Abstract
Semaphorins, a large family of membrane-bound and secreted proteins, signal through their transmembrane receptors, the plexins. Semaphorins and plexins share structural homologies with scatter factor receptors, a family of tyrosine kinase receptors for which Met is the prototype. Semaphorins have been studied primarily in the developing nervous system, where they act as repelling cues in axon guidance. However, they are widely expressed in several tissues, and their role in epithelial morphogenesis has been recently established. Not much is known about their role in angiogenesis, a key step during embryonic development and adulthood. Here we demonstrate that a semaphorin, Sema4D, is angiogenic in vitro and in vivo and that this effect is mediated by its high-affinity receptor, Plexin B1. Moreover, we prove that biologic effects elicited by Plexin B1 require coupling and activation of the Met tyrosine kinase. In sum, we identify a proangiogenic semaphorin and provide insight about the signaling machinery exploited by Plexin B1 to control angiogenesis.
Introduction
Semaphorins belong to a large family of transmembrane, glycosylphosphatidylinositol (GPI)–anchored, and secreted proteins that have been divided into subclasses according to their structural features.1 Semaphorins act by binding their cognate receptors, the plexins, a family of transmembrane molecules sharing structural homology with semaphorins themselves.2 All plexins have a large cytoplasmic domain, highly conserved during evolution, that is endowed with R-Ras GAP activity3 and is able to interact, directly or indirectly, with small G proteins, thus exerting control over cytoskeletal structures.4-7 Semaphorins were originally characterized in the nervous system, where they are involved in the establishment of a correct neuronal network, steering axon growth cones and dendrites to their final targets.8 According to the widespread expression of semaphorins and plexins, an increasing amount of data involve these 2 protein families in the development of different tissues and organs beyond the nervous system. In particular, a significant role for these molecules has been established in cardiac and skeletal development,9 immune response,10,11 epithelial morphogenesis,12 and tumor growth and metastasis.13,14
Although secreted class 3 semaphorins have been involved in the control of endothelial cell (EC) adhesion, motility, and vascular development, the role of semaphorins belonging to other classes remains largely unknown. Indeed, it has been shown that the prototypic secreted class 3 semaphorin, Sema3A, inhibits EC migration and in vitro angiogenesis.15 Furthermore, during vascular development and experimental angiogenesis, ECs generate autocrine chemorepulsive signals, driven by class 3 semaphorins, that inhibit integrin activity, thus endowing the vascular system with the plasticity required for reshaping.16
We focused our interest on Sema4D, a semaphorin originally identified for its activity in the immune system, where it promotes B cell aggregation and survival, and T-cell activation.17-19 This semaphorin exists as a 150-kDa transmembrane molecule17 and as a soluble form of 120 kDa that originates from cleavage of the extracellular moiety near the transmembrane portion.20 Experimental evidence shows that the soluble form of the semaphorin can promote many of the biologic effects observed in the immune system. The activities of Sema4D are mediated by 2 receptors, Plexin B1, the high-affinity receptor widely expressed throughout different tissues and organs,2 and CD72, a low-affinity receptor found in cells of the hemangiopoietic lineage.21 Sema4D and Plexin B1 share structural homology with scatter factor receptors, a family of transmembrane molecules endowed with tyrosine kinase activity.22,23 The prototype of this family is the receptor for hepatocyte growth factor (HGF), encoded by the Met gene.24,25 On HGF binding, Met becomes active and elicits pleiotropic biologic responses, ultimately leading to a complex sequence of events called invasive growth.23 Met activation has been implicated in many embryologic events, such as epithelial morphogenesis,26,27 migration of myogenic precursors,28 chemoattraction, survival and proliferation of different types of neurons,29 and notably EC migration, proliferation, and organization into new blood vessels during the angiogenic process.30,31
We have previously demonstrated12 that Sema4D is able to activate the invasive growth program in epithelial cells, a complex process including dissociation of cell-to-cell adhesive contacts, anchorage-independent growth, and branching morphogenesis. We found also that in primary ECs, Sema4D binding to Plexin B1 results in the stimulation of Met tyrosine kinase activity required to elicit Sema4D-induced biologic responses.
A sort of invasive growth can be observed during the angiogenic process, a complex multistep morphogenetic event during which ECs, stimulated by major determinants of vascular remodeling, dynamically modify their cell-to-cell and cell-to-matrix contacts and move directionally to be reorganized into a mature vascular tree.32-34 The formation of new blood vessels is a key step during embryo development, but it also occurs in adults in physiologic and in pathologic conditions, such as retinopathy, rheumatoid arthritis, ischemia, and particularly tumor growth and metastasis.35
The present work discloses a novel biologic function of Sema4D: its activity as a proangiogenic factor. We demonstrate that this semaphorin stimulates EC migration, formation of capillary-like tubes, and cytoskeletal reorganization in vitro and that it is angiogenic in vivo. Moreover, we show that these activities are mediated by its high-affinity receptor, Plexin B1, which on Sema4D stimulation couples to the Met tyrosine kinase receptor, whose activity is required to elicit the angiogenic properties of this semaphorin.
Materials and methods
Cells and reagents
For the in vitro experiments, we used human ECs from umbilical cord vein.30 Cells were cultured on 1% gelatin-coated dishes in M199 medium (Sigma, St Louis, MO) supplemented with 20% fetal bovine serum (FBS) (Euroclone, Leeds, United Kingdom), heparin sodium salt (50 mg/L; Sigma), and endothelial growth factors (100 μg/mL) and were used at early passages (I-V).
VEGF and HGF were purchased from R&D Systems (Minneapolis, MN). Purified Sema4D-GST and AP-Sema4D-GST were obtained as previously described.12
All the antibodies used were from Santa Cruz Biotechnology (Santa Cruz, CA) except the monoclonal α-Plexin B1 antibody, obtained by recombinant protein immunization of mice,36,37 and the α-green fluorescent protein (anti-GFP) antibody Roche (Indianapolis, IN).
The lipophilic red-emitting fluorescence dye used was 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Fluka, Buchs, Switzerland).
DNA constructs and siRNAs
Met-GFP or GFP was subcloned in the lentiviral vector p156RRLsin-PPThCMVMCSpre as previously described.12
cDNA encoding PDZ-RhoGef dominant-negative construct (F1), previously described,37 was subcloned in p156RRLsinPPThCMVMCSpre lentiviral vector.
siRNAs were produced according the manufacturer's protocol (siRNA Construction Kit; Ambion, Austin, TX). The sequence of the 2 different siRNAs against Met is reported in Pennacchietti et al.38 We produced the following siRNA against Plexin B1: oligo sense, 5′-AAG CAG GCA TCT CGC GTG CGA CCT GTC TC-3′. The control siRNA was designed against the GFP protein: 5′-AAC TTG AAG AAG TCG TGC TGC CCT GTC TC-3′.
Cell stimulation
Unless indicated otherwise, for cell stimulation with mock we used dilutions of the elution buffer used to purify Sema4D-GST from Sepharose-glutathione chromatography. For reverse transcription–polymerase chain reaction (RT-PCR), human umbilical vein endothelial cells (HUVECs) were treated for 24 hours with soluble Sema4D (10 nM) or with a mock in the standard culture medium (20% FBS). Cell stimulation with Sema4D (10 nM) or HGF (400 U/mL) was performed as described.12
Cell infection, immunoprecipitation, and Western blot
Lentiviruses were produced by transient transfection of 293T cells. HUVECs were infected by overnight incubation with lentiviruses, in M199 medium, 20% FBS supplemented with 8 μg/mL polybrene.
Cells were lysed with EB buffer (1% Triton X100, 20 mM Tris pH 7.4, 5 mM ethylenediaminetetraacetic acid [EDTA], 10% glycerol, 150 mM NaCl) in the presence of a mixture of protease inhibitors (phenylmethylsulfonyl fluoride, leupeptin, aprotinin, pepstatin) and immunoprecipitated with the appropriate antibodies. When amounts of the same protein had been compared among different samples, the quantity of total proteins present in the lysates was checked using the BCA Protein Assay Kit (Pierce, Rockford, IL) according the manufacturer's protocol. Immunoprecipitated or total lysate proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Western blotted, stained with specific primary antibodies followed by secondary antibodies (Amersham, Arlington Heights, VA), and detected with the ECL system (Amersham). Quantification of phosphotyrosine staining was performed with the Quantity One Bio-Rad software (Bio-Rad, Hercules, CA).
RNA extraction and RT-PCR
mRNA from stimulated, freshly cultured HUVECs was extracted with the RNAqueous kit (Ambion) according to the manufacturer's protocol. The obtained mRNA, first annealed to oligo-dT, was retrotranscribed with 10 U/μL M-MLV reverse transcriptase RNase H minus, point mutant (Promega, Madison, WI) in the presence of M-MLV buffer, 1 mM dNTPs, 10 mM dithiothreitol (DTT), and 1 U/μL RNase inhibitor (Promega) in H2O diethylpyrocarbonate (DEPC). The mix was incubated for 1 hour at 37°C, followed by 1 hour at 42°C. PCR was performed with the Pfu DNA polymerase (Promega) in the GeneAmp PCR System 2400 (Applied Biosystems, Weiterstadt, Germany) for 30 cycles with the following primers: Ang-2 sense, 5′-GATAGAAATAGGGACAAACC-3′; Ang-2 antisense, 5′-AAGATCAAGAAGTTACTACC-3′; vascular endothelial growth factor-A (VEGF-A) sense, 5′-GGGTGCATTGGAGCCTTG-3′; VEGF-A antisense, 5′-CATTCACATTTGTTGTGCTGTAGG-3′; HGF sense, 5′-CGAAATCCT CGAGGGAAGAAG-3′; HGF antisense, 5′-AATTGCACAGTACTCCCAGCGGG-3′; Plexin B1 sense, 5′-CCTTCACGGGCACGCCCTGGGCCT-3′; Plexin B1 antisense, 5′-AAGAACCCC AAGCTGATGCTGCGCAGG-3′; CD72 sense, 5′-AGCTCCGCCTCAAGATAAC-3′; CD72 antisense, 5′-CTCCTCTGTTGCTCCTCA-3′.
Migration assay
HUVECs (4 × 104), detached with 15 mM EDTA to avoid trypsin digestion, were seeded on the upper surface of a polycarbonate 8-μm porous Transwell membrane, previously coated on both sides with 3000 ng/mL fibronectin. M199 medium was supplemented with 2% FBS in both chambers, whereas chemoattractants were added in the lower chamber (6 nM Sema4D; 4 nM HGF; 4 nM VEGF-A165). After 6 hours of incubation, cells on the upper surface of the membrane were mechanically removed; cells that migrated were fixed in 11% glutaraldehyde for 10 minutes and then stained with crystal violet for 5 minutes. Stained cells were quantified by diluting the dye with 10% acetic acid, and the A560 absorbance was read in a microplate reader.
Sprouting assay
The assay was performed according to protocols in the literature.39,40 Cells (800 per well) were seeded in 0.24% methylcellulose M199 medium during overnight incubation to form a single spheroid. Spheroids were collected, centrifuged at low speed, and seeded in M199 medium supplemented with rat tail collagen (0.6 mg/mL; Boehringer, Mannheim, Germany), 20% FBS, 0.7% methylcellulose, NaOH 15 mM, 10 mM HEPES (N-2-hydroxyethylenepiperazine-N'-2-ethanesulfonic acid) pH 7.3, and the stimulating factor (3.5 nM Sema4D; 2 nM VEGF). In samples with VEGF, 3 U/mL heparin sodium salt was added to the solution mix. Twenty-four hours after collagen polymerization, spheroids were analyzed. Sprout outgrowth was visualized by observing EC spheroids in phase contrast with an inverted photomicroscope (model DM IRB HC; Leica Microsystems, Wetzlar, Germany). Phase-contrast images on different focus planes of 1024 × 1024 pixels were captured using a cooled digital CCD Hamamatsu ORCA camera (Hamamatsu Photonics Italia, Milan, Italy), digitally recorded, and quantified with ImageProPlus 4.0 imaging software (Media Cybernetics, Silver Spring, MD). After choosing the appropriate spatial calibration scale, capillary sprouts were measured as follows. Two point features, 1 at the base and 1 at the distal tip of each sprout, were generated by means of the “point” tool of ImageProPlus 4.0. The measurement of the distance between these 2 point features was quantified by means of the “distance measurement” tool. Results were expressed as the mean capillary length (in micrometers).
Immunofluorescence
HUVECs (8 × 104) were plated on gelatin-coated glasses in a 24-well microplate. After stimulation, cells were fixed in 3.7% paraformaldehyde for 20 minutes at room temperature (RT) and then permeabilized for 3 minutes in 0.1% Triton X100. After incubation for 1 hour in blocking solution (goat serum 3% in phosphate-buffered saline [PBS]), cells were kept for 30 minutes at RT with the primary antibody against β-catenin (1 μg/mL). HUVECs were incubated for 30 minutes at RT with fluorescein phalloidin and the secondary rhodamine-conjugated antimouse antibody (2 μg/mL; Alexa Fluor 546; Molecular Probes, Eugene, OR). All immunofluorescence images were taken with the confocal microscope Radiance 2100 (Bio-Rad) with a 60 × lens.
Binding assay
Binding assay was performed as previously described.2 Cells were washed and incubated for 1.5 hours at RT in HBHA solution (20 mM HEPES, 150 mM NaCl, 0.1% azide, 5 g/L bovine serum albumin [BSA], 5 mM CaCl2, 1 mM MgCl2), supplemented with Sema 4D-AP (10 nM) or with the AP alone (3 U/mL; Calbiochem, San Diego, CA). Cells were fixed for 30 minutes in 60% acetone/3% formaldehyde and then were incubated for 20 minutes at 65°C. The presence of the cell-bound AP was detected by the NBT/BCIP system (Promega), diluted in AP buffer (100 mM Tris-HCl pH 9.5, 100 mM NaCl, 5 mM MgCl2).
CAM assay
Fertilized white Leghorn chicken eggs were incubated under conditions of constant humidity at 37°C. On the third day of incubation, a square window was opened in the eggshell after removal of 2 to 3 mL albumen so as to detach the developing chicken chorioallantoic membrane (CAM) from the shell. The window was sealed with a glass of the same size, and the eggs were returned to the incubator. At day 8, 1 mm3 sterilized gelatin sponges (Gelfoam; Upjohn, Kalamazoo, MI) adsorbed with granulocyte macrophage–colony-stimulating factor (GM-CSF) dissolved in 3 μL PBS were implanted on the tops of growing CAMs under sterile conditions within a laminar flow hood.41 Sema4D was delivered at 1.2 pmol/implant, whereas sponges containing HEPES-glutathione, basic fibroblast growth factor (bFGF) (30 pmol/sponge), or HGF (1.8 pmol/sponge) were used as negative and positive controls. CAMs were examined daily until day 12 and photographed in ovo under a Zeiss (Oberkochen, Germany) stereomicroscope SR equipped with an MC 63 Camera System (Zeiss).
Matrigel plug assay in mice
Five hundred microliters of Matrigel (Becton Dickinson, Franklin Lakes, NJ) containing either 200 ng/mL of the 146-amino acid variant of bFGF (R&D Systems) or 300 ng/mL of affinity-purified Sema4D, or an equivalent amount of PBS (for control purposes), was injected subcutaneously in the dorsal area of C57bl6 mice. Three mice per point (2 implants/mouse) were used. After 7 days, animals were killed, and implants were collected and immediately processed for paraffin embedding. Multiple sections from each sample were then histologically analyzed by hematoxylin-eosin staining, and the angiogenic response was quantified (square micrometer of blood vessel area/field) by means of Image-Pro Plus (Media Cybernetics).
Results
Sema4D is a proangiogenic factor for HUVECs
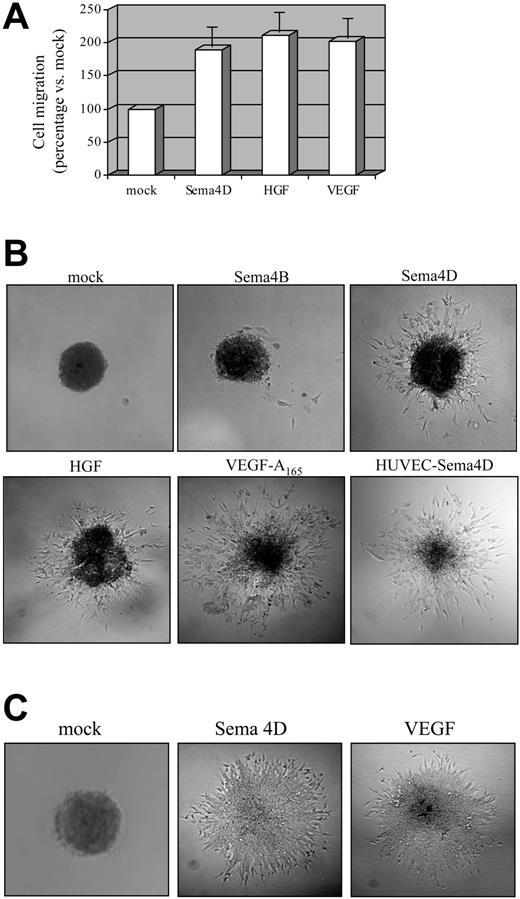
To evaluate whether Sema4D can elicit a functional response on ECs, we carried out in vitro experiments on HUVECs. We first investigated whether Sema4D exerted mitogenic activity on ECs by performing a migration assay in a Transwell system. Both sides of the Transwell filter were coated with fibronectin (3 μg/μL; see “Materials and methods”) to allow suitable integrin engagement,42 and a chemoattractant was added in the lower chamber. For these and the following experiments, we used soluble Sema4D affinity purified from the supernatant of COS cells transfected with Sema4D cDNA (for the purification procedure, see “Materials and methods”). As shown in Figure 1A, purified Sema4D exerted a clear chemoattraction on HUVECs, comparable to that elicited by vascular endothelial growth factor A165 (VEGF-A165) or HGF used as positive control.
It is well known that in vitro angiogenic factors stimulate ECs to sprout from cell spheroids embedded in a collagen matrix and that they form capillarylike tubular structures. This sprouting assay represents a powerful tool to evaluate in vitro the angiogenic potential of molecules because it correlates well with the ability of ECs to form blood vessels in vivo, in response to the same stimuli.39,40 We thus carried out sprouting assays in which EC spheroids were grown in a type I collagen gel, with or without the addition of Sema4D or of other factors. After 24 hours, the biologic response was evaluated in terms of length, number, morphology, and 3-dimensional arrangement of sprouts. As shown in Figure 1B, Sema4D, but not other semaphorins such as Sema4B or Sema6A (not shown), elicited the formation of numerous sprouts from HUVEC spheroids. The pattern of Sema4D-mediated sprouting was similar to that induced by VEGF-A165 (Figure 1B). In addition, the optimal stimulating concentrations of both Sema4D and VEGF-A165 were in the same range (3.5 nM and 2 nM, respectively).
Soluble Sema4D shows proangiogenic activity on ECs. (A) Mitogenic ability of Sema4D on HUVECs was evaluated in Transwell assay. Cells that underwent migration in response to the different chemoattractants were stained and solubilized, and the absorbance was calculated (see “Materials and methods”). The obtained values for stimulation with mock were normalized to 100, and migration in response to different factors was calculated as a percentage increase with respect to the mock. VEGF-A165 (4 nM) and HGF (4 nM) were used as positive controls. Data represent the mean of at least 6 independent experiments. As shown, Sema4D (6 nM) is endowed with mitogenic activity on HUVECs, comparable to those of VEGF and HGF. Data are shown as mean ± SD. (B) To evaluate the in vitro angiogenic activity, spheroids of ECs were grown in a collagen matrix in the absence or in the presence of angiogenic molecules. HUVECs-Sema4D are HUVECs infected with a lentivirus containing the Sema4D full-length cDNA. Infected cells overexpressed Sema4D full length, as observed in Western blot analysis (not shown). As shown, soluble Sema4D (3.5 nM) and the positive controls HGF (4 nM) and VEGF-A165 (2 nM) elicited strong morphogenetic responses. Moreover, HUVECs expressing Sema4D full-length underwent spontaneous sprouting, without requiring any other stimulating factors. Images were captured with a 10×/0.40 NA air objective lens. (C) Spheroid assay on microendothelial cells grown as in panel B, in the presence of the indicated growth factors. These cells show an angiogenic response in the presence of Sema4D. Images were captured with a 20×/0.70 NA air objective lens.
Soluble Sema4D shows proangiogenic activity on ECs. (A) Mitogenic ability of Sema4D on HUVECs was evaluated in Transwell assay. Cells that underwent migration in response to the different chemoattractants were stained and solubilized, and the absorbance was calculated (see “Materials and methods”). The obtained values for stimulation with mock were normalized to 100, and migration in response to different factors was calculated as a percentage increase with respect to the mock. VEGF-A165 (4 nM) and HGF (4 nM) were used as positive controls. Data represent the mean of at least 6 independent experiments. As shown, Sema4D (6 nM) is endowed with mitogenic activity on HUVECs, comparable to those of VEGF and HGF. Data are shown as mean ± SD. (B) To evaluate the in vitro angiogenic activity, spheroids of ECs were grown in a collagen matrix in the absence or in the presence of angiogenic molecules. HUVECs-Sema4D are HUVECs infected with a lentivirus containing the Sema4D full-length cDNA. Infected cells overexpressed Sema4D full length, as observed in Western blot analysis (not shown). As shown, soluble Sema4D (3.5 nM) and the positive controls HGF (4 nM) and VEGF-A165 (2 nM) elicited strong morphogenetic responses. Moreover, HUVECs expressing Sema4D full-length underwent spontaneous sprouting, without requiring any other stimulating factors. Images were captured with a 10×/0.40 NA air objective lens. (C) Spheroid assay on microendothelial cells grown as in panel B, in the presence of the indicated growth factors. These cells show an angiogenic response in the presence of Sema4D. Images were captured with a 20×/0.70 NA air objective lens.
Because it is known that VEGF promotes EC growth, we investigated the effects of Sema4D on the proliferation rate of HUVECs. Cells were seeded in 96-well plates and were stimulated for 7 days. At daily intervals, cells were fixed and stained, and their numbers were evaluated. No difference in proliferation rate was observed between HUVECs treated with Sema4D and unstimulated cells (data not shown).
Taken together, these experiments demonstrate that soluble Sema4D promotes EC migration and induces their organization in capillarylike tubes, mimicking the pivotal events occurring in vivo during the angiogenic process. It must be noted that the angiogenic activity of Sema4D was not restricted to HUVECs but was also shown on microendothelial cells (Figure 1C).
To investigate whether autocrine production of Sema4D could induce EC sprouting, we cloned HA (N-terminal tag)–Sema4D full-length cDNA in a lentiviral vector and used this lentivirus to infect HUVECs (HUVECs-Sema4D; Figure 1B). As shown in the figure, these cells displayed constitutive sprouting in a collagen matrix, stimulated by the soluble Sema4D secreted in the medium (data not shown). This experiment indicates that Sema4D is sufficient to induce an angiogenic response in vitro.
Sema4D induces cytoskeletal rearrangement in HUVECs
Because Sema4D induces EC migration and branching morphogenesis and because both activities require dramatic cytoskeletal rehandling, we analyzed the effects of Sema4D stimulation on cytoskeletal structures. HUVECs were stimulated for increasing lengths of time (2, 5, 10, 20, and 60 minutes), fixed, and stained to analyze F-actin organization. Mock-treated cells displayed numerous stress fibers, mainly aligned along the major cell axis (Figure 2). Sema4D stimulation resulted in a dramatic reorganization of actin network. Stress fibers were progressively dismantled, and the effect was already evident after 5 minutes of stimulation. At the same time, actin started polymerizing at the cell edge, leading to the formation of evident peripheral ruffles. These changes were accomplished after a few minutes of stimulation and did not change significantly at later times (Figure 2; data not shown).
It is known that one of the first changes observed in angiogenic endothelium is the disruption of cadherin-based cell-to-cell junctions associated with the internalization of β-catenin.43,44 We thus analyzed β-catenin localization of HUVECs after Sema4D stimulation. As shown in Figure 2 (lower panels), challenging ECs with Sema4D resulted in the disruption of intercellular junctions and in the rapid relocalization of β-catenin from cell-to-cell contacts into the cytoplasm. These experiments show that Sema4D induces cytoskeletal changes that are consistent with an angiogenic endothelium.
Immunofluorescence analysis of Sema4D-induced cytoskeletal rearrangement. In wild-type HUVECs (left panels), soluble Sema4D triggered dramatic cytoskeletal rearrangements, characteristic of an angiogenic endothelium. On Sema4D stimulation (20 minutes), we observed the disappearance of stress fibers, the formation of membrane ruffles (phalloidin-FITC [fluorescein isothiocyanate] staining, top row), the dismantling of focal adhesions, and the internalization of β-catenin (α-βcatenin staining, lower rows). Reduced expression of Plexin B1, obtained as a consequence of HUVEC transfection with siRNA specific for Plexin B1, resulted in a lack of response to Sema4D stimulation for the overall actin reorganization and for the β-catenin relocalization (right column). Images were captured with a 63×/1.32 oil objective lens.
Immunofluorescence analysis of Sema4D-induced cytoskeletal rearrangement. In wild-type HUVECs (left panels), soluble Sema4D triggered dramatic cytoskeletal rearrangements, characteristic of an angiogenic endothelium. On Sema4D stimulation (20 minutes), we observed the disappearance of stress fibers, the formation of membrane ruffles (phalloidin-FITC [fluorescein isothiocyanate] staining, top row), the dismantling of focal adhesions, and the internalization of β-catenin (α-βcatenin staining, lower rows). Reduced expression of Plexin B1, obtained as a consequence of HUVEC transfection with siRNA specific for Plexin B1, resulted in a lack of response to Sema4D stimulation for the overall actin reorganization and for the β-catenin relocalization (right column). Images were captured with a 63×/1.32 oil objective lens.
Sema4D has angiogenic activity in vivo
Having shown that Sema4D induces the in vitro migration of ECs and their assembly into capillarylike tubes, we then explored its in vivo angiogenic activity. To this aim, we carried out a CAM assay. Gelatin sponges, implanted into 8-day chicken embryo CAMs, were soaked with different soluble factors. Three days after sponge implantation, the presence and orientation of newly formed blood vessels were evaluated. Although in control specimens no vascular reaction was detectable (Figure 3A, panel i), sponges containing purified Sema4D (1.2 pmol/sponge) were surrounded by newly formed thin allantoic vessels radiating toward the implant in a typical “spoked-wheel” pattern (Figure 3A, panel ii). This reaction was similar to that observed around sponges containing either HGF (1.8 pmol/sponge) or bFGF (30 pmol/sponge) (Figure 3A, panels iii, iv). No angiogenic activity was observed in the presence of sponges containing Sema3A (data not shown). To further confirm the in vivo angiogenic activity of Sema4D, we performed a Matrigel plug assay in mice. Sema4D, bFGF, or PBS was incorporated in Matrigel, a matrix of reconstituted basement membrane, and injected subcutaneously into mice. After 7 days, mice were killed and plugs were analyzed histologically. Sema4D and bFGF induced abundant vascularization of Matrigel plugs, as shown in Figure 3B. Together these data show that Sema4D is proangiogenic not only in vitro but also in vivo.
Sema4D-dependent angiogenic activity is not mediated by the up-regulation of major angiogenic factors
Next, we investigated the molecular mechanisms responsible for the observed biologic effects. We first determined whether Sema4D was able to up-regulate the expression of major angiogenic factors, such as angiopoietin-2, VEGF-A, or HGF. mRNA was extracted from untreated or Sema4D-treated HUVECs and was analyzed by semiquantitative RT-PCR. As shown in Figure 4, there was no significant difference in expression of angiopoietin-2, VEGF-A (all splicing variants), and HGF between unstimulated and Sema4D-stimulated ECs. This observation suggests that Sema4D-elicited angiogenic effects are not mediated by the production of these major factors, which are often up-regulated during the angiogenic process.
Sema4D displays angiogenic activity in vivo. (A) CAM assays were performed to test the angiogenic effect of Sema4D. Mock (i), Sema4D (1.2 pmol/sponge; ii), HGF (1.8 pmol/sponge; iii), or bFGF (30 pmol/sponge; iv) were loaded on sponges and implanted into 8-day-old chicken embryos. Seventy-two hours after factor soaking, the angiogenic activity of the different factors was evaluated. Numerous newly formed capillaries, growing straight toward the angiogenic source, were observed in the presence of Sema4D. In size and number, they were comparable to those observed in the presence of HGF and bFGF. (B) Matrigel plug assay in mice. Subcutaneously injected Matrigel plugs containing either PBS (Control), bFGF, or Sema4D were isolated after 7 days and analyzed histologically. When compared with control, bFGF and Sema4D elicited strong angiogenic invasion of Matrigel plugs. Images were captured with a 10×/0.40 air objective lens. Mean ± SD blood vessel area (square micrometers) per observed field is shown in the graph.
Sema4D displays angiogenic activity in vivo. (A) CAM assays were performed to test the angiogenic effect of Sema4D. Mock (i), Sema4D (1.2 pmol/sponge; ii), HGF (1.8 pmol/sponge; iii), or bFGF (30 pmol/sponge; iv) were loaded on sponges and implanted into 8-day-old chicken embryos. Seventy-two hours after factor soaking, the angiogenic activity of the different factors was evaluated. Numerous newly formed capillaries, growing straight toward the angiogenic source, were observed in the presence of Sema4D. In size and number, they were comparable to those observed in the presence of HGF and bFGF. (B) Matrigel plug assay in mice. Subcutaneously injected Matrigel plugs containing either PBS (Control), bFGF, or Sema4D were isolated after 7 days and analyzed histologically. When compared with control, bFGF and Sema4D elicited strong angiogenic invasion of Matrigel plugs. Images were captured with a 10×/0.40 air objective lens. Mean ± SD blood vessel area (square micrometers) per observed field is shown in the graph.
Sema4D stimulation does not up-regulate the expression of angiopoietin-2, VEGF-A, or HGF. mRNAs extracted from unstimulated (first lane) and Sema4D-stimulated (second lane) HUVECs, were retrotranscribed and amplified for the angiopoietin-2 (Ang-2), VEGF-A, and HGF transcripts (see “Materials and methods”). Plasmid DNA (third lane) was used as positive control. No increase in expression of these genes was observed on Sema4D stimulation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification was performed as a control for all the extracted RNAs. No differences were observed; one of these controls is shown.
Sema4D stimulation does not up-regulate the expression of angiopoietin-2, VEGF-A, or HGF. mRNAs extracted from unstimulated (first lane) and Sema4D-stimulated (second lane) HUVECs, were retrotranscribed and amplified for the angiopoietin-2 (Ang-2), VEGF-A, and HGF transcripts (see “Materials and methods”). Plasmid DNA (third lane) was used as positive control. No increase in expression of these genes was observed on Sema4D stimulation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification was performed as a control for all the extracted RNAs. No differences were observed; one of these controls is shown.
Sema4D angiogenic activity is mediated by its high-affinity receptor Plexin B1
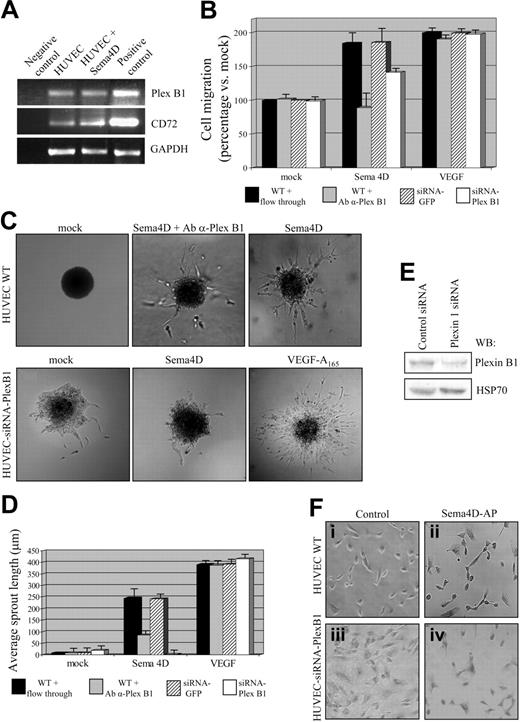
As previously mentioned, 2 different Sema4D receptors have been identified thus far: a high-affinity receptor, Plexin B1, and a low-affinity receptor, CD72.2,21 By means of RT-PCR experiments, we first demonstrated that Plexin B1 and CD72 mRNAs are expressed in HUVECs and that their levels of expression did not change after Sema4D stimulation (Figure 5A).
Plexin B1 is the endothelial receptor required for Sema4D-angiogenic activity. (A) mRNAs obtained from untreated and Sema4D-treated HUVECs were retrotranscribed and amplified with Plexin B1 or CD72-specific primers (as described in “Materials and methods”). cDNA obtained from lymphocytes of a patient with chronic lymphocytic leukemia was used as positive control for CD72; MDA-MB-435 cells expressing Plexin B1 were used as positive control for Plexin B1. As shown, Plexin B1 (top blot) and CD72 (middle blot) are expressed in HUVECs, and Sema4D stimulation did not significantly up-regulate their expression. (B) Migration assay of wild-type HUVECs and HUVECs transfected with Plexin B1–specific siRNA. As shown, an impairment of cell migration in response to Sema4D was observed in wild-type cells in the presence of inhibitory α-Plexin B1 antibodies and in cells displaying decreased expression of Plexin B1 because of siRNA expression. The negative controls varied by experimental condition: in the experiments with inhibitory α-Plexin B1 antibodies, as a control, wild-type (WT) cells were treated with flow-through; in the experiments with Plexin B1–specific siRNAs, control cells were transfected with GFP-specific siRNA. Neither the antibody treatment nor the expression of siRNA affected HUVEC response to VEGF-A165. Data are shown as mean ± SD. (C) Sprouting assay performed in the same conditions described in panel B. A reduction of Sema4D-induced sprouting was observed in the presence of inhibitory α-Plexin B1 antibodies and in cells expressing Plexin B1 siRNAs. In addition, in this assay, response to VEGF-A165 was not affected. (D) Quantification of the sprouting assay shown in panel C. Data are shown as mean ± SD. (E) Western blot analysis of HUVECs transfected with Plexin B1–specific siRNAs. The reduction of Plexin B1 has been quantified at approximately 70%. HSP70 indicates heat shock protein 70. (F) Binding assay. HUVECs transfected with siRNAs specific for Plexin B1 displayed a strong impairment in Sema4D-AP binding ability (iv), compared with wild-type HUVECs (ii). The AP enzyme alone was used as negative control (i,iii). Images were captured with a 10×/0.40 air objective lens.
Plexin B1 is the endothelial receptor required for Sema4D-angiogenic activity. (A) mRNAs obtained from untreated and Sema4D-treated HUVECs were retrotranscribed and amplified with Plexin B1 or CD72-specific primers (as described in “Materials and methods”). cDNA obtained from lymphocytes of a patient with chronic lymphocytic leukemia was used as positive control for CD72; MDA-MB-435 cells expressing Plexin B1 were used as positive control for Plexin B1. As shown, Plexin B1 (top blot) and CD72 (middle blot) are expressed in HUVECs, and Sema4D stimulation did not significantly up-regulate their expression. (B) Migration assay of wild-type HUVECs and HUVECs transfected with Plexin B1–specific siRNA. As shown, an impairment of cell migration in response to Sema4D was observed in wild-type cells in the presence of inhibitory α-Plexin B1 antibodies and in cells displaying decreased expression of Plexin B1 because of siRNA expression. The negative controls varied by experimental condition: in the experiments with inhibitory α-Plexin B1 antibodies, as a control, wild-type (WT) cells were treated with flow-through; in the experiments with Plexin B1–specific siRNAs, control cells were transfected with GFP-specific siRNA. Neither the antibody treatment nor the expression of siRNA affected HUVEC response to VEGF-A165. Data are shown as mean ± SD. (C) Sprouting assay performed in the same conditions described in panel B. A reduction of Sema4D-induced sprouting was observed in the presence of inhibitory α-Plexin B1 antibodies and in cells expressing Plexin B1 siRNAs. In addition, in this assay, response to VEGF-A165 was not affected. (D) Quantification of the sprouting assay shown in panel C. Data are shown as mean ± SD. (E) Western blot analysis of HUVECs transfected with Plexin B1–specific siRNAs. The reduction of Plexin B1 has been quantified at approximately 70%. HSP70 indicates heat shock protein 70. (F) Binding assay. HUVECs transfected with siRNAs specific for Plexin B1 displayed a strong impairment in Sema4D-AP binding ability (iv), compared with wild-type HUVECs (ii). The AP enzyme alone was used as negative control (i,iii). Images were captured with a 10×/0.40 air objective lens.
To analyze the role of Plexin B1 in Sema4D-induced biologic responses, we exploited 2 different approaches, the use of inhibitory antibodies against Plexin B1 and RNA interference technology (siRNA). We thus took advantage of antibodies directed against the extracellular portion of Plexin B1, able to interfere with receptor activation.37 HUVECs were stimulated with different factors, either in the absence or in the presence of these antibodies, and the biologic outcome was evaluated. As shown in Figure 5B, anti–Plexin B1 antibodies completely abrogated the mitogenic response promoted by Sema4D. Similarly, in the presence of anti–Plexin B1 antibodies, sprouts arising from spheroids stimulated with Sema4D were much thinner and greatly decreased in number and length (35.4% of wild-type average sprout length) compared with Sema4D-stimulated spheroids (Figure 5C-D). Anti–Plexin B1 antibodies did not interfere with the angiogenic process per se because they did not alter the ability of VEGF-A165 to induce migration or sprouting of ECs (Figure 5B-D).
As an alternative approach to evaluate the role of Plexin B1 in Sema4D-induced angiogenesis, we transfected HUVECs with siRNAs recognizing the Plexin B1 transcript. These siRNAs, targeting the corresponding mRNA, greatly impaired the expression of Plexin B1, as shown by Western blot analysis and the reduced ability of HUVECs to bind soluble, purified Sema4D, fused to the alkaline phosphatase (Sema4D-AP; Figure 5E-F). As shown in Figure 5B-D, the decrease of Plexin B1 expression strongly interfered with Sema4D-stimulated (but not with VEGF-induced) migration and sprouting of ECs. Similarly, HUVECs expressing low levels of Plexin B1 did not show any cytoskeletal rearrangement after Sema4D stimulation, either in terms of F-actin organization or of the β-catenin localization pattern (Figure 2, right panels). These 2 experimental approaches demonstrate that Plexin B1 is the endothelial receptor that mediates Sema4D proangiogenic activity.
Because it has recently been shown that Plexin B family members can signal through the Rho/Rho kinase pathway by direct interaction of their C-termini with the PDZ domain of a subgroup of RhoGef proteins, we aimed to analyze whether this pathway was involved in the Sema4D-induced responses in ECs. We thus infected HUVECs with a lentiviral construct encoding for a dominant-negative form of PDZ-RhoGef (F1).37 After evaluating the expression of the exogenous protein by Western blot analysis, we studied the mitogenic response of these cells to Sema4D. As shown in supplemental Figure 1 (see the Supplemental Figure link at the top of the online article), we did not detect any difference in motility in cells expressing the dominant-negative PDZ-RhoGef compared with control cells.
Sema4D activates Met receptor in HUVECs
After showing the importance of Plexin B1 in mediating Sema4D angiogenic activity, we investigated how Plexin B1 transduces the angiogenic stimulus. First we analyzed whether Plexin B1 interacts with endothelial-specific receptors, such as VEGFR-2. Cell lysates from HUVECs were immunoprecipitated with anti–Plexin B1 or anti–VEGFR-2 antibodies, and Western blots were stained with both antibodies. In our experimental conditions, we did not detect a significant interaction between the 2 endogenous receptors (data not shown).
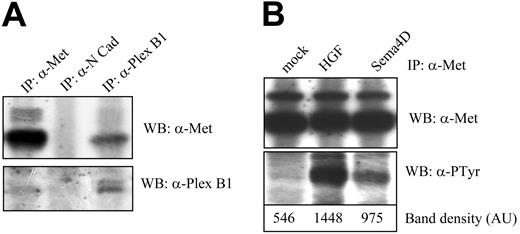
We and others30,31 have previously shown that the HGF/Met system is endowed with angiogenic activity. Moreover, we have recently demonstrated a structural and functional association between Plexin B1 and Met receptor in epithelial cells12 and have also shown that siRNA down-regulation of Plexin B1 prevents Met phosphorylation.45 To explore the Plexin B1–Met interaction in ECs, we performed a coimmunoprecipitation assay and confirmed a basal interaction between endogenous Plexin B1 and Met receptors (Figure 6A). Furthermore, Sema4D stimulation of HUVECs resulted in Met tyrosine phosphorylation (Figure 6B) without modifying the interaction between Plexin B1 and Met (data not shown). These data prove that Plexin B1 and Met interact in ECs and that Sema4D stimulation results in Met activation and tyrosine phosphorylation.
Met is essential to mediate Sema4D-induced angiogenic activity
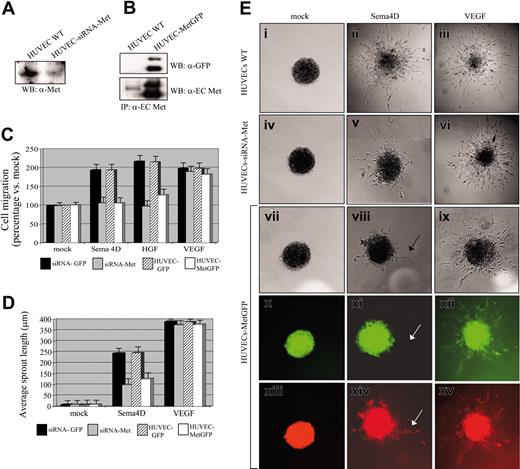
Recently, we demonstrated that Met activation is strictly required to elicit Sema4D-induced biologic activity in epithelial cells.12 Having also shown that Plexin B1 and Met associate in ECs in a complex and that Sema4D stimulation results in Met phosphorylation, we investigated the role of Met in Sema4D-induced angiogenic activity. We took 2 different approaches: the use of siRNA technology and the expression of dominant-interfering mutants of Met. HUVECs transfected with siRNAs against Met exhibited a strong reduction in Met expression compared with controls (Figure 7A). In a migration assay, HUVECs depleted of endogenous Met were unable to move toward HGF and Sema4D chemoattractants (Figure 7C). Similarly, siRNA-transfected HUVECs analyzed in a sprouting assay gave rise to a poor response in the presence of Sema4D; most of the sprouts were monocellular, not organized into tubular structures, and were generally shorter than the ones observed in controls (38.65% of wild-type average sprout length; Figure 7D-E, panels ii, v). Expression of siRNAs against Met did not affect the cellular response to VEGF-A165 stimulation, either in migration (Figure 7C) or in sprouting assays (Figures 7D-E, panel vi).
To further validate the requirement of Met in Sema4D angiogenic activity, we used a dominant-negative Met construct in which the full cytoplasmic region of Met was deleted and substituted with the green fluorescent protein (GFP) moiety (MetGFP12 ). We produced lentiviruses containing this construct and used them to infect HUVECs. Cell extracts obtained from Mock and MetGFP HUVECs were immunoprecipitated with antiextracellular Met antibodies and blots were stained with anti-Met or anti-GFP antibodies (Figure 7B). We carried out a migration assay, and we observed a strong reduction of the mitogenic response to HGF and Sema4D in MetGFP-expressing cells compared with controls (Figure 7C). Finally, we analyzed HUVEC-MetGFP cells by means of a sprouting assay. We found that few ECs sprouted on Sema4D stimulation, generating occasional, but well-organized, pluricellular structures, as shown in Figure 7E, panel viii. When the same spheroids were observed under fluorescent light to evaluate Met-GFP expression, we found that none of the few cells sprouted in response to Sema4D expressed the MetGFP protein. On the contrary, the expression of MetGFP did not affect the ability of cells to sprout in response to VEGF-A165 (Figure 7E, panels ix, xii, and xv). These 2 approaches demonstrate that Sema4D-induced angiogenic activity requires Met receptor activation.
Sema4D stimulation induces Met tyrosine phosphorylation in HUVECs. (A) HUVEC lysates were immunoprecipitated using the indicated antibodies, and Western blot was probed with Met-specific antibodies (top blot) or Plexin B1 antibodies (bottom blot). As shown, Met and Plexin B1 coprecipitated, whereas an unrelated protein, such as N-cadherin, did not show association with Met. (B) HUVECs were stimulated for 15 minutes with HGF or Sema4D, and cellular extracts were immunoprecipitated with α-Met antibodies. Probing of the blot with antiphosphotyrosine antibodies showed that Sema4D treatment triggered Met tyrosine phosphorylation (top blot). The control of the amount of the immunoprecipitated protein is shown in the intermediate panel. Quantification of tyrosine phosphorylation is shown in the bottom blot.
Sema4D stimulation induces Met tyrosine phosphorylation in HUVECs. (A) HUVEC lysates were immunoprecipitated using the indicated antibodies, and Western blot was probed with Met-specific antibodies (top blot) or Plexin B1 antibodies (bottom blot). As shown, Met and Plexin B1 coprecipitated, whereas an unrelated protein, such as N-cadherin, did not show association with Met. (B) HUVECs were stimulated for 15 minutes with HGF or Sema4D, and cellular extracts were immunoprecipitated with α-Met antibodies. Probing of the blot with antiphosphotyrosine antibodies showed that Sema4D treatment triggered Met tyrosine phosphorylation (top blot). The control of the amount of the immunoprecipitated protein is shown in the intermediate panel. Quantification of tyrosine phosphorylation is shown in the bottom blot.
Met is required for Sema4D-induced angiogenic activity. (A) HUVECs were transfected with siRNAs specific for Met, and the level of the Met protein present in total cellular extracts was evaluated. The figure shows a reduction of Met expression in transfected cells. (B) HUVECs were infected with lentiviruses encoding a MetGFP chimera, previously shown to act in a dominant-negative manner. Cell extracts were immunoprecipitated with antibodies directed against the extracellular portion of Met (α-extracellular Met), and Western blot was probed with either α-GFP (top blot) or α-extracellular Met (bottom blot) antibodies. The figure shows that MetGFP is expressed in infected cells at a level higher than endogenous Met. (C) Migration assay of control HUVECs, HUVECs transfected with Met-specific siRNAs, and HUVECs expressing the dominant-negative MetGFP. Controls varied according to experimental conditions: HUVECs transfected with siRNAs against GFP were used in the experiments performed on cells treated with Met-specific siRNAs; HUVECs infected with a lentivirus containing the GFP sequence were used in experiments performed with HUVECs-MetGFP. As shown, an impairment of cell migration in response to Sema4D was observed in cells displaying a decreased expression of Met because of siRNA treatment and in cells expressing MetGFP. No reduction of mitogenic ability was observed in these cells in response to VEGF-A165. (D) Quantification of the sprouting assay shown in panel E. Data in panels C and D indicate means ± SD. (E) A reduction of Sema4D-induced sprouting was observed in cells displaying decreased expression of Met because of siRNA treatment and in cells expressing MetGFP. Spheroids of cells expressing MetGFP were observed under ultraviolet light: sprouts formed by cells in response to VEGF-A165 appeared green (xii), indicating that the expression of MetGFP did not interfere with the response to VEGF-A165. The few sprouts observed in response to Sema4D were not fluorescent (x, white arrow) and were thus thought to have originated from cells that did not express the dominant-negative MetGFP. Spheroids were also stained with a vital red-emitting fluorochrome to allow detection of all sprouts (xiii-xv). Images were captured with a 20×/0.70 air objective lens.
Met is required for Sema4D-induced angiogenic activity. (A) HUVECs were transfected with siRNAs specific for Met, and the level of the Met protein present in total cellular extracts was evaluated. The figure shows a reduction of Met expression in transfected cells. (B) HUVECs were infected with lentiviruses encoding a MetGFP chimera, previously shown to act in a dominant-negative manner. Cell extracts were immunoprecipitated with antibodies directed against the extracellular portion of Met (α-extracellular Met), and Western blot was probed with either α-GFP (top blot) or α-extracellular Met (bottom blot) antibodies. The figure shows that MetGFP is expressed in infected cells at a level higher than endogenous Met. (C) Migration assay of control HUVECs, HUVECs transfected with Met-specific siRNAs, and HUVECs expressing the dominant-negative MetGFP. Controls varied according to experimental conditions: HUVECs transfected with siRNAs against GFP were used in the experiments performed on cells treated with Met-specific siRNAs; HUVECs infected with a lentivirus containing the GFP sequence were used in experiments performed with HUVECs-MetGFP. As shown, an impairment of cell migration in response to Sema4D was observed in cells displaying a decreased expression of Met because of siRNA treatment and in cells expressing MetGFP. No reduction of mitogenic ability was observed in these cells in response to VEGF-A165. (D) Quantification of the sprouting assay shown in panel E. Data in panels C and D indicate means ± SD. (E) A reduction of Sema4D-induced sprouting was observed in cells displaying decreased expression of Met because of siRNA treatment and in cells expressing MetGFP. Spheroids of cells expressing MetGFP were observed under ultraviolet light: sprouts formed by cells in response to VEGF-A165 appeared green (xii), indicating that the expression of MetGFP did not interfere with the response to VEGF-A165. The few sprouts observed in response to Sema4D were not fluorescent (x, white arrow) and were thus thought to have originated from cells that did not express the dominant-negative MetGFP. Spheroids were also stained with a vital red-emitting fluorochrome to allow detection of all sprouts (xiii-xv). Images were captured with a 20×/0.70 air objective lens.
Discussion
Compared with the older, simplistic concept of angiogenesis as a mere sprouting event during which ECs degrade the extracellular matrix, migrate, and proliferate, our current view of angiogenesis is increasingly complex. Actually, angiogenesis is a multistep process involving a number of different biologic mechanisms. During this morphogenetic event, ECs of preexisting blood vessels, challenged by appropriate biochemical and physical cues, dynamically remodel their cell-to-cell and cell-to-matrix adhesions and reorganize into a mature, hierarchically organized system of hollow endothelial tubes that then acquire arteriovenous identity and are eventually stabilized by interaction with pericytes. Although the first generation of angiogenic growth factors, eg VEGF and bFGF, fits well into the concept of a sprouting capillary, additional factors must be identified to provide a molecular basis supporting blood vessel assembly, remodeling, maturation, and differentiation. Angiopoietins, ephrins,34 and class 3 semaphorins16 are prominent examples of this new class of morphoangiogenic molecules. In the present work, we demonstrate that a class 4 semaphorin, namely Sema4D, is endowed with proangiogenic activity. First, we show that Sema4D promotes directional motility of ECs, a key step in all events of vascular morphogenesis46,16 and a common feature of all proangiogenic molecules. This activity is paralleled by the ability of Sema4D to induce cytoskeletal rearrangements that are considered pathognomonic of an angiogenic endothelium, such as F-actin reorganization, breakage of intercellular junctions, and relocalization of β-catenin. We then demonstrated that, on Sema4D stimulation, migrating ECs gave rise to numerous, well-organized tubular structures, thus recapitulating the events that occur in vivo during angiogenesis.39,40 Our in vitro results were strongly supported by in vivo assays exploited on the chick chorioallantoic membrane and in the murine Matrigel angiogenic assay, where Sema4D displayed an activity similar, in terms of molar concentration, to that of known angiogenic factors such as bFGF.
We were then interested in understanding the molecular events responsible for the angiogenic activity elicited by this semaphorin. First we investigated which cellular receptor might be responsible for the Sema4D-induced biologic activities in ECs because its high-affinity receptor, Plexin B1, and its low-affinity receptor, CD72, are expressed on HUVECs. Through 2 different experimental approaches—use of blocking antibodies and of RNA interference—we demonstrated that Plexin B1 was the Sema4D receptor mediating the angiogenic effect. We then analyzed the molecular mechanisms through which the plexin receptor exerted the angiogenic activity. We showed that Plexin B1 activation did not induce transcription of the most common angiogenic factors, such as VEGF-A and angiopoietin-2. Moreover, we demonstrated that the activation of the Rho/Rho kinase pathway is not strictly required, at least in human primary ECs. This finding prompted us to speculate that the observed outcomes could be a direct effect of the ability of Plexin B1 to activate a signal transduction pathway leading to angiogenesis. In this work we show that in ECs, Plexin B1 forms a constitutive complex with the tyrosine kinase receptor Met and that Sema4D-mediated Plexin B1 activation results in stimulation of Met tyrosine kinase activity. Moreover, Met activation is an absolute requirement for Sema4D stimulation of angiogenesis, as shown by experiments in which we interfered with Met function by using a dominant-negative form of Met or RNA interference. We thus show that Plexin B1 forms a complex with Met and that this interaction is required to elicit the angiogenic activity in response to Sema4D. Given that Plexin B1 interacts with Met in a ligand-independent manner, we can hypothesize that Sema4D binding results in plexin clustering, driving Met oligomerization and activation. As we have previously observed in epithelial cells,12 Sema4D-induced Met phosphorylation is weaker than that induced by the cognate ligand HGF. We believe that this can be attributed either to the amount of Met receptor recruited by the activated plexin in comparison to HGF or to the stoichiometry of multimerization. We have also observed that Met activation induced by the 2 ligands differs in terms of kinetics because it is delayed in the case of Sema4D. The semaphorin thus may behave like a partial agonist, such as NK247 or monoclonal antibodies (ie, DN3048 ), unable to induce a full phosphorylation of Met receptor or to allow the accomplishment of the full spectrum of HGF-induced biologic activities. Like partial agonists, in fact, Sema4D induces motility and morphogenesis, but it does not promote proliferation.
It has been previously shown that Met activation, induced by its own ligand, HGF, results in strong angiogenic activity.30 Nevertheless, though they converge on the same receptor, Sema4D and HGF do not have completely overlapping effects because HGF displays mitogenic activity on ECs whereas we did not observe it in Sema4D and because HGF up-regulates VEGF expression48 whereas Sema4D does not. However, VEGF induction is not strictly required for Met-mediated angiogenesis because it has been demonstrated that Met is angiogenic, independently of VEGF up-regulation.49-51
It is important to emphasize that HGF is a soluble ligand that, diffused in the extracellular environment, can activate ECs in a fairly extensive environment and induce widespread angiogenic effects. On the contrary, semaphorins, such as Sema4D, can exert their activity in a more localized manner, acting either as membrane-bound or locally released proteins. Furthermore, plexin receptors, through their ability to act on the cytoskeleton, might induce specific cytoskeletal rearrangements in different cellular portions, thus conferring polarization to EC movements and contributing to the 3-dimensional assembly. Therefore, HGF and Sema4D, despite their ability to activate the same receptor, might exert different functions in vivo.
Our data point to Sema4D as a new morphoangiogenic factor whose unique modality of signaling could represent a finer molecular strategy to modulate the spatial responses necessary to generate the complex and sensitive arborization of the vascular tree. Angiogenesis is a key step during embryo development, but it also occurs in some pathologic conditions such as atherosclerosis, arthritis, diabetic retinopathy, psoriasis, and tumor growth and invasion. Although tissue-specific molecules can have a major role in the angiogenic process, it has been clearly demonstrated that the final response depends on a finely modulated balance between several proangiogenic and antiangiogenic factors, each of which must be spatially and temporally expressed in the right amount. On these bases, identifying a novel angiogenic molecule and its downstream pathway contributes to an understanding of the process of angiogenesis and provides a further key to intervene with disease.
Note added in proof. After the submission of our manuscript, similar results showing that class IV semaphorins promote angiogenesis were published by Basile et al.52 Although most aspects are aligned with our findings, our work underlines the importance of the interaction between Plexin B1 and Met tyrosine kinase in eliciting the angiogenic response.
Prepublished online as Blood First Edition Paper, January 4, 2005; DOI 10.1182/blood-2004-07-2885.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (S.G., F.B., P.M.C., L.T.), Ministero dell'Universita' e della Ricerca Scientifica, MIUR, (S.G., F.B.), Istituto Superiore di Sanita', CNR, Fondi Incentivazione sulla Ricerca di Base (F.B.), and Telethon–Italy (G.S.).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Naldini for lentiviral constructs, G. Morterra for kindly providing HUVECs, FAMAARCO S.p.A. and SUSATRAS-PORTI S.p.A. for white Leghorn chicken eggs, and our colleagues S. Artigiani, D. Barberis, and P. Fazzari for helpful discussions. We also thank L. Palmas and R. Albano for their excellent technical assistance. We thank Antonella Cignetto for secretarial help and Elaine Wright for carefully reading the manuscript.

![Figure 2. Immunofluorescence analysis of Sema4D-induced cytoskeletal rearrangement. In wild-type HUVECs (left panels), soluble Sema4D triggered dramatic cytoskeletal rearrangements, characteristic of an angiogenic endothelium. On Sema4D stimulation (20 minutes), we observed the disappearance of stress fibers, the formation of membrane ruffles (phalloidin-FITC [fluorescein isothiocyanate] staining, top row), the dismantling of focal adhesions, and the internalization of β-catenin (α-βcatenin staining, lower rows). Reduced expression of Plexin B1, obtained as a consequence of HUVEC transfection with siRNA specific for Plexin B1, resulted in a lack of response to Sema4D stimulation for the overall actin reorganization and for the β-catenin relocalization (right column). Images were captured with a 63×/1.32 oil objective lens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-07-2885/6/m_zh80110579130002.jpeg?Expires=1765895857&Signature=xTJdVIZnIz11LUgTgFo5IlsjSvZ7Xw3Tx6z3j0AJ8fQTqe4api3GWd4vqDnw94WtAU7ClLyRFybOz4dWE99qdDeKLQuHfsDdvfUoZ0glrFq~~PDbhNVoKKL6tteR78prkvdcYr5vUJ-ZfuF-KhkPWL4SBqYbjnwQaO3OzEupM2unAqYNp6FBKgAMWSO2c37ODZdT2vT6gKxN8HxCU1GeShi3MEhR71IEx1gG4V~qYxZmQmxzbZ30iUuk7LrTWJvMyhyvUcQuCw1mLxgdgjWatTKdES5yFc8g396BWFWe0DgsTTtCA~9Sf2vny~kzahvPXY5wboT5UpcR~uIzD191Tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal