule BAD, the present data suggest that these 2 pathways can be distinguished by the mechanisms through which they promote increased translation. Translation is tightly coupled to cellular growth and proliferation and is regulated by a variety of oncogenes. Deregulated translation is an increasingly appreciated feature of cellular transformation. It has been demonstrated that the deregulation of cellular signaling components, such as Ras/MAPK and Akt, leads to the translation of pathway-specific mRNAs associated with tumor growth.31 Although a well-defined pathway has been described for the Akt-mediated regulation of translation involving the modulation of mTOR activity, more work is needed to better understand the rapamycin-insensitive regulation of cell growth and translation by Pim-2. The observed effects of Pim-2 on 35S incorporation may result from differences in amino acid uptake, size of amino acid pools, and rate of protein synthesis. Better understanding of the effects of Pim-2 on cell growth would allow for the generation of therapeutic agents to target translation in tumors in which Pim kinases are activated or in which rapamycin resistance is encountered. Several agents that target eIF4E are now in development,32 and it would be of interest to use these to target tumors in which Pim kinases are overexpressed.
In conclusion, our data support the idea that functional overlap between families of kinases rather than overlap in the function of individual members of a given family can contribute to the minimal phenotypes observed in some single-gene deletions. Akt-1 deletion or rapamycin treatment reveals a required role for Pim-2 in the compensatory regulation of critical cellular functions. It is possible that this overlap in the control of hematopoietic cell growth and survival was selected during evolution to safeguard against microbial inhibition of individual pathways, as exemplified here by the bacterially derived compound rapamycin.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-09-3706.
Supported in part by grants from the National Institutes of Health and the National Cancer Institute and by a Cancer Research Institute predoctoral training grant.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank T. Lindsten for the generation of the Pim-2 transgenic mouse. We thank N. Sonenberg and P. Rothman for sharing reagents and mice. We thank D. Plas and D. Bauer for helpful editorial comments.


![Figure 5. Pim-2 and Akt cooperate to promote increases in cell size, cell survival, and tumorigenesis in transgenic animals. (A) Lysates prepared from thymuses of littermate control, Akt1 transgenic (Akt TG), Pim-2 transgenic (Pim-2 TG), and double-transgenic animals were probed for the expression of phosphothreonine 37/46 4EBP-1 (p-37/46 4EBP-1), phospho-threonine 70 4EBP-1, 4EBP-1, phospho-serine 209 eIF4E (p-209 eIF4E), eIF4E, Pim-2, phospho-serine 473 Akt (p-473 Akt), and Actin. A nonspecific band is observed above Pim-2 in all lanes of the sixth blot. (B) Cell size measurements are shown from peripheral T cells isolated from wild-type (WT), Pim-2 transgenic (Pim-2), Akt transgenic (Akt), and compound transgenic (Akt + Pim-2) cells. Data represent mean ± SD of 3 independent samples. *Significance (P < .05) compared with wild-type cells (analysis of variance [ANOVA]). (C) Viability is shown for transgenic T cells 24 hours after isolation. Mean ± SD of triplicate measurements are depicted. *Significance (P < .05) compared with wild-type cells (ANOVA). (D) Incidence of lethal lymphoma in transgenic mice of the following genotypes: WT, Pim-2 TG, Akt TG, and Akt TG + Pim-2 TG. Ten mice are included for each genotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-09-3706/6/m_zh80110579270005.jpeg?Expires=1769254827&Signature=IMSmAEFsMC2IwhWbhXmpwdu-Arni1MaXWUsqLxg39B7wsmqgVM0IAG7xYUYj-Hb1YfUudIunfXDUFQ6YLD5X2na99LHBOPQX-ZlCwxROhjE9nvPz5jIc-RV3LnnxYdb6MYS715iKlN4ryXrUGr0eG3gG4YH0dAMM5GWy1VZxImpb5mowZiD9PWe54X~vSIJ6hU6XkQt5YQCWk9Sj8VsbRBcpBIaYJ9Uy6bIA~fzR9cc1fYiCt0IU03Br3XX6Zm1Zor7l12SE81175Nka7JlfmS0grKAsSIyhqMCHIsHmQdburGumXKoZkcy-GQR43I2oPG-ZUrUPFNpgIkVJY43Frg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal