Abstract
Multiple myeloma (MM) is a B-cell neoplasm that is characterized by the clonal expansion of malignant plasma cells and is frequently associated with chromosomal translocations placing an oncogene under the control of the immunoglobulin heavy chain enhancer. Despite these pathogenic translocations, MM cells remain dependent on external cues for survival. We present evidence that brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, and its high-affinity receptor, tropomyosin receptor kinase B (TrkB), contribute to these survival cues. MM cells express TrkB, and respond to BDNF by activating mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase–a PI3K target (PI3K/Akt) signaling cascades. Addition of BDNF protects human MM cell lines (HMCLs) from apoptosis induced by dexamethasone or bortezomib and prolongs the survival of primary MM cells cultured alone or with human bone marrow (BM) stroma. As BDNF and TrkB are expressed by osteoblasts, stromal cells, and endothelial cells within the BM microenvironment, a BDNF-TrkB axis may be critical to the interactions of MM with bone and stroma that contribute to MM tumor progression. Finally, BDNF is expressed by malignant plasma cells isolated from a subset of patients with MM, as well as by most HMCLs, suggesting a potential role for this neurotrophin axis in autocrine as well as paracrine support of MM.
Introduction
Multiple myeloma (MM) is a B-cell neoplasm, characterized by the clonal expansion of malignant plasma cells, and frequently associated with chromosomal translocations placing an oncogene under the control of the immunoglobulin heavy chain enhancer.1 These translocations typically occur during immunoglobulin heavy chain switch recombination, but they may also occur as a result of errors in somatic hypermutation. Recurrent translocation partners deregulated in MM include cyclin D1 and cyclin D3, the transcription factors c-musculoaponeurotic fibrosarcoma (c-MAF) and myeloma SET (single exon transfer) domain (MMSET), and fibroblast growth factor receptor 3 (FGFR3). Despite these pathogenic translocations, malignant plasma cells retain their dependency on external signals for survival. Such signals include interleukin 6 (IL-6), insulin-like growth factor-1 (IGF-1), hemopoietic growth factor, basic fibroblast growth factor, B-lymphocyte stimulator (BLyS), and integrin-mediated cellular contact.2-7 We present evidence that neurotrophin-receptor interactions, involving brain-derived neurotrophic factor (BDNF) and its high-affinity receptor, tropomyosin receptor kinase B (TrkB), contribute to the survival signals necessary for MM tumor progression.
The neurotrophin family of growth factors includes BDNF, NGF (nerve growth factor), NT-3 (neurotrophin-3) and NT-4/5 (neurotophin-4/5). Each neurotrophin binds to a specific Trk: NGF binds to TrkA, BDNF and NT-4/5 bind to TrkB, and NT-3 binds to TrkC. In addition, neurotrophins share a common receptor, p75NTR, a member of the tumor necrosis factor (TNF) receptor superfamily.8 Although structurally related to TNF, neurotrophins are secreted as homodimers rather than trimers and induce receptor dimerization rather than trimerization. Trk receptor dimerization triggers kinase activation and the initiation of signaling cascades, including MEK/MAPK (mitogen-activated protein kinase kinase–mitogen-activated protein kinase) and PI3K/Akt (phosphatidylinositol-3 kinase–a PI3K target), that favor cellular survival.9 In contrast, p75NTR engagement triggers divergent pathways to modulate survival, cell cycle regulation, and cytoskeletal rearrangement.9 Thus, while p75NTR binding can increase the affinity and specificity of Trk-neurotrophin interactions, p75NTR can also induce apoptosis when Trk activation is reduced or absent.10,11 The overall cellular response to neurotrophin exposure reflects a balance between p75NTR and Trk engagement.
Neurotrophins are synthesized as proneurotrophins and are enzymatically cleaved to yield mature proteins. Cleavage can occur either intracellularly, by the action of furin or proconvertase, or extracellularly, by the action of plasmin, matrix metalloproteinase 7 (MMP-7) or MMP-9.11,12 Both mature neurotrophins and proneurotrophins are biologically active, with divergent roles that reflect differing receptor affinities: proneurotrophins display greater affinities for p75NTR, whereas mature neurotrophins display greater affinities for their respective Trks.11 Thus, regulation of proneurotrophin processing adds additional control over the balance between p75NTR and Trk engagement.
Both BDNF and TrkB are expressed by T and B lymphocytes, as well as by vascular endothelial cells and platelets.13-20 Within bone marrow (BM), BDNF has been found in osteoblasts, megakaryocytes, endothelial cells, and adventitial reticular cells, a subset of BM stroma characterized by p75NTR expression and close anatomic association with nonmalignant plasma cells.21,22
We find that malignant plasma cells from most patients with MM, and most human MM cell lines (HMCLs), express TrkB and do not express p75NTR. Further, we find that BDNF activation of TrkB on malignant plasma cells initiates prosurvival signals, resulting in phosphorylation of prosurvival factors CREB (cAMP [cyclic adenosine monophosphate] response element binding protein), Akt, and MAPK p42/44. Consistent with a prosurvival role for TrkB signaling in MM, we find that BDNF protects HMCL RPMI 8226 from dexamethasone-induced apoptosis, delays onset of bortezomib-induced apoptosis in HMCL JJN3, and prolongs the survival of primary MM plasma cells cultured alone or with the human BM stromal cell line HS5. As BDNF and TrkB are expressed by osteoblasts, stromal cells, and endothelial cells within the marrow microenvironment, a BDNF/TrkB axis may contribute to the interactions of MM with bone and stroma that contribute to MM tumor progression. We also find that BDNF is expressed by malignant plasma cells isolated from a subset of patients with MM, as well as by most HMCLs, suggesting a potential role for this neurotrophin axis in autocrine as well as paracrine support of MM.
Materials and methods
Cell lines and reagents
HMCL National Cancer Institute (NCI) H929 and Roswell Park Memorial Institute (RPMI) 8226 were obtained from the American Type Culture Collection (Manassas, VA); LP1, KMS11, UTMC2, and JJN3 were provided by Leif Bergsagel (Mayo Clinic, Scottsdale, AZ); OPM1 and OPM2 were provided by Edward Thompson (University of Texas, Galveston, TX); ARP-1 was provided by Rena Feinman (University of Medicine and Dentistry of New Jersey, Newark, NJ). Human Waldentrom macroglobulinemia cell line, WSU-WM, was a gift of Ramzi Mohammad (Wayne State University, Detroit, MI).23 PC12-TrkB (PC12 rat pheochromocytoma cell line transfected with TrkB) was the gift of Pantelis Tsoulfas (University of Miami School of Medicine, Miami, FL). Human BM stromal cell line HS5 was provided by Beverly Torok-Storb (Fred Hutchinson Cancer Research Center, Seattle, WA). Primary MM, HMCL, WSU-WM, and HS5 were cultured in RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and 50 μM β-mercaptoethanol (Sigma, St Louis, MO). PC12 and PC12-TrkB were cultured in 45% Dulbecco modified Eagle medium (Mediatech), 45% Ham solution (Mediatech), 5% FBS, and 5% horse serum (Invitrogen). PC12-TrkB cells were maintained in 400 μg/mL G418 sulfate (Invitrogen). Recombinant human IGF-1, BDNF, NGF, osteoprotegerin (OPG), and chicken anti–human BDNF were obtained from R&D Systems (Minneapolis, MN). Mouse anti–human p75NTR (7F10) was obtained from Novocastra Laboratories (Newcastle, United Kingdom). Rabbit anti-p75NTR (9992) was obtained from Promega (Madison, WI). Rabbit anti-BDNF (N-20), rabbit anti-TrkB (H-181), rabbit anti-Trk kinase (C-14), and mouse anti-Trk kinase (B-3) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). LY294002 and mouse anti–human BDNF were obtained from Calbiochem (La Jolla, CA). Donkey anti–rabbit IgG-HRP (horseradish peroxidase) and donkey anti–mouse IgG-HRP were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Rabbit anti-Akt (9272), anti-phosphoAkt (9271), anti-phosphoCREB/ATF (activating transcription factor) (9191), phospho-CREB blocking peptide (1090), and anti-phosphoMAPK (9101) were obtained from Cell Signaling Technology (Beverly, MA). Fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody to human CD138 (clone BB4) was obtained from Biosource International (Camarillo, CA). Biotinylated goat anti–chicken IgY, biotinylated goat anti–rabbit IgG, biotinylated goat anti–mouse IgG, goat serum, Vectashield, Vectastain Elite ABC Kit, Vector VIP kit, Vector DAB, Avidin/Biotin Block, and hematoxylin were obtained from Vector Laboratories (Burlingame, CA). Avidin-rhodamine and DAPI (4′,6-diamidino-2-phenylindole) were from Molecular Probes (Eugene, OR). Fetal calf serum, TRIzol, trypan blue stain, and random primers were obtained from Invitrogen. Immobilon-P transfer membranes were obtained from Millipore (Bedford, MA). Enhanced chemiluminescence (ECL) reagent, Protein A-Sepharose, Sephadex G50, Ficoll-Paque Plus, and Iodine-125 were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Avian myeloblastosis virus (AMV) reverse transcriptase was obtained from Boehringer Mannheim (Mannheim, Germany). Taq polymerase was obtained from Serological Corp (Atlanta, GA). Lactoperoxidase, TWEEN20, and hydrogen peroxide were obtained from Sigma.
Isolation of primary MM plasma cells
This study was performed in accordance with federal and institutional guidelines for human subject research. Plasma cells were isolated from heparinized BM aspirates obtained from patients at The Center for Myeloma and Lymphoma of Cornell University after informed consent under a protocol approved by the institutional review board of Cornell University. The mononuclear cell fraction was separated by Ficoll density centrifugation. Plasma cells were isolated from the mononuclear fraction using anti–human CD138 microbeads (Miltenyi Biotec, Auburn, CA) by AutoMACS according to manufacturer's instructions. Purity of plasma cells ranged between 60% and 90%, based on fluorescence-activated cell sorting (FACS) analysis of CD138 expression (FACSCaliber; BD Biosciences, San Jose, CA).
Immunohistochemistry for BDNF
Cytospins of Ficoll-separated BM aspirates were fixed for 15 minutes with 2% paraformaldehyde in phosphate-buffered saline (PBS). Endogenous peroxidase activity was inhibited by incubating samples with 0.1% hydrogen peroxide in methanol for 30 minutes at -20°C. Samples were blocked for 1 hour at room temperature, using blocking solution (5% bovine serum albumin [BSA], 1% Triton-X100, 5% goat serum in PBS), and then incubated overnight at 4°C in blocking solution with chicken anti–human BDNF (5 μg/mL) in blocking buffer. Chicken anti–human BDNF precleared with BDNF (10 μg/mL) was used as negative control. After washing, samples were incubated for 1 hour at room temperature in blocking solution containing biotinylated goat anti–chicken IgY (1:200). The samples were then incubated with Vectastain Elite ABC substrate kit to form avidin-biotin complexes and visualized by either Vector DAB or Vector VIP according to manufacturer's instructions. Hematoxylin was used as counterstain. Slides were coverslipped and sealed using cytoseal (Richard Allen Scientific, Kalamazoo, MI). Images were captured using an Olympus BX51 microscope (Olympus America, Melville, NY), and Olympus UPLNFLN 100×/1.30 NA oil immersion objective, and a Regita EX CCD digital camera (QImaging, Burnaby, BC, Canada). Images were processed using QCapture Suite 2.68 (QImaging) and Photoshop 6.0 (Adobe Systems, San Jose, CA).
Immunofluorescence
TrkB/CD138. Cytospins of Ficoll-separated BM aspirates were fixed for 10 minutes at -20°C using methanol-acetone (1:1), incubated for 1 hour at room temperature in blocking solution (5% BSA, 1% Triton-X100, 5% goat serum in PBS), and then incubated in Avidin/Biotin Block (Vector Laboratories) according to manufacturer's instructions. Samples were then incubated overnight at 4°C in blocking solution with rabbit anti-TrkB or rabbit IgG control at 10 μg/mL. After washing, samples were incubated for 1 hour at room temperature in blocking solution containing biotinylated goat anti–rabbit IgG at 5 μg/mL. Samples were then incubated for 1 hour at room temperature with avidin-rhodamine (1:500 in 0.15 M NaCl, 0.1 M bicarbonate, pH 8.3). Samples were again blocked for 30 minutes at room temperature, after which samples were incubated with either mouse anti–CD138-FITC (1:1) or mouse IgG1-FITC control. Samples were then incubated in DAPI (100 nM), coverslipped, sealed using Vectashield mounting media.
PhosphoAkt/CD138 and phosphoCREB/CD138. BM mononuclear cells were incubated in RPMI 1640 with or without BDNF (50 ng/mL) for 5 minutes at 37°C and then centrifuged onto glass slides. Cytospins were fixed in 4% paraformaldehyde for 10 minutes, permeabilized with 2% Triton X-100 in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) for 5 minutes, blocked for 1 hour at room temperature using 5.5% goat serum/TBST (0.1% Triton X-100), and incubated overnight at 4°C with either rabbit anti-phosphoAkt or rabbit anti-phosphoCREB (1:250 in 3% BSA/TBS). After washing, samples were incubated for 1 hour at room temperature in 3% BSA/TBS containing biotinylated goat anti–rabbit IgG at 5 μg/mL. Samples were then incubated with avidin-rhodamine, blocked for 30 minutes at room temperature, incubated with mouse anti–CD138-FITC, coverslipped, and sealed using Vectashield mounting media.
Fluorescence microscopy. Images were captured using an Olympus BX51 microscope, and Olympus UPNFLN 40×/1.30 NA oil immersion objective, and a Regita EX CCD digital camera. Images were processed using QCapture Suite 2.68 and Photoshop 6.0.
BDNF ELISA
Samples of heparinized BM plasma were collected and stored at -20°C until the day of assay. Enzyme-linked immunosorbent assay (ELISA) was performed in triplicate using the BDNF Emax ImmunoAssay System (Promega) according to the manufacturer's instruction. Results were analyzed using the Mann-Whitney test.
Western blotting
Cells (107) were serum-starved overnight, exposed to ligand for 5 or 20 minutes at 37°C, resuspended in Triton lysis buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 0.6% Triton X-100; 10% glycerol; 5 mM ethylenediaminetetraacetic acid, pH 8.0; 2 mM dithiothreitol; 4 mM MgCl2; 50 mM NaF; 2 mM NaVO3; beta-glycerol phosphate [2 mg/mL]; Pepstatin, Leupeptin, and Aprotinin [5 μg/mL]; Benzamidine [30 μg/mL]; and 2 mM phenylmethyl-sulfonyl fluoride) and extracted for 30 minutes at 4°C on a rotator. Extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membrane, blocked in TBS with 0.05% TWEEN20, 5% nonfat dry milk (or 1% BSA for phospho-specific antibodies), and incubated overnight at 4°C with primary antibody at 1:1500. Membranes were then incubated for 1 hour at room temperature with appropriate HRP-conjugated secondary antibody at 1:6500 and developed using ECL.
RT-PCR
TRIzol was used to isolate total RNA from HMCL and WSU-WM cultured in RPMI 1640 supplemented with 10% FBS. First-strand cDNA synthesis was generated by using AMV reverse transcriptase and random primers. Polymerase chain reaction (PCR) was performed for 36 cycles (94°C for 1 minute, 62°C for 1 minute, 72°C for 1minute), followed by an extension step of 72°C for 10 minutes, using Taq polymerase. The primer pairs used for PCR were BDNF (5′-AACAATAAGGACGCAGACTT-3′/5′-TGCAGTCTTTTTGTCTGCCG-3′), TrkB (5′-AGGGCAACCCGCCCACGGAA-3′/5′-GGATCGGTCTGGGGAAAAG-3′), p75NTR (5′-CTGGACAGCCTGACGTTCTCC-3′/5′-CTGCCACCGTGCTGGCTATGA-3′), OPG (5′-TGCTGTTCCTACAAAGTTTACG-3′/5′-CTTTGAGTGCTTTAGTGCGTG-3′), and β-actin (5′-GTGACGAGGCCCAGAGCAAGAG-3′/5′-AGGGGCCGGACTCATCGTACTC-3′).
PC12 differentiation
Conditioned media were harvested from 5-day-old cultures of HMCLs plated at an initial density of 2 × 105 in RPMI 1640 supplemented with 1% FBS. PC12 and PC12-TrkB were cultured with HMCL conditioned media for 5 days with or without neutralizing monoclonal anti-BDNF antibody (10 μg/mL). Images were captured using a Nikon Axiophot inverted microscope (Nikon, Melville, NY), a Nikon 20×/0.40 NA PH1 ADL objective, and a Nikon COOLPIX 5700 digital camera. Images were processed using NikonView 6.0 and Photoshop 6.0. Neurite outgrowth was determined by morphologic examination and scored as the number of neurites with length greater than 2 soma.
Binding, internalization, and release of [125I]-BDNF
BDNF was iodinated to a specific activity of 2000 cpm/fmol, using a modification of the lactoperoxidase method, and separated from unincorporated 125I using Sephadex G50 column chromatography.24 [125I]-BDNF demonstrated 25% bioactivity compared with unlabeled BDNF when assayed for induction of neurite outgrowth using PC12-TrkB. Binding and internalization of [125I]-BDNF by human MM cell lines was analyzed as described.25 Briefly, JJN3 cells were cultured overnight in serum-free RPMI 1640; resuspended at 106 cells/mL in PBS supplemented with 1 mg/mL glucose, 2 mM CaCl, 1 mg/mL BSA; and divided into 200-μL aliquots. For binding studies, cells were then incubated with 0.1 nM [125I]-BDNF in the presence or absence of increasing concentrations of unlabeled BDNF (0-500 nM) for 1 hour at 4°C. Free and bound radioactivities were separated by centrifugation through calf serum at 4°C. Each condition was assessed in triplicate. Results were corrected for nonspecific binding, determined in parallel incubations with 500-fold excess BDNF, and are expressed as the mean ± standard deviation. GraphPad Prism (GraphPad Software, San Diego, CA) was used to generate the IC50 (inhibitory concentration 50%). Kd (distribution coefficient) was determined using the equation of Cheng and Prusoff.26 For internalization studies, cells were incubated with 0.1 nM [125I]-BDNF for variable times at either 4°C or 37°C, treated with 0.2 M acetic acid to strip surface-bound [125I]-BDNF, and pelleted. Each condition was assessed in triplicate, and the results are expressed as the mean ± standard deviation.
Cell growth and survival assay
Primary MM. CD138-immunoselected plasma cells were plated in RPMI 1640 supplemented with 1% FBS at a density of 5 × 104/mL. HS5 cells were used at a density of 104/cm2. BDNF (50 ng/mL), NGF (100 ng/mL), and osteoprotegerin (OPG) (500 ng/mL) were replenished every 3 days. RPMI 1640 supplemented with 10% FBS served as control. Cell viability was determined by trypan blue exclusion. Presented are the means ± standard deviations of results using cells isolated from 5 patients. Student t test was used to determine significance.
HMCL RPMI 8226. HMCL RPMI 8226 cells were plated in RPMI 1640 supplemented with 1% FBS at a density of 105/mL in 96-well plates with increasing concentrations of dexamethasone. BDNF (50 ng/mL), NGF (100 ng/mL), or OPG (500 ng/mL) was added on days 1 and 3. After 5 days, cell viability was determined by trypan blue exclusion. Each condition was assessed in triplicate, and the results are expressed as the mean plus or minus standard deviation. Student t test was used to determine significance.
HMCL JJN3. HMCL JJN3 cells were plated in RPMI 1640 supplemented with 1% FBS at a density of 105/mL in 24-well plates. BDNF (50 ng/mL), IGF (100 ng/mL), and LY294002 (5 μM) were added 12 hours prior to bortezomib (10 nM). Cells were harvested 24 hours after addition of bortezomib, stained with annexin V–FITC (BD Pharmingen, San Jose, CA), and analyzed by FACS. Each condition was assessed in triplicate, and the results are expressed as the mean ± standard deviation. Student t test was used to determine significance.
Results
Expression of BDNF by MM
BM aspirates from 25 patients with MM were analyzed for BDNF expression using immunohistochemistry. In 12 MM samples, malignant plasma cells exhibited robust BDNF immunoreactivity (Figure 1A-B), whereas erythrocyte and myeloid precursors were negative or weakly positive. Nonmalignant plasma cells enriched by CD138 immunoselection from BM aspirates of individuals without MM were also negative (data not shown). Expression of both mature BDNF protein, as well as uncleaved pro-BDNF, was evident in HMCL lysates by Western blot using either of 2 anti-BDNF antibodies (Figure 1C). Expression of BDNF mRNA by HMCL was confirmed by RT-PCR (Figure 1D).
To determine whether BDNF might be expressed and secreted by primary MM cells in vivo, heparinized BM plasma was isolated from 17 patients with MM and 9 patients without MM and analyzed for BDNF using ELISA (Figure 1E). The mean BDNF concentration in BM plasma from patients without MM was 72 pg/mL, consistent with prior studies documenting low levels in plasma from peripheral blood specimens.27 In contrast, 8 of 17 patients with MM had levels of BDNF significantly higher than patients without MM (150-900 pg/mL). However, this assay does not determine the cellular origin of BDNF within the BM. Elevated levels of BDNF in BM plasma of patients with MM may reflect production by the malignant clone or an effect of MM on surrounding cellular elements, including stromal fibroblasts, osteoblasts, endothelial cells, and megakaryocytes.
Expression of BDNF by malignant plasma cells. (A-B) Immunohistochemistry demonstrating BDNF expression by primary MM plasma cells. Representative cytospins of BM mononuclear cells freshly isolated from 2 patients with MM. Plasma cells show strong immunoreactivity using chicken anti–human BDNF but are not stained by chicken anti–human BDNF precleared with BDNF (inset). (C) Western blot demonstrating expression of both mature and pro-BDNF protein by HMCL. Expression was evident using either mouse anti-BDNF (shown) or rabbit (N-20) (not shown) antibodies. (D) Reverse transcriptase (RT)–PCR demonstrating BDNF mRNA expression by HMCL. No PCR products were evident in the absence of RT (not shown). (1) NCI H929, (2) RPMI 8226, (3) LP1, (4) ARP1, (5) OPM1, (6) KMS11, (7) JJN3, and (8) OPM2. (E) ELISA demonstrating increased BDNF in BM plasma of MM patients. Mean value for MM patients was 274 ± 71; patients without MM, 72 ± 22 (P < .05, Mann-Whitney test). * indicates significantly different from non-MM mean.
Expression of BDNF by malignant plasma cells. (A-B) Immunohistochemistry demonstrating BDNF expression by primary MM plasma cells. Representative cytospins of BM mononuclear cells freshly isolated from 2 patients with MM. Plasma cells show strong immunoreactivity using chicken anti–human BDNF but are not stained by chicken anti–human BDNF precleared with BDNF (inset). (C) Western blot demonstrating expression of both mature and pro-BDNF protein by HMCL. Expression was evident using either mouse anti-BDNF (shown) or rabbit (N-20) (not shown) antibodies. (D) Reverse transcriptase (RT)–PCR demonstrating BDNF mRNA expression by HMCL. No PCR products were evident in the absence of RT (not shown). (1) NCI H929, (2) RPMI 8226, (3) LP1, (4) ARP1, (5) OPM1, (6) KMS11, (7) JJN3, and (8) OPM2. (E) ELISA demonstrating increased BDNF in BM plasma of MM patients. Mean value for MM patients was 274 ± 71; patients without MM, 72 ± 22 (P < .05, Mann-Whitney test). * indicates significantly different from non-MM mean.
Secretion of functional BDNF by malignant plasma cells was confirmed using the rat pheochromocytoma cell line PC12 transfected with TrkB. PC12-TrkB cells exhibit robust neurite outgrowth in response to BDNF, whereas parental PC12 cells, which do not express TrkB, do not respond to BDNF. Media conditioned by 8 of 8 HMCLs triggered neurite outgrowth by PC12-TrkB cells; neurite outgrowth elicited by JJN3-conditioned media is shown in Figure 2. Neurite outgrowth did not occur in the presence of neutralizing antibody to BDNF and was not elicited from parental PC12 cells, confirming the presence of BDNF in HMCL-conditioned media. These results suggest that MM cells can synthesize and secrete significant levels of functional BDNF and that enhanced secretion of BDNF accompanies malignant transformation.
Malignant plasma cells secrete functional BDNF. Media conditioned by HMCL JJN3 triggers neurite outgrowth by TrkB-expressing PC12 cells (PC12-TrkB) (A) but not by parental PC12 cells, which lack TrkB (B) and not by PC12-TrkB in the presence of neutralizing anti-BDNF antibody (C).
Malignant plasma cells secrete functional BDNF. Media conditioned by HMCL JJN3 triggers neurite outgrowth by TrkB-expressing PC12 cells (PC12-TrkB) (A) but not by parental PC12 cells, which lack TrkB (B) and not by PC12-TrkB in the presence of neutralizing anti-BDNF antibody (C).
Expression of TrkB by MM
As BDNF is a survival factor for TrkB-expressing neurons and endothelial cells, it might also provide autocrine support for TrkB-expressing MM cells. To determine whether malignant plasma cells are capable of responding to BDNF, TrkB expression by primary MM and HMCL was evaluated using RT-PCR, Western blotting, and immunohistochemistry. TrkB mRNA was detected by RT-PCR in CD138+ cells isolated from 2 of 2 patients with MM, and in 8 of 9 HMCLs, but it was not detected in a B-cell line generated from a patient with Waldenstrom macroglobulinemia (Figure 3A). Expression of TrkB protein by HMCLs was detected by Western blotting of whole-cell lysates (8226, JJN3, OPM1, and U266 shown in Figure 3B). KMS11, 8226, and LP1 show constitutive expression of TrkB protein. In contrast, OPM1, U266, JJN3, H929, and UTMC2 exhibit low basal expression of TrkB but increase their expression on exposure to dexamethasone. Dexamethasone was evaluated for its effect on TrkB expression because it had previously been shown to increase expression of other prosurvival receptors, including IGF receptor and gp130.28 TrkB protein is also present on primary MM. TrkB was detected immunohistochemically in 24 of 25 BM aspirates from MM patients, in which malignant plasma cells, but not erythrocyte or myeloid precursors, demonstrated immunoreactivity (Figure 3C). In contrast, p75NTR, the low-affinity receptor for BDNF, was not detected on HMCL or primary MM plasma cells by either RT-PCR or immunohistochemistry (data not shown).
Expression of TrkB by MM. (A) RT-PCR demonstrating TrkB mRNA expression by 8 of 9 HMCLs (lanes 1-9) and by 2 primary MM samples (lanes 11-12) but not by a Waldenstrom cell line (lane 10). No PCR products were evident in the absence of RT (not shown). HMCLs were (1) NCI H929, (2) LP1, (3) RPMI 8226, (4) ARP1, (5) U266, (6) OPM1, (7) UTMC2, (8) KMS11, and (9) JJN3. (B) Western blot of whole-cell lysates stained with anti-Trk (C-14), demonstrating TrkB expression by 4 HMCLs. RPMI 8226 constitutively express TrkB, whereas JJN3, OPM1, and U266 express TrkB after 24-hour exposure to 100 nM dexamethasone. (C) Immunofluorescence demonstrating TrkB expression by primary MM plasma cells. Representative cytospins of BM mononuclear cells freshly isolated from a patient with MM and stained with antibodies to TrkB (rhodamine) and CD138 (FITC). CD138+ plasma cells show strong immunoreactivity for TrkB, seen in the merged image. (Insets) No staining is evident when nonimmune rabbit IgG (for TrkB) or mouse IgG (for CD138) are used as primary antibodies.
Expression of TrkB by MM. (A) RT-PCR demonstrating TrkB mRNA expression by 8 of 9 HMCLs (lanes 1-9) and by 2 primary MM samples (lanes 11-12) but not by a Waldenstrom cell line (lane 10). No PCR products were evident in the absence of RT (not shown). HMCLs were (1) NCI H929, (2) LP1, (3) RPMI 8226, (4) ARP1, (5) U266, (6) OPM1, (7) UTMC2, (8) KMS11, and (9) JJN3. (B) Western blot of whole-cell lysates stained with anti-Trk (C-14), demonstrating TrkB expression by 4 HMCLs. RPMI 8226 constitutively express TrkB, whereas JJN3, OPM1, and U266 express TrkB after 24-hour exposure to 100 nM dexamethasone. (C) Immunofluorescence demonstrating TrkB expression by primary MM plasma cells. Representative cytospins of BM mononuclear cells freshly isolated from a patient with MM and stained with antibodies to TrkB (rhodamine) and CD138 (FITC). CD138+ plasma cells show strong immunoreactivity for TrkB, seen in the merged image. (Insets) No staining is evident when nonimmune rabbit IgG (for TrkB) or mouse IgG (for CD138) are used as primary antibodies.
Expression of functional TrkB by MM: binding and internalization of ligand. (A) Binding of [125I]-BDNF. Dilutions of unlabeled BDNF were assayed for their ability to displace [125I]-BDNF from JJN3 HMCL. Each condition was assessed in triplicate. Results were corrected for nonspecific binding, determined in parallel incubations with 500-fold excess BDNF, and are presented as the mean ± standard deviation. The Kd for binding of [125I]-BDNF to JJN3 was determined as 0.1 nM, using the equation of Cheng and Prusoff.26 (B) Internalization of [125I]-BDNF. JJN3 HMCL was incubated with 0.1 nM [125I]-BDNF at either 4°C (♦) or 37°C (▪). Internalization was determined as counts remaining after acid stripping of surface-bound BDNF. Each condition was assessed in triplicate, and the results are presented as the mean ± standard deviation.
Expression of functional TrkB by MM: binding and internalization of ligand. (A) Binding of [125I]-BDNF. Dilutions of unlabeled BDNF were assayed for their ability to displace [125I]-BDNF from JJN3 HMCL. Each condition was assessed in triplicate. Results were corrected for nonspecific binding, determined in parallel incubations with 500-fold excess BDNF, and are presented as the mean ± standard deviation. The Kd for binding of [125I]-BDNF to JJN3 was determined as 0.1 nM, using the equation of Cheng and Prusoff.26 (B) Internalization of [125I]-BDNF. JJN3 HMCL was incubated with 0.1 nM [125I]-BDNF at either 4°C (♦) or 37°C (▪). Internalization was determined as counts remaining after acid stripping of surface-bound BDNF. Each condition was assessed in triplicate, and the results are presented as the mean ± standard deviation.
Expression of functional TrkB by MM: intracellular signaling in response to BDNF. (A-B) BDNF triggers phosphorylation of Akt and MAPK by HMCL. HMCLs were serum starved overnight, exposed to ligand for 5 minutes at 37°C, and lysed. Whole-cell lysates were subjected to SDS-PAGE, blotted, and probed with antibodies specific to Akt, phosphoAkt, MAPK, or phosphoMAPK. Blots were developed with ECL, and the resulting images were scanned and analyzed using Scion Image for Windows (Frederick, MD). The density of each phosphospecific band was corrected for variance in loading, using the density of the corresponding nonphosphorylated band. The fold induction was then calculated as the ratio of densities between stimulated and unstimulated phosphorylated protein. (A) BDNF triggers phosphorylation of Akt in HMCL. The ability of NGF (100 ng/mL), BDNF (50 ng/mL), or IL-6 (100 ng/mL) to activate PI3K/Akt signaling in HMCL was assessed using antibodies specific to Akt and phosphoAkt. (B) BDNF triggers phosphorylation of MAPK in HMCL. Antibodies specific to MAPK and phosphoMAPK p42/44 were used to assess activation of Ras/Raf/MEK signaling by BDNF (50 ng/mL) in HMCLs OPM1, U266, and KMS11. (C-D) BDNF triggers phosphorylation of Akt and CREB in primary MM cells. BM mononuclear cells were stimulated for 10 minutes at 37°C with BDNF (50 ng/mL), spun onto microscope slides, and stained for CD138 (FITC) and phosphoAkt (rhodamine), or CD138 (FITC) and phosphoCREB (rhodamine). (C) Akt phosphorylation by primary MM. CD138+ cells (green) stain for cytoplasmic phosphoAkt (red) after stimulation with BDNF (merged image is yellow). (D) CREB phosphorylation by primary MM. CD138+ cells (green) show nuclear localization of pCREB (red) after stimulation with BDNF.
Expression of functional TrkB by MM: intracellular signaling in response to BDNF. (A-B) BDNF triggers phosphorylation of Akt and MAPK by HMCL. HMCLs were serum starved overnight, exposed to ligand for 5 minutes at 37°C, and lysed. Whole-cell lysates were subjected to SDS-PAGE, blotted, and probed with antibodies specific to Akt, phosphoAkt, MAPK, or phosphoMAPK. Blots were developed with ECL, and the resulting images were scanned and analyzed using Scion Image for Windows (Frederick, MD). The density of each phosphospecific band was corrected for variance in loading, using the density of the corresponding nonphosphorylated band. The fold induction was then calculated as the ratio of densities between stimulated and unstimulated phosphorylated protein. (A) BDNF triggers phosphorylation of Akt in HMCL. The ability of NGF (100 ng/mL), BDNF (50 ng/mL), or IL-6 (100 ng/mL) to activate PI3K/Akt signaling in HMCL was assessed using antibodies specific to Akt and phosphoAkt. (B) BDNF triggers phosphorylation of MAPK in HMCL. Antibodies specific to MAPK and phosphoMAPK p42/44 were used to assess activation of Ras/Raf/MEK signaling by BDNF (50 ng/mL) in HMCLs OPM1, U266, and KMS11. (C-D) BDNF triggers phosphorylation of Akt and CREB in primary MM cells. BM mononuclear cells were stimulated for 10 minutes at 37°C with BDNF (50 ng/mL), spun onto microscope slides, and stained for CD138 (FITC) and phosphoAkt (rhodamine), or CD138 (FITC) and phosphoCREB (rhodamine). (C) Akt phosphorylation by primary MM. CD138+ cells (green) stain for cytoplasmic phosphoAkt (red) after stimulation with BDNF (merged image is yellow). (D) CREB phosphorylation by primary MM. CD138+ cells (green) show nuclear localization of pCREB (red) after stimulation with BDNF.
Functional analysis of TrkB
Binding and internalization of [125I]-BDNF by MM. HMCL JJN3 was used to demonstrate saturable binding of radioiodinated BDNF (Figure 4A). Competition with unlabeled BDNF gave an estimate of binding affinity of 0.1 nM, consistent with published results for TrkB.29 In addition, JJN3 cells internalize BDNF by a temperaturesensitive mechanism (Figure 4B), consistent with TrkB-mediated uptake of BDNF described for neurons and megakaryocytes.26,30 HMCLs thus express on their surface functional TrkB, capable of binding BDNF with high affinity and of triggering receptor internalization on ligand engagement.
Intracellular signaling. Functional TrkB expression should allow BDNF to initiate prosurvival signals in MM, as it does in neuronal systems.31 In neurons, BDNF triggers PI3K/Akt and Ras/Raf/MEK signaling, resulting in phosphorylation and activation of CREB/ATF.32,33 Activated CREB/ATF enhances transcription of prosurvival genes, including MCL1.34 In HMCLs, BDNF stimulates phosphorylation of both Akt (Figure 5A) and MAPK p42/44 (Figure 5B), the more robust phosphorylation of Akt suggesting preferential activation of PI3K/Akt by TrkB. Alternatively, this difference may reflect the high constitutive phosphorylation of MAPK seen in HMCLs. Similarly, BDNF did not increase CREB/ATF phosphorylation over background, possibly because of the high constitutive phosphorylation of CREB/ATF seen in most HMCLs (data not shown).
Primary MM cells also respond to BDNF. Fresh BM aspirates from 5 patients with MM were exposed to BDNF, transferred to microscope slides, and stained with antibodies to phosphoAkt, phosphoCREB/ATF, and CD138. BDNF triggered increased cytoplasmic immunoreactivity for phosphoAkt (Figure 5C) and increased nuclear immunoreactivity for phosphoCREB/ATF (Figure 5D) in CD138+ cells from 2 of the 5 aspirates. The ability to detect BDNF induction of CREB/ATF phosphorylation may reflect lower constitutive activation of Ras/Raf/MEK signaling in primary MM than in HMCLs.
BDNF is a survival factor for malignant plasma cells
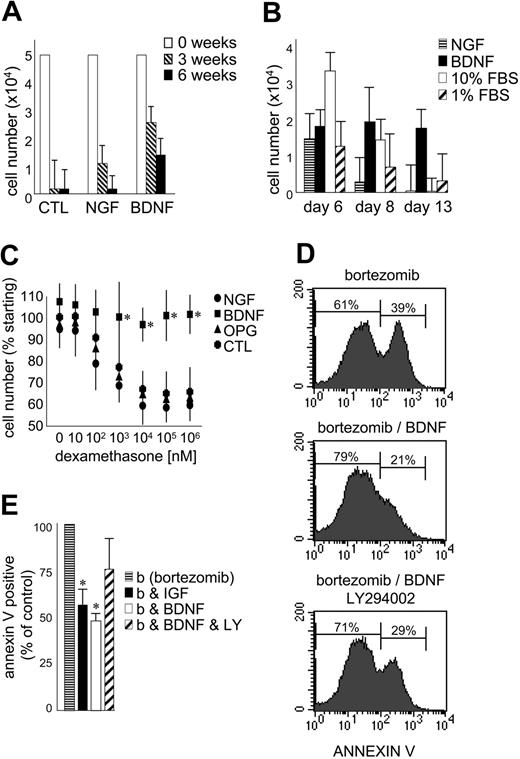
The effect of exogenous BDNF on the growth and survival of primary MM was evaluated using CD138-selected cells isolated from 5 patients with MM (Figure 6A-B). The numbers of CD138+ cells surviving after 3 and 6 weeks in coculture with the human BM stromal cell line HS5 are presented in Figure 6A. BDNF did not stimulate an expansion of CD138-selected cells in vitro but did consistently prolong their survival at both 3 and 6 weeks (P < .05). This demonstrates the ability of BDNF to enhance survival of primary MM cells already receiving stromal support. While less potent than BDNF, NGF also prolonged the survival of primary MM in coculture. MM cells do not express p75NTR and infrequently express TrkA, the high-affinity receptor for NGF. This highlights the potential for neurotrophins to indirectly support MM by stimulating stromal cells that express p75NTR, TrkA, TrkB, and TrkC. Neurotrophins may thus participate in the reciprocal interactions that exist between MM and its supporting stroma.
BDNF promotes MM-cell survival. (A) Survival of primary MM cultured with BM stroma. CD138-immunoselected primary MM plasma cells were cultured in 1% serum on HS5 stromal cells. BDNF (50 ng/mL) and NGF (100 ng/mL) were added every 3 days. Viable cells were counted at 0 (□),3(▧), and 6 weeks (▪). The results using aspirates taken from 5 patients are presented as mean ± standard deviation. (B) Survival of primary MM cultured alone. CD138-immunoselected primary MM plasma cells (5 × 104) were cultured without stroma in 1% FBS without (▨) or with BDNF (50 ng/mL; ▪) or NGF (100 ng/mL; ▤). 10% FBS (□) was used as comparator. Viable cells were counted at days 6, 8, and 13. The results using aspirates taken from 5 patients are presented as mean ± standard deviation. (C) BDNF protects HMCL RPMI from dexamethasone-induced death. RPMI 8226 cells in 1% FBS were exposed to increasing concentrations of dexamethasone ± BDNF (50 ng/mL) or NGF (100 ng/mL) or OPG (500 ng/mL). Viable cell numbers were assayed at 5 days using trypan blue exclusion. Each condition was assessed in triplicate, and the results are expressed as the mean ± standard deviation. (D-E) BDNF delays bortezomib-induced death of HMCL JJN3. JJN3 cells in 1% FBS were exposed to bortezomib (10 nM) for 24 hours, stained with annexin V–FITC, and analyzed by FACS. BDNF (50 ng/mL), IGF (100 ng/mL), or LY294002 (5 μM) was added 12 hours prior to bortezomib. (D) FACS profiles of a representative experiment. After 24-hour exposure to bortezomib, 39% of untreated, 21% of BDNF-treated, and 29% of BDNF/LY294002-treated JJN3 cells bind annexin V. Each condition was assessed in triplicate, and the results are presented in panel E as the mean ± standard deviation. ▤ indicates bortezomib alone; ▪, plus IGF; □ plus BDNF; and ▨, plus BDNF and LY294002. Student t test was used to determine significance (P < .05).
BDNF promotes MM-cell survival. (A) Survival of primary MM cultured with BM stroma. CD138-immunoselected primary MM plasma cells were cultured in 1% serum on HS5 stromal cells. BDNF (50 ng/mL) and NGF (100 ng/mL) were added every 3 days. Viable cells were counted at 0 (□),3(▧), and 6 weeks (▪). The results using aspirates taken from 5 patients are presented as mean ± standard deviation. (B) Survival of primary MM cultured alone. CD138-immunoselected primary MM plasma cells (5 × 104) were cultured without stroma in 1% FBS without (▨) or with BDNF (50 ng/mL; ▪) or NGF (100 ng/mL; ▤). 10% FBS (□) was used as comparator. Viable cells were counted at days 6, 8, and 13. The results using aspirates taken from 5 patients are presented as mean ± standard deviation. (C) BDNF protects HMCL RPMI from dexamethasone-induced death. RPMI 8226 cells in 1% FBS were exposed to increasing concentrations of dexamethasone ± BDNF (50 ng/mL) or NGF (100 ng/mL) or OPG (500 ng/mL). Viable cell numbers were assayed at 5 days using trypan blue exclusion. Each condition was assessed in triplicate, and the results are expressed as the mean ± standard deviation. (D-E) BDNF delays bortezomib-induced death of HMCL JJN3. JJN3 cells in 1% FBS were exposed to bortezomib (10 nM) for 24 hours, stained with annexin V–FITC, and analyzed by FACS. BDNF (50 ng/mL), IGF (100 ng/mL), or LY294002 (5 μM) was added 12 hours prior to bortezomib. (D) FACS profiles of a representative experiment. After 24-hour exposure to bortezomib, 39% of untreated, 21% of BDNF-treated, and 29% of BDNF/LY294002-treated JJN3 cells bind annexin V. Each condition was assessed in triplicate, and the results are presented in panel E as the mean ± standard deviation. ▤ indicates bortezomib alone; ▪, plus IGF; □ plus BDNF; and ▨, plus BDNF and LY294002. Student t test was used to determine significance (P < .05).
To discern the direct effect of BDNF on primary MM cells, 5 × 104 CD138-selected cells were cultured without stromal support in 1% FBS (10% FBS served as positive control). Five experiments were conducted using CD138+ cells isolated from 5 different BM aspirates. The mean numbers of cells surviving after 6, 8, and 13 days in culture are presented in Figure 6B. At day 6, cells cultured in 10% FBS demonstrated enhanced survival. By day 13, only cells exposed to BDNF exhibited enhanced survival (P < .05), confirming a direct prosurvival effect of BDNF on MM plasma cells. The prosurvival activity of BDNF can also be demonstrated using HMCLs. RPMI 8226, which undergoes apoptosis when exposed to dexamethasone at micromolar concentrations, is protected by BDNF during 5-day exposure to dexamethasone at concentrations as high as 1 mM (Figure 6C). Neither NGF nor OPG, a decoy receptor for TRAIL (TNF-related apoptosis-inducing ligand)35 demonstrate this protection. Similarly, BDNF delays bortezomib-triggered apoptosis of HMCL JJN3, evident as reduced annexin V binding (Figure 6D-E). Both BDNF and IGF-1 reduce by half the number of JJN3 cells that bind annexin V following 24-hour exposure to 10 nM bortezomib. However, neither BDNF nor IGF-1 prevents bortezomib-induced apoptosis, as all JJN3 cells are apoptotic after 48-hour exposure to 10 nM bortezomib. The ability of BDNF to delay bortezomib-triggered apoptosis is partially blocked by LY294002, suggesting that PI3K activation contributes to, but is not fully responsible for, BDNF protection. This is consistent with TrkB activating multiple signaling cascades in MM.
Discussion
Trk receptors were originally identified as proto-oncogenes in colon and thyroid carcinomas and later, myeloid leukemia. Expression of TrkB has been shown to increase metastatic potential by suppressing anoikis and has been implicated in the pathogenesis of a number of neuronal and non-neuronal tumors, including neuroblastoma, medulloblastoma, Wilms tumor, and adenocarcinomas of the lung, thyroid, prostate, and pancreas.36-50 TrkB has been found on B-cell lymphomas and Epstein-Barr virus–transformed B lymphocytes.51,52 However, the significance of this expression has not been determined.
Neurotrophins, originally identified because of their ability to promote neuronal survival, are critical to the development and survival of multiple extraneuronal tissues.53 This is highlighted by the lethal vascular hemorrhage that occurs in perinatal mice lacking either BDNF or NT-3.20 A role for neurotrophins in hematopoiesis has yet to be confirmed using conditional knockouts. Nonetheless, cells of all hematopoietic lineages, as well as marrow stroma, express neurotrophins and their receptors.22 In the immune system, neurotrophins have been shown to influence all phases of lymphocyte development, including T- and B-cell growth and survival, development of immunologic memory, and immunoglobulin production.53-59 Best studied are NGF and its high-affinity receptor, TrkA. Both are expressed by memory B cells and function in an autocrine loop to promote the survival of memory B cells.55,60 B and T lymphocytes also express BDNF and its high-affinity receptor, TrkB, and increase their expression of both TrkB and BDNF on activation by Staphylococcus aureus Cowan strain 1 or phytohemagglutinin.17,51,61,62 Similar to the prosurvival role played by NGF and TrkA, BDNF has been shown to enhance the survival of both double-negative thymocytes and B-cell lines after serum deprivation.61,63 We do not find expression of TrkA or NGF by most malignant plasma cells (data not shown) but do find expression of BDNF and TrkB.
We find BDNF activation of TrkB increases the survival of primary MM cells in culture and protects HMCL RPMI 8226 from dexamethasone- and JJN3 from bortezomib-induced apoptosis. BDNF acts as a survival factor for MM cells but does not trigger MM cell proliferation, consistent with the established role of neurotrophins as mediators of neuronal and endothelial cell survival, and with the low proliferative index characteristic of malignant plasma cells. Also suggestive of a prosurvival role for TrkB in MM, we find that most HMCLs increase their expression of TrkB, as they do other prosurvival receptors, when exposed to dexamethasone.28 We find BDNF activation of TrkB induces phosphorylation of Akt and CREB/ATF in primary MM cells and phosphorylation of Akt and MAPK in HMCLs. While Akt and MAPK are recognized mediators of prosurvival signaling in MM, members of the ATF/CREB family of transcription factors have yet to be implicated in MM cell survival. Nonetheless, the high constitutive phosphorylation of CREB/ATF that we observe in most HMCLs (data not shown) suggests that these transcription factors may participate in the pathogenesis of MM. Furthermore, CREB is known to regulate expression of MCL-1, an antiapoptotic protein also thought to be involved in MM pathogenesis.34,64
Neither BDNF nor TrkB appears to be expressed by normal plasma cells, and mechanisms underlying their aberrant expression by MM are unknown. As TrkB and BDNF are expressed by activated CD19+ lymphocytes, their expression by malignant plasma cells may reflect cellular activation associated with transformation. However, the fact that TrkB and BDNF are not always elevated in the same clinical sample suggests that separate mechanisms underlie their expression by MM. Reciprocal translocations involving the BDNF or TrkB loci have not been described in MM. Nevertheless, the complex MM karyotype frequently includes trisomy 9 (TrkB locus 9q22), and trisomy 11 (BDNF locus 11p13), raising the possibility that alterations in gene dosage are responsible.65
The frequent expression of TrkB by MM plasma cells suggests an important role for BDNF, known to be expressed by osteoblasts, BM endothelial cells, and BM stroma, in the paracrine support provided MM by its microenvironment. Autocrine production of BDNF by MM plasma cells may contribute additional support for tumor cell survival. Further, expression of p75NTR and TrkB by osteoblasts, endothelial cells, and BM stroma may allow malignant plasma cells to use BDNF to influence its microenvironment. We do not find elevated levels of BDNF in BM plasma from all patients with MM. However MM is a heterogeneous disease, reflecting the karyotypic instability that contributes both to its initiation and to its evolution to more aggressive stages.1 Thus, despite its common clinical presentation as a clonal expansion of plasma cells within BM, multiple pathways, including a BDNF-TrkB axis, are likely to contribute to malignant plasma cell survival. Nonetheless, our results suggest that TrkB and BDNF are expressed by MM to generate prosurvival signals, making BDNF-TrkB signaling a potential therapeutic target.
Prepublished online as Blood First Edition Paper, January 18, 2005; DOI 10.1182/blood-2004-08-3096.
Supported by the Burroughs Wellcome Fund, The Leukemia and Lymphoma Society of America, and the Sidney Kimmel Foundation for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Chao, R. Kramer, K. Teng, and C. Siao for comments; R. Lee for assistance with BDNF binding studies; L. Firme for technical assistance; R. Niesvizky, K. Pekle, J. Lane, M. Coleman, H. Cho, M. Schuster, B. Jalilizeinali, S. Ely and members of the Center for Lymphoma and Myeloma for clinical samples; J. Carlin and D. Knowles for their generous support.




![Figure 4. Expression of functional TrkB by MM: binding and internalization of ligand. (A) Binding of [125I]-BDNF. Dilutions of unlabeled BDNF were assayed for their ability to displace [125I]-BDNF from JJN3 HMCL. Each condition was assessed in triplicate. Results were corrected for nonspecific binding, determined in parallel incubations with 500-fold excess BDNF, and are presented as the mean ± standard deviation. The Kd for binding of [125I]-BDNF to JJN3 was determined as 0.1 nM, using the equation of Cheng and Prusoff.26 (B) Internalization of [125I]-BDNF. JJN3 HMCL was incubated with 0.1 nM [125I]-BDNF at either 4°C (♦) or 37°C (▪). Internalization was determined as counts remaining after acid stripping of surface-bound BDNF. Each condition was assessed in triplicate, and the results are presented as the mean ± standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3096/6/m_zh80090577860004.jpeg?Expires=1765925933&Signature=W82fZi6TtzUnPMRy3eXimOkzPJHhRxmoyF8vl1GGljIRcJv2vUl3~FN9Rrlw-UbxKc7LExbvn4G50hXKKUH~FBbblyUn4dojS5V59dAz827iRA~nnzIwgnAYnvPG6KkhsNCCEnlN7PLi5-ijAfhmkbpbZJEzR9qZfrgsn9LsBBjGiYp-yqrIKcHe40xDIGFL329l06O9fpdvnxN7h-vAwKxsb~jwKEwOwCVxPll8-sTTglIHOBfBvd5zJMdWSKt9UbJyjF3rtvksGDFdA820mhmVwKvC9xazWMXuBk57ozSnIuFEwYlec7bZBd9gdydEfYZ3yg-1E9Njnu5BIID3~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal