Abstract
Mice lacking SHP-1 exhibit a plethora of perturbations in their hematopoietic and immune systems. To reveal the primary effects resulting from SHP-1 deficiency, we used embryonic stem (ES) cells to study the role of SHP-1 in developmental hematopoiesis. We expressed wild-type (WT) and dominant-negative (R459M) forms of SHP-1 in ES cells and used ES/OP-9 coculture and embryoid body development followed by hematopoietic colony assays to demonstrate that SHP-1 acts at multiple stages of hematopoietic differentiation to alter lineage balance. Expression of WT SHP-1 reduced myeloid colony numbers while increasing the numbers of secondary embryoid bodies and mixed hematopoietic colonies obtained. Conversely, expression of R459M SHP-1 resulted in a significant increase in the numbers and sizes of myeloid colonies observed while reducing the numbers of colonies derived from undifferentiated cells or hematopoietic precursor cells. Confining the expression of WT or R459M SHP-1 to the early phases of differentiation decreased and increased progenitor cell numbers, respectively, and influenced colony formation. Overall, our results are consistent with SHP-1 acting during multiple stages of hematopoietic development, and they suggest that the increases in granulocytes and macrophages observed in motheaten mice arise as the result of a cell autonomous effect early during development.
Introduction
The SH2-domain containing tyrosine phosphatase SHP-1 is expressed almost exclusively in hematopoietic cells.1-3 Two strains of mice harbor mutations within the SHP-1 gene (Hcph) and have been used extensively to characterize SHP-1 as a negative regulator of hematopoietic and immune cell function.4-9 The murine motheaten mutation (Hcphme, abbreviated gene symbol me) results in a complete absence of SHP-1 activity, whereas the motheaten viable mutation (Hcphme-v, abbreviated gene symbol mev) retains 10% to 20% of SHP-1 activity. Ectopic expression of wild-type (WT) and inactive forms of SHP-1 in various hematopoietic cell lines has also demonstrated that SHP-1 is a negative regulator of terminally differentiated hematopoietic and immune cells.10-13
Me/me and mev/mev mice experience a plethora of abnormalities, including autoimmunity, hypergammaglobulinemia, precocious thymic involution, splenomegaly, and glomerulonephritis.6,14-17 Pulmonary infiltration of monocytes and macrophages leads to fatal pneumonitis and pulmonary hemorrhage after approximately 3 and 9 weeks in me/me and mev/mev mice, respectively.4,5 Within the erythroid and myeloid compartments, there is a pronounced shift of erythroid and granulocyte/macrophage precursors from the bone marrow to the spleen in mev/mev mice.17 In addition, splenic erythroid colony-forming units (CFU-Es) form spontaneously in the absence of erythropoietin in mev/mev mice.17 Several lines of evidence suggest that granulocytes and macrophages from motheaten mouse strains are hyperresponsive. For example, splenic macrophages from me/me mice proliferate in the absence of exogenous cytokines.14,15 Hyperproliferative responses of me/me and mev/mev bone marrow macrophages have been reported in response to granulocyte macrophage–colony-stimulating factor (GM-CSF),18 G-CSF (mev/mev macrophages),19 and CSF-1.20 In addition, me/me and mev/mev mice exhibit extensive defects in immune development and function, resulting in severe immunodeficiency.21,22 However, the myeloid cell defects do not appear to require T and B cells in mev/mev mice,23 and the production of transforming growth factor β1 (TGFβ1) by mev/mev macrophages24 may account for some of the reported effects on suppression of lymphopoiesis and natural killer cell development.21,25
The myriad abnormalities observed in me/me and mev/mev mice have made it difficult to distinguish clearly between the primary causative, cell autonomous effects and the secondary consequences resulting from the imbalance of the hematopoietic system. The self-renewal, distribution, and differentiation capacity of hematopoietic stem cells (HSCs) are reported to be normal in me/me mice,16 and bone marrow transplantation studies have suggested the phenotype is conferred by hematopoietic progenitors.16 However, studies have not been performed to examine hematopoiesis during different stages of development in me/me or mev/mev mice, and the possibility remains that SHP-1 may act at early developmental stages to perturb hematopoiesis. In this study, we have used 2 in vitro murine embryonic stem (ES) cell hematopoietic differentiation models to characterize the role of SHP-1 in regulating hematopoietic development to a level of detail that studying me/me and mev/mev mice has not allowed. ES cells26,27 remain pluripotent when cultured in the presence of leukemia inhibitory factor (LIF).28 The process of hematopoiesis that occurs during ES cell differentiation, either in coculture with the OP-9 stromal cell line or in embryoid body aggregates, most closely reflects yolk sac (ie, primitive hematopoiesis); hence, data generated from their study are not necessarily directly transferable to adult hematopoiesis. Despite this caveat, ES cells represent a useful model system in which to study developmental (embryonic) phases of in vivo hematopoietic development and the cellular and molecular events involved in the early stages of lineage determination.29-31 We have characterized the expression and localization of SHP-1 in undifferentiated ES cells, and, using the regulated expression of WT and catalytically inactive, nonsubstrate trapping (R459M) SHP-1, we have revealed a function for SHP-1 at far earlier stages of hematopoietic development than was previously recognized. These studies have allowed us to shed light on the likely cell autonomous defects in motheaten mice.
Materials and methods
Routine cell culture
E14tg2a murine ES cells32 were cultured on tissue culture plates (Nunc, Roskilde, Denmark) coated with 0.1% (wt/vol) gelatin (Sigma, Dorset, United Kingdom) in knockout Dulbecco modified Eagle medium (DMEM; Invitrogen, Paisley, Scotland) in the presence of 15% (vol/vol) knockout serum replacement (Invitrogen), 0.1 mM mercaptoethanol, 2 mM glutamine, 0.1 nM nonessential amino acids, and 1000 U/mL murine LIF (Chemicon, Hampshire, United Kingdom), as described previously.33 Cells were trypsinized and replated every second day. The OP-9 stromal cell line34,35 was cultured in alpha minimal essential medium (Invitrogen) supplemented with 20% (vol/vol) fetal bovine serum (FBS), 2 mM glutamine, and 0.1 mM mercaptoethanol. Ba/F3 cells were cultured as described previously.36
Generation of stable transfectants
The tetracycline (Tet)–off regulated expression system was used to express WT and catalytically inactive R459M SHP-1, described previously.10 E14tg2a cells expressing the Tet-sensitive transactivator (tTA) were the kind gift of Dr O. Witte (University of California at Los Angeles).37 tTA ES cells (1 × 107) were electroporated at 800 mV/3.0 μF with 10 μg linearized response plasmid encoding either WT or R459M SHP-1. Selection in the presence of 1000 U/mL LIF, 1 μg/mL tetracycline, and 200 μg/mL G418 was initiated 48 hours after transfection, and individual clones were picked after 5 to 6 days. For screening, 1 × 105 cells were plated into 60-mm plates in the presence or absence of 500 ng/mL Tet. After 24 hours, lysates were prepared and immunoblotted for expression of the exogenous SHP-1 protein. Independent clones (termed E14WTSHP-1, -3, -7, and -15 and E14RMSHP-1, -20, -43, and -48) exhibiting very low to undetectable basal expression and good inducible expression on the removal of Tet were selected for further analyses.
Cell lysates, immunoprecipitation, and immunoblotting
Cell lysates and cytosolic/membrane and nuclear fractionations were prepared as described previously.38 Protein concentrations were determined using a Bio-Rad (Hercules, CA) DC protein estimation kit according to the manufacturer's instructions, and 20 μg of each lysate was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted onto nitrocellulose.39 Immunoprecipitations were performed as described previously10 using 3 μg anti–SHP-1 (sc-287; Santa Cruz Biotechnology, Santa Cruz, CA), 2μg anti–SHP-2 (sc-280), or 1 μg 9E10 (M4439; Sigma, St Louis, MO). Primary antibodies were used at the following dilutions for immunoblotting: 1:1000 for anti–SHP-1, 1:2000 for anti-gp130 (sc-656), anti–Oct-4 (sc-9081), anti-extracellular regulated kinase (ERK1) (sc-91), and anti-Stat3 (sc-482); 1:10 000 for anti–SHP-2 and antiphosphotyrosine (4G10; Upstate Biotechnology, Milton Keynes, United Kingdom), and 0.25 μg/mL for the antibody recognizing the myc epitope tag (9E10). Goat antirabbit or mouse secondary antibodies, conjugated to horseradish peroxidase (HRP; DAKO, Cambridgeshire, United Kingdom), were used at 1:20 000 dilution, and blots were developed using enhanced chemiluminescence (ECL; Amersham Biosciences, Bucks, United Kingdom). Blots were stripped and reprobed as described previously.39
Differentiation protocols and hemopoietic colony assays
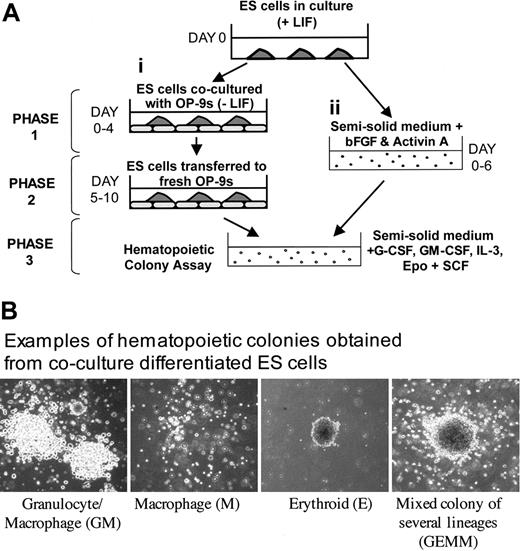
The ES cell/OP-9 coculture procedure was adapted from Era and Witte37,40 and is summarized in Figure 1A(i). ES cell transfectants (1 × 105) were plated, in the presence or absence of 500 ng/mL Tet, onto 80% confluent OP-9 feeder cells in OP-9 medium. After 4 to 5 days, cocultures were trypsinized, neutralized, and passed though a pipette 10 times to generate a single-cell suspension. Cells were plated in OP-9 medium onto tissue culture dishes for 30 minutes to allow OP-9 to adhere before the nonadherent, differentiated ES cells were removed, and 2 × 106 cells were plated onto fresh OP-9. After another 4 to 6 days, nonadherent progenitor cells were removed by gentle pipetting and were plated into hemopoietic colony assays. Embryoid body differentiation was adapted from Keller et al30 and from Kennedy et al41 (Figure 1A(ii)). ES cells (1 × 105) were added to 10 mL embryoid body medium (1% (wt/vol) ES Cult base methylcellulose (Stem Cell Technologies, Vancouver, Canada), 15% (vol/vol) FBS (Pierce, Cheshire, United Kingdom), 0.2 mg/mL transferrin, 0.1 mM 2-mercaptoethanol, 50 μg/mL l-ascorbic acid, 2.5 μg/mL insulin in Iscove modified Dulbecco medium (IMDM) supplemented with 2.5 ng/mL basic fibroblast growth factor (bFGF) and 12.5 ng/mL activin A, plated into bacterial Petri dishes in the presence or absence of 500 ng/nL Tet, and incubated at 37°C for 3 to 6 days. Embryoid bodies (EBs) were harvested by 3 washings in phosphate-buffered saline (PBS), followed by trypsinization for 3 to 5 minutes. Cells were passed through a 20-gauge needle before they were plated into a second round of colony formation or hemopoietic colony assays or were subjected to fluorescence-activated cell sorter (FACS) analysis. For secondary colony formation, cells derived from primary EBs were replated into methylcellulose and were supplemented with 5 ng/mL vascular endothelial growth factor (VEGF) and 100 ng/mL stem cell factor (SCF), as described previously.41 For hemopoietic colony assays (HCAs) (Figure 1A), 1 × 105 cells harvested from OP-9 cocultures or EB-derived cells were plated in Methocult (Stem Cell Technologies) containing 1% (wt/vol) methylcellulose, 15% (vol/vol) FBS, 1% (wt/vol) bovine serum albumin (BSA), 10 μg/mL insulin, 0.2 mg/mL transferrin, 0.1 mM 2-mercaptoethanol, 2 mM glutamine, 10 ng/mL GM-CSF, 10 ng/mL G-CSF, 10 ng/mL interleukin-3 (IL-3), 10 ng/mL SCF (all from Peprotech, London, United Kingdom), and 1.0 U/mL erythropoietin (EPO; R&D Systems, Oxfordshire, United Kingdom) in IMDM, into 35-mm Petri dishes and were incubated at 37°C for 5 to 15 days. Examples of colonies are shown in Figure 1B. Images were visualized using an Olympus (Tokyo, Japan) XI51 inverted microscope and objective lenses (apertures and magnifications are specified in each figure legend) and captured using an Olympus Camedia C4040 digital camera. For dark-field images, an Olympus SZ6045TR stereomicroscope was used. Images were downloaded into iPhoto (Apple, Cupertino, CA) and used with no further processing.
Hematopoietic differentiation of ES cells. (A) Schematic showing the 2 ES cell differentiation protocols used in this study. ES cells were placed in a 3-phase coculture differentiation system or an embryoid body differentiation system. For a full description, see “Materials and methods.” (B) Examples of the hematopoietic colony types obtained in this study, original magnification ×100. A 10×/0.4 objective lens was used.
Hematopoietic differentiation of ES cells. (A) Schematic showing the 2 ES cell differentiation protocols used in this study. ES cells were placed in a 3-phase coculture differentiation system or an embryoid body differentiation system. For a full description, see “Materials and methods.” (B) Examples of the hematopoietic colony types obtained in this study, original magnification ×100. A 10×/0.4 objective lens was used.
Cytospins and immunocytochemistry
Cytospins were prepared from 1 × 104 cells using a Shandon Cytospin 3 at 500 rpm (300 × g) for 10 minutes and were fixed in 90% (vol/vol) ethanol for 15 minutes before rehydration in Tris-buffered saline (TBS) (pH 7.5) for 10 minutes. Cells were blocked in TBS + 1% BSA + 0.2% ovalbumin (blocking solution; Sigma) for 1 hour at room temperature and then incubated in blocking solution + 2 μg/mL primary antibody overnight at 4°C. After a 5-minute wash in TBS, cells were incubated in peroxidase blocking reagent (DAKO) for 15 minutes, washed in TBS, and incubated in BS + HRP-conjugated secondary antibody (1:100) (DAKO) for 1 hour at room temperature. Cells were washed, incubated in DAB-HRP substrate for 10-30 minutes, counterstained, and dehydrated before they were mounted in Pertex nonaqueous mounting medium.
Results
Studies on Hcphme and Hcphme-v mice have demonstrated that SHP-1 is a negative regulator of the hematopoietic and immune systems.6,7 However, because of the complex network of interactions that regulate hematopoiesis, it has not been clearly established which abnormalities of motheaten mice arise as a result of cell autonomous effects and which arise as part of a secondary response to the primary defects. It has also not been conclusively determined whether SHP-1 affects only terminally differentiated cells or whether it also acts during development to perturb hematopoiesis. To address these issues, we have used murine ES cells as a model of in vitro hematopoietic differentiation,30,31 which most closely reflects primitive hematopoiesis.
SHP-1 expression and localization in ES cells
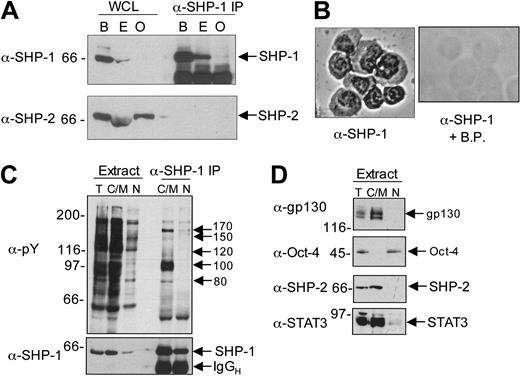
In the adult mouse, SHP-1 is expressed almost exclusively in hematopoietic lineages and in some epithelial lineages. As shown in Figure 2A, and confirming an earlier report,42 undifferentiated ES cells express SHP-1, though at lower levels than BaF/3 cells. OP-9 cells did not express detectable levels of SHP-1, whereas all cells expressed the related tyrosine phosphatase SHP-2. Immunohistochemical staining demonstrated the presence of SHP-1 in the cytosol and the nucleus of ES cells (Figure 2B), confirmed by biochemical fractionation (Figure 2C, lower panel). Immunoprecipitation of SHP-1 from the NP40 soluble fraction (containing cytosolic and membrane proteins) led to coprecipitation of a number of tyrosine-phosphorylated proteins of approximately 80, 100, 120, 150, and 170 kDa with SHP-1 (Figure 2C, upper panel). Interestingly, nuclear SHP-1 did not coprecipitate to any great extent with phosphotyrosyl proteins. The purity of our fractions was assessed by immunoblotting. As shown in Figure 2D, gp130, a component of the LIF receptor, was localized exclusively in the NP40 soluble fraction, as was SHP-2. Oct-4, a transcription factor expressed in undifferentiated ES cells, was localized exclusively in the nuclear fraction, whereas signal transducer and activator of transcription 3 (STAT3) was located in the NP40-soluble and nuclear fractions. These findings indicate that SHP-1 is expressed in undifferentiated ES cells, where it is coupled to tyrosine-phosphorylated proteins, suggesting that it may play a functional role in these cells.
Characterization of SHP-1 expression and localization in ES cells. (A) Whole cell lysate (WCL) was prepared from 5 × 106 cells (B = Ba/F3, E = E14tg2a ES cells, O = OP-9), and SHP-1 immunoprecipitates (α-SHP-1 IP) were prepared. These immunoprecipitates were separated by SDS-PAGE and immunoblotted for SHP-1. Blots were stripped and reprobed for SHP-2. Arrows indicate the positions of SHP-1 and SHP-2. (B) ES cells (1 × 104) were cytospun onto a slide, and immunocytochemistry was performed using anti–SHP-1 antibody. As a control, a duplicate slide was prepared and probed with α-SHP-1 + blocking peptide (α-SHP-1 + B.P.) original magnification ×400, a 40×/10.65 objective lens was used. (C) Total cell extracts (T), NP40 soluble extracts comprising cytosolic and membrane proteins (C/M), and nuclear fractions (N) were generated from 1 × 107 ES cells, and anti–SHP-1 immunoprecipitate (IP) was prepared. Extracts and precipitates were separated by SDS-PAGE and probed with the antiphosphotyrosine antibody 4G10 (α-pY, top blot). The equivalent of 1 × 105 cells was loaded per lane for total extract, and the equivalent of 2 × 105 cells was loaded per lane for the cytosolic and nuclear fractions (ie, double the amount of total extract separated). The blot was stripped and reprobed with an anti–SHP-1 antibody (bottom blot). (D) Extracts from each subcellular fraction (loaded in a manner equivalent to that in panel C) were separated on duplicate gels, 7.5% acrylamide for anti-gp130 immunoblotting and 10% acrylamide for anti–Oct-4. These blots were stripped and reprobed with antibodies against STAT3 and SHP-2, respectively. The positions of tyrosine-phosphorylated proteins coprecipitating with SHP-1 are indicated. Molecular weight standards are indicated on the left side of the panels. IgGH indicates immunoglobin heavy chain.
Characterization of SHP-1 expression and localization in ES cells. (A) Whole cell lysate (WCL) was prepared from 5 × 106 cells (B = Ba/F3, E = E14tg2a ES cells, O = OP-9), and SHP-1 immunoprecipitates (α-SHP-1 IP) were prepared. These immunoprecipitates were separated by SDS-PAGE and immunoblotted for SHP-1. Blots were stripped and reprobed for SHP-2. Arrows indicate the positions of SHP-1 and SHP-2. (B) ES cells (1 × 104) were cytospun onto a slide, and immunocytochemistry was performed using anti–SHP-1 antibody. As a control, a duplicate slide was prepared and probed with α-SHP-1 + blocking peptide (α-SHP-1 + B.P.) original magnification ×400, a 40×/10.65 objective lens was used. (C) Total cell extracts (T), NP40 soluble extracts comprising cytosolic and membrane proteins (C/M), and nuclear fractions (N) were generated from 1 × 107 ES cells, and anti–SHP-1 immunoprecipitate (IP) was prepared. Extracts and precipitates were separated by SDS-PAGE and probed with the antiphosphotyrosine antibody 4G10 (α-pY, top blot). The equivalent of 1 × 105 cells was loaded per lane for total extract, and the equivalent of 2 × 105 cells was loaded per lane for the cytosolic and nuclear fractions (ie, double the amount of total extract separated). The blot was stripped and reprobed with an anti–SHP-1 antibody (bottom blot). (D) Extracts from each subcellular fraction (loaded in a manner equivalent to that in panel C) were separated on duplicate gels, 7.5% acrylamide for anti-gp130 immunoblotting and 10% acrylamide for anti–Oct-4. These blots were stripped and reprobed with antibodies against STAT3 and SHP-2, respectively. The positions of tyrosine-phosphorylated proteins coprecipitating with SHP-1 are indicated. Molecular weight standards are indicated on the left side of the panels. IgGH indicates immunoglobin heavy chain.
Generation and characterization of stable ES cell transfectants that express WT SHP-1 and an inactive mutant of SHP-1
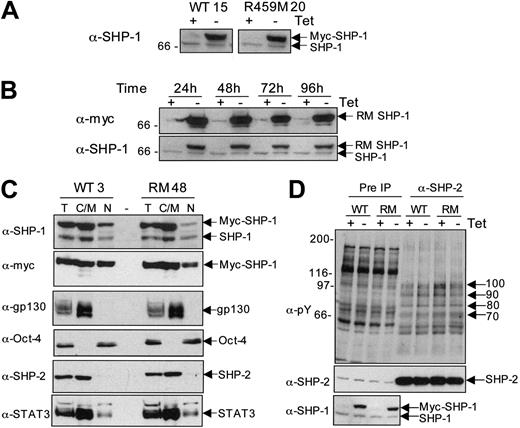
To examine the role of SHP-1 during early stages of murine hematopoietic differentiation, we decided to use the Tet-off–regulated expression system to express variants of SHP-1 in ES cells because this system allows the effects with and without expression to be observed within the same cell clone. We predicted that expression of WT SHP-1 should enable the consequences of expressing elevated levels of the SHP-1 on hematopoietic development to be examined. Expression of a mutated form of SHP-1, in which R 459 has been replaced by M, creating a dominant-negative, nontrapping form of SHP-1,10,43 was predicted to model the phenotype of motheaten mice because of competition of the inactive R459M SHP-1 mutant with endogenous SHP-1. Stable ES cell transfectants were generated, and Figure 3A demonstrates the expression in 2 representative clones. The reduced electrophoretic mobility is attributed to the addition of an N-terminal myc-epitope tag to the SHP-1 variants. Expression was maintained for more than 96 hours after Tet removal for both RM SHP-1–expressing (Figure 3B) and WT SHP-1–expressing (data not shown) clones. Immunocytochemical staining using an anti-myc tag antibody demonstrated that WT and R459M SHP-1 were expressed in more than 95% of cells in clones 3 and 48 (data not shown). Subcellular fractionation, shown in Figure 3C, demonstrated that the expressed SHP-1 variants were located in the nucleus and in the cytosol/membrane fractions, similar to parental ES cells, though the highest levels were present in the cytosol. Expression of the SHP-1 variants did not appreciably or consistently perturb interactions of SHP-2 with phosphotyrosyl proteins in undifferentiated ES cells in exponentially growing cultures (Figure 3D) or after acute stimulation with LIF (data not shown). Three independent clones expressing each SHP-1 variant were selected for further analyses.
Generation of stable transfectants inducibly expressing WT and R459M SHP-1 variants. ES cell transfectants were generated as described in “Materials and methods.” (A) Transfectants were cultured for 24 hours in the presence or absence of 500 ng/mL Tet, and lysates were prepared. Lysates were separated by SDS-PAGE and immunoblotted for SHP-1. WT clone 15 and R459M clone 20 are shown as examples. Arrows indicate the positions of the expressed and endogenous proteins. (B) Transfectants were cultured as in panel A, but the induction was extended for 96 hours. Lysates were taken after each 24-hour period. Lysates were separated by SDS-PAGE and immunoblotted with anti-myc epitope tag antibody (9E10) and then reprobed for SHP-1. R459M clone 20 is shown as an example. Arrows indicate the positions of the expressed and endogenous proteins. (C) Total cell extracts (T), NP40 soluble extracts comprising cytosolic and membrane proteins (C/M), and nuclear fractions (N) were prepared from 1 × 107 ES cells from WT clone 3 and R459M clone 48. For the total cell extracts, the equivalent of 1 × 105 cells was loaded per lane and the equivalent of 2 × 105 cells was loaded per lane for the cytosolic and nuclear fractions (ie, double the amount of total extract separated). Duplicate gels were run, and 7.5% acrylamide gels were probed with the anti–SHP-1 antibody (α-SHP-1) or the anti-gp130 antibody (α-gp130) and subsequently were stripped and reprobed with anti-myc tag or anti-STAT3 antibodies. The 10% acrylamide gel was probed for Oct-4 and then stripped and reprobed for SHP-2. (D) WT clone 3 and R459M clone 48 ES cell transfectants were plated in the presence and absence of 500 ng/mL Tet for 48 hours. Extracts from cells growing exponentially were prepared, and 1 mg cell extract (Pre IP) was used to generate SHP-2 immunoprecipitates (α-SHP2). Immunoblotting was performed with 4G10 (α-pY), and the blot was stripped and reprobed with anti–SHP-2 antibodies to control for loading and then probed for SHP-1 to demonstrate expression. Arrows indicate the molecular weights of tyrosine-phosphorylated proteins coprecipitating with SHP-2.
Generation of stable transfectants inducibly expressing WT and R459M SHP-1 variants. ES cell transfectants were generated as described in “Materials and methods.” (A) Transfectants were cultured for 24 hours in the presence or absence of 500 ng/mL Tet, and lysates were prepared. Lysates were separated by SDS-PAGE and immunoblotted for SHP-1. WT clone 15 and R459M clone 20 are shown as examples. Arrows indicate the positions of the expressed and endogenous proteins. (B) Transfectants were cultured as in panel A, but the induction was extended for 96 hours. Lysates were taken after each 24-hour period. Lysates were separated by SDS-PAGE and immunoblotted with anti-myc epitope tag antibody (9E10) and then reprobed for SHP-1. R459M clone 20 is shown as an example. Arrows indicate the positions of the expressed and endogenous proteins. (C) Total cell extracts (T), NP40 soluble extracts comprising cytosolic and membrane proteins (C/M), and nuclear fractions (N) were prepared from 1 × 107 ES cells from WT clone 3 and R459M clone 48. For the total cell extracts, the equivalent of 1 × 105 cells was loaded per lane and the equivalent of 2 × 105 cells was loaded per lane for the cytosolic and nuclear fractions (ie, double the amount of total extract separated). Duplicate gels were run, and 7.5% acrylamide gels were probed with the anti–SHP-1 antibody (α-SHP-1) or the anti-gp130 antibody (α-gp130) and subsequently were stripped and reprobed with anti-myc tag or anti-STAT3 antibodies. The 10% acrylamide gel was probed for Oct-4 and then stripped and reprobed for SHP-2. (D) WT clone 3 and R459M clone 48 ES cell transfectants were plated in the presence and absence of 500 ng/mL Tet for 48 hours. Extracts from cells growing exponentially were prepared, and 1 mg cell extract (Pre IP) was used to generate SHP-2 immunoprecipitates (α-SHP2). Immunoblotting was performed with 4G10 (α-pY), and the blot was stripped and reprobed with anti–SHP-2 antibodies to control for loading and then probed for SHP-1 to demonstrate expression. Arrows indicate the molecular weights of tyrosine-phosphorylated proteins coprecipitating with SHP-2.
SHP-1 negatively regulates hematopoietic differentiation of ES cells in OP-9 coculture
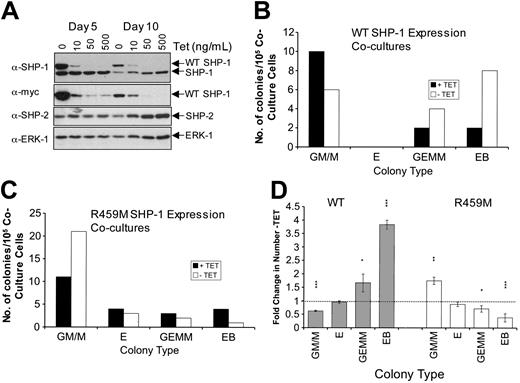
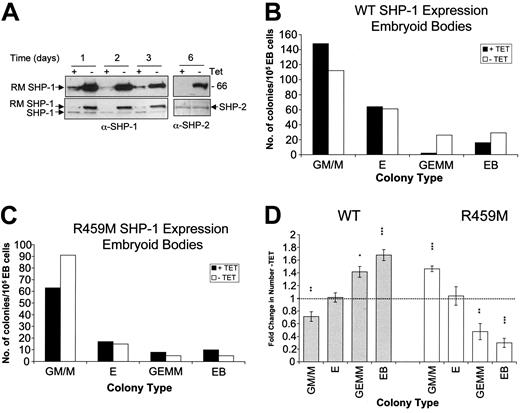
We examined whether the expression of WT or R459M SHP-1 during OP-9 coculture–facilitated hematopoietic differentiation of ES cells altered the numbers or lineages of the hematopoietic colonies obtained. ES cells are directed to differentiate along mesodermal lineages when placed in coculture with the CSF-1–deficient stromal cell line OP-9, such that hematopoietic progenitors are obtained after 8 to 10 days of culture.34,35,37,40 The capacity of these progenitors to form mature hematopoietic colonies is then assayed quantitatively in in vitro hemopoietic colony-forming assays (HCAs). The cytokine cocktail included in our HCAs was selected for its ability to support myeloid and erythroid development. In this set of analyses, the SHP-1 variants were maintained in the presence or absence of Tet throughout the 3 phases of differentiation. In Figure 4A, we demonstrate that the expression of SHP-1 is maintained in the -Tet samples at days 5 and 10, with levels of exogenous SHP-1 relative to endogenous SHP-1 similar, though overall levels of endogenous and exogenous SHP-1 decreased during differentiation, consistent with the restricted pattern of SHP-1 expression in adult mice. Colonies obtained in the HCAs were scored for lineage type and number. Results of representative experiments are shown for WT SHP-1 (Figure 4B) and R459M SHP-1 (Figure 4C) and were replicated for at least 2 independent clones in each case. Data from at least 3 independent experiments using different WT- or R459M-expressing ES cell clones were combined to produce the data shown in Figure 4D. Significantly, expression of WT SHP-1 reduced the number of granulocyte/macrophage and macrophage (GM/M)–type colonies, indicative of SHP-1 playing a role in control of this lineage. Interestingly, we observed a 3.8-fold increase in the number of secondary embryoid bodies that formed in the HCAs, and this was highly significant (Figure 4B, D). These data indicate that a significant number of undifferentiated ES cells must have been plated into the HCAs, suggesting that elevated SHP-1 activity slows the differentiation of ES cells. Conversely, the expression of dominant-negative R459M SHP-1 reduced the number of secondary embryoid bodies in the HCA (Figure 4C-D). This was mirrored by an increase of approximately 1.5-fold in the number of myeloid colonies that formed when R459M SHP-1 was expressed. These results are consistent with the increases in granulocytes and macrophages observed in me and mev mice,4,17 and they indicate to us that this aspect of the me phenotype is likely to result from a cell autonomous effect. Interestingly, we observed no significant effects on erythroid colony formation.
Effect of inducible WT and R459M SHP-1 expression on hematopoietic differentiation of ES cells in OP-9 coculture. (A) ES cell transfectants were placed in coculture with OP-9 stromal cells in the presence of the indicated doses of Tet or in the absence of Tet. Lysates were prepared at the end of the first (day 5) and second (day 10) phases and were separated by SDS-PAGE on duplicate gels. Immunoblotting was performed first with anti–SHP-1 or anti-myc tag antibodies. The anti–SHP-1 blot was stripped and subsequently blotted with anti–SHP-2 and then anti-ERK1 antibodies. Anti–SHP-2 and ERK-1 blots indicate that the 10, 50, and 500 ng/mL Tet day 10 samples had more protein loaded than all the day 5 and day 10 no Tet samples. (B-D) ES cell transfectants were placed in coculture with OP-9 stromal cells in the presence or absence of 500 ng/mL Tet for the duration of the coculture and HCA. Representative results are shown using WT (B) and R459M (C) SHP-1–expressing transfectants. Numbers of GM/M, erythroid (E), GEMM, and secondary EB colonies obtained are shown. (D) Average data from experiments using 2 independent WT (n = 4; ▦) and R459M (n = 3; □) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of each colony type obtained -Tet compared with +Tet has been calculated and the average is shown, with standard errors indicated. Data were subjected to paired Student t test (***P < .005; **P < .05; *P < .1).
Effect of inducible WT and R459M SHP-1 expression on hematopoietic differentiation of ES cells in OP-9 coculture. (A) ES cell transfectants were placed in coculture with OP-9 stromal cells in the presence of the indicated doses of Tet or in the absence of Tet. Lysates were prepared at the end of the first (day 5) and second (day 10) phases and were separated by SDS-PAGE on duplicate gels. Immunoblotting was performed first with anti–SHP-1 or anti-myc tag antibodies. The anti–SHP-1 blot was stripped and subsequently blotted with anti–SHP-2 and then anti-ERK1 antibodies. Anti–SHP-2 and ERK-1 blots indicate that the 10, 50, and 500 ng/mL Tet day 10 samples had more protein loaded than all the day 5 and day 10 no Tet samples. (B-D) ES cell transfectants were placed in coculture with OP-9 stromal cells in the presence or absence of 500 ng/mL Tet for the duration of the coculture and HCA. Representative results are shown using WT (B) and R459M (C) SHP-1–expressing transfectants. Numbers of GM/M, erythroid (E), GEMM, and secondary EB colonies obtained are shown. (D) Average data from experiments using 2 independent WT (n = 4; ▦) and R459M (n = 3; □) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of each colony type obtained -Tet compared with +Tet has been calculated and the average is shown, with standard errors indicated. Data were subjected to paired Student t test (***P < .005; **P < .05; *P < .1).
SHP-1 negatively regulates hematopoietic differentiation of ES cells in embryoid bodies
As an alternative approach to examine the effects of WT and R459M SHP-1 expression on hematopoietic differentiation, we used the development of EBs to facilitate mesodermal differentiation (Figure 1A(ii)). After the removal of LIF, ES cells spontaneously differentiate as 3-dimensional aggregates termed EBs, with hemangioblasts or blast colony-forming cells developing within 3 to 5 days that differentiate into progenitors of all hematopoietic lineages.41 The potential of these progenitors can be assayed by plating dissociated EB-derived cells into HCAs. WT and R459M SHP-1 ES cell transfectants were induced to form EBs for 6 days, in the presence or absence of 500 ng/mL Tet, throughout the period of EB formation and HCAs. Expression levels during EB formation are shown in Figure 5A for RM SHP-1, with similar expression patterns seen with WT SHP-1 (data not shown). Expression of WT SHP-1 led to a reduction in the number of GM/M colonies, coupled with 1.5- and 1.75-fold increases in the number of secondary EBs and mixed hemopoietic (GEMM) colonies obtained, respectively. A representative experiment is shown in Figure 5B, with combined analyses shown in Figure 5D. In contrast, the expression of R459M SHP-1 increased the number of GM/M type colonies obtained by 1.5-fold, whereas the numbers of GEMM colonies and EBs were reduced to 0.5- and 0.3-fold their original levels (Figure 5C-D). As with the results of the OP-9 cocultures, no significant effects on erythroid colony numbers were observed. These results are consistent with the effects of WT and R459M SHP-1 expression in OP-9 coculture-facilitated differentiation and support a role for SHP-1 in regulating hematopoietic differentiation.
Effect of inducible WT and R459M SHP-1 expression on hematopoietic differentiation of ES cells in EBs. ES cell transfectants were placed in EB culture in the presence or absence of 500 ng/mL Tet for the duration of EB development and HCAs. (A) Lysates were prepared from EBs at the times shown and were immunoblotted with the anti-myc epitope tag antibody 9E10 (top row). The position of R459M SHP-1 is indicated. Blots were stripped and reprobed for anti–SHP-1 or anti–SHP-2 (bottom row). Representative results are shown for experiments performed using WT (B) and R459M (C) SHP-1–expressing transfectants. Numbers of GM/M, erythroid (E), GEMM, and secondary EB colonies obtained are shown. (D) Combined data from experiments using WT (n = 3; ▦) and R459M (n = 3; □) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of each colony type obtained -Tet compared with +Tet was calculated, the average was taken, and standard errors are shown. Data were subjected to paired Student t test (***P < .005; **P < .05; *P < .1).
Effect of inducible WT and R459M SHP-1 expression on hematopoietic differentiation of ES cells in EBs. ES cell transfectants were placed in EB culture in the presence or absence of 500 ng/mL Tet for the duration of EB development and HCAs. (A) Lysates were prepared from EBs at the times shown and were immunoblotted with the anti-myc epitope tag antibody 9E10 (top row). The position of R459M SHP-1 is indicated. Blots were stripped and reprobed for anti–SHP-1 or anti–SHP-2 (bottom row). Representative results are shown for experiments performed using WT (B) and R459M (C) SHP-1–expressing transfectants. Numbers of GM/M, erythroid (E), GEMM, and secondary EB colonies obtained are shown. (D) Combined data from experiments using WT (n = 3; ▦) and R459M (n = 3; □) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of each colony type obtained -Tet compared with +Tet was calculated, the average was taken, and standard errors are shown. Data were subjected to paired Student t test (***P < .005; **P < .05; *P < .1).
SHP-1 acts at multiple stages of ES cell hematopoietic differentiation
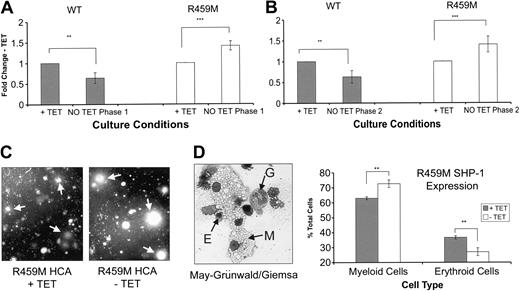
Our results reveal that the level of SHP-1 activity within differentiating ES cells affects their ability to develop along hematopoietic lineages. However, these results do not distinguish whether SHP-1 is acting during all phases of differentiation or whether SHP-1 action is limited to more specific stages. The Tet-regulated system allows us to investigate this by limiting expression to just 1 phase of differentiation. Initially, we examined the effects on proliferation during each phase of OP-9 coculture. Expression of WT SHP-1 during phase 1 reduced proliferation, whereas the expression of R459M SHP-1 significantly increased cell numbers (Figure 6A). Expression of WT SHP-1 during phase 2 alone also reduced proliferation, whereas again the expression of R459M SHP-1 significantly increased proliferation (Figure 6B). Consistent with increased proliferation during phase 3, the GM/M colonies formed by cells expressing R459M SHP-1 were consistently larger than those obtained from cells plated in the presence of Tet (Figure 6C). Furthermore, when all cells from the HCAs were harvested and distinct cell types were enumerated, the proportion of myeloid cells was increased, and there was a corresponding reduction in the proportion of erythrocytes obtained in cells expressing R459M SHP-1 (Figure 6D). These findings are consistent with the increases in the numbers and sizes of GM/M colonies obtained after the expression of R459M SHP-1 and with the phenotype of mev mice in which granulocytes and macrophages exhibit enhanced cytokine responsiveness.18,19
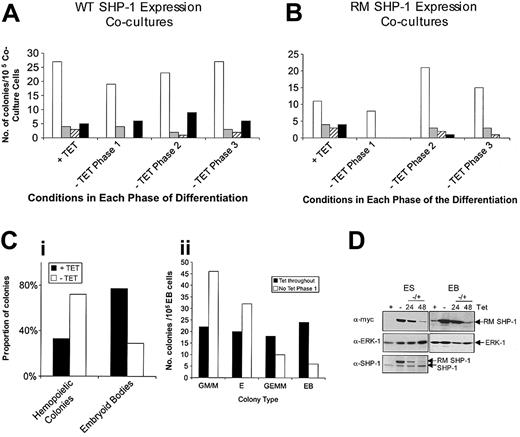
Next, we assessed whether hematopoiesis was affected by perturbing SHP-1 during the early stages of hematopoietic development. Expression of WT SHP-1, during the first or the second phase of OP-9 coculture, reduced the number of GM/M colonies and increased the number of secondary EBs obtained, whereas differences in GEMM colony number were not consistently observed. (Figure 7A). Expression during phase 3 alone had little overall effect. Expression of R459M SHP-1 during the second phase of the coculture increased the number of GM/M colonies and reduced the number of secondary EBs obtained (Figure 7B). Expression of R459M SHP-1 during the first phase of coculture consistently suppressed the formation of EBs and of E and GEMM colonies, though an increase in GM/M colonies was not always observed. Given these effects and those we observed on expression of WT SHP-1 during phase 1, we investigated the effects of R459M SHP-1 further. The results shown in Figure 7C(i) demonstrate that the expression of R459M SHP-1 during days 0 to 3 of EB formation was sufficient to accelerate the appearance of hematopoietic colonies. If these EB-derived cells were replated into methylcellulose for another 3 days, with Tet added back to suppress R459MSHP-1 expression, the number of GM/M colonies in the HCA doubled compared with cells maintained in Tet throughout (Figure 7C(ii)). In ES cells and during secondary EB development, the readdition of Tet led to a reduction in expression by 24 hours, which was reduced to basal levels by 48 hours (Figure 7D). These findings indicate that SHP-1 can act at early stages of hematopoietic differentiation to result in enhanced myelopoiesis.
We were interested in defining the molecular mechanisms underlying the effects of SHP-1 variant expression on hematopoiesis and analyzed the expression of a limited set of genes associated with particular stages of differentiation, including the pluripotency ES cell marker Oct-4,44 the early mesodermal markers brachyury and Flk-1, and hemopoietic markers c-kit,30 Cdx4, and HoxB4. No significant effects in mRNA expression of any of these markers were observed, and the surface expression of Flk-1 and c-kit remained unchanged in cells expressing WT or R459M SHP-1 at days 3 to 6 of EB development (data not shown).
Effect of inducible WT and R459M SHP-1 expression on the growth of ES cells differentiating in coculture with OP-9 stromal cells. (A) WT and R459M SHP-1 ES cell transfectants were placed in coculture for 4 days in the presence (+ TET) or absence (NO TET) of 500 ng/mL Tet. OP-9 cells were removed by differential adhesion, and the numbers of differentiated ES cell derivatives obtained were determined. Data from experiments using WT (n = 4; ▦) and R459M (n = 4; □) SHP-1–expressing transfectants are shown. (B) Cells derived as in panel A in the presence of Tet were placed in coculture for another 6 days in the presence or absence of 500 ng/mL Tet, and the numbers of cells obtained were determined. Data from experiments using WT (n = 3) and R459M (n = 6) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of cells obtained -Tet compared with +Tet was calculated, the average was taken, and standard errors are shown. Data were subjected to paired Student t test (***P < .005; **P < .05). (C) Dark-field digital photomicrograph of HCAs derived from hemopoietic progenitors generated from R459M SHP-1/OP-9 coculture in the presence or absence of 500 ng/mL Tet for the duration of the coculture and HCA. Arrows indicate the positions of granulocyte/macrophage colonies, original magnification ×15. (D, left) Cells in HCAs generated from R459M SHP-1 transfectants differentiated in EB culture were removed from the HCA, and cytospins were prepared. Cells were stained with May-Grünwald/Giemsa stain, and the relative proportions of myeloid cell types and erythrocytes were determined (right). Original magnification ×400, a 40×/0.65 objective lens was used. Results are the averages of triplicate counts, and error bars are standard deviations. Data were subjected to paired Student t test (**P < .05).
Effect of inducible WT and R459M SHP-1 expression on the growth of ES cells differentiating in coculture with OP-9 stromal cells. (A) WT and R459M SHP-1 ES cell transfectants were placed in coculture for 4 days in the presence (+ TET) or absence (NO TET) of 500 ng/mL Tet. OP-9 cells were removed by differential adhesion, and the numbers of differentiated ES cell derivatives obtained were determined. Data from experiments using WT (n = 4; ▦) and R459M (n = 4; □) SHP-1–expressing transfectants are shown. (B) Cells derived as in panel A in the presence of Tet were placed in coculture for another 6 days in the presence or absence of 500 ng/mL Tet, and the numbers of cells obtained were determined. Data from experiments using WT (n = 3) and R459M (n = 6) SHP-1–expressing transfectants are shown. For each experiment, the fold change in the number of cells obtained -Tet compared with +Tet was calculated, the average was taken, and standard errors are shown. Data were subjected to paired Student t test (***P < .005; **P < .05). (C) Dark-field digital photomicrograph of HCAs derived from hemopoietic progenitors generated from R459M SHP-1/OP-9 coculture in the presence or absence of 500 ng/mL Tet for the duration of the coculture and HCA. Arrows indicate the positions of granulocyte/macrophage colonies, original magnification ×15. (D, left) Cells in HCAs generated from R459M SHP-1 transfectants differentiated in EB culture were removed from the HCA, and cytospins were prepared. Cells were stained with May-Grünwald/Giemsa stain, and the relative proportions of myeloid cell types and erythrocytes were determined (right). Original magnification ×400, a 40×/0.65 objective lens was used. Results are the averages of triplicate counts, and error bars are standard deviations. Data were subjected to paired Student t test (**P < .05).
SHP-1 acts at early and intermediate stages of differentiation. (A) WT and (B) R459M SHP-1 ES cell transfectants were differentiated according to the 3-phase coculture protocol (Figure 1A(i)). Cells were plated in the presence of 500 ng/mL Tet for the duration, or Tet was removed for 1 of the 3 phases. Culture conditions over the 3 phases are indicated. Numbers of colonies present in the HCAs (phase 3) were determined, and representative results are shown. □ indicates GM/M; ▦, E; ▨, GEMM; and ▪, EB. (C) R459M SHP-1 ES cell transfectants were placed in EB culture for 3 days in the presence or absence of 500 ng/mL Tet. EBs were dissociated, and cells (i) were plated directly into HCAs or (ii) were subjected to a second round of embryoid body formation, in the presence of Tet in both cases, before plating into HCAs. Numbers of colonies present in the HCAs were determined, and representative results are shown. (D) R459M clone 20 ES cells were plated in the presence (+) or absence (-) of 500 ng/mL Tet for 24 hours in the case of undifferentiated ES cells (ES) or for 72 hours in the case of primary embryoid body formation (EB). After these periods of time, cells were washed and 500 ng/mL Tet was added back to the ES cell liquid cultures for 24 and 48 hours (-/+). Primary embryoid bodies were dissociated and plated into a second round of embryoid body formation with the readdition of 500 ng/mL Tet. Lysates were prepared at the times indicated, and immunoblotting was performed with anti–SHP-1 antibodies first. For EB samples, the amount of protein obtained was too low to detect a clear SHP-1 signal. Blots were stripped and reprobed with anti-myc antibodies and then stripped and reprobed with anti-ERK1 antibody as a loading control. The position of proteins is indicated.
SHP-1 acts at early and intermediate stages of differentiation. (A) WT and (B) R459M SHP-1 ES cell transfectants were differentiated according to the 3-phase coculture protocol (Figure 1A(i)). Cells were plated in the presence of 500 ng/mL Tet for the duration, or Tet was removed for 1 of the 3 phases. Culture conditions over the 3 phases are indicated. Numbers of colonies present in the HCAs (phase 3) were determined, and representative results are shown. □ indicates GM/M; ▦, E; ▨, GEMM; and ▪, EB. (C) R459M SHP-1 ES cell transfectants were placed in EB culture for 3 days in the presence or absence of 500 ng/mL Tet. EBs were dissociated, and cells (i) were plated directly into HCAs or (ii) were subjected to a second round of embryoid body formation, in the presence of Tet in both cases, before plating into HCAs. Numbers of colonies present in the HCAs were determined, and representative results are shown. (D) R459M clone 20 ES cells were plated in the presence (+) or absence (-) of 500 ng/mL Tet for 24 hours in the case of undifferentiated ES cells (ES) or for 72 hours in the case of primary embryoid body formation (EB). After these periods of time, cells were washed and 500 ng/mL Tet was added back to the ES cell liquid cultures for 24 and 48 hours (-/+). Primary embryoid bodies were dissociated and plated into a second round of embryoid body formation with the readdition of 500 ng/mL Tet. Lysates were prepared at the times indicated, and immunoblotting was performed with anti–SHP-1 antibodies first. For EB samples, the amount of protein obtained was too low to detect a clear SHP-1 signal. Blots were stripped and reprobed with anti-myc antibodies and then stripped and reprobed with anti-ERK1 antibody as a loading control. The position of proteins is indicated.
Discussion
In this study, we have examined the role of SHP-1 in early developmental hematopoiesis using ES cells as a model for primitive hematopoietic differentiation. We demonstrate that the expression of dominant-negative SHP-1 during in vitro differentiation increases the formation of myeloid colonies, effectively recapitulating the increases in granulocytes and macrophages characteristic of motheaten mice.4 Importantly, the expression of WT SHP-1 had the opposite effect and reduced the number of GM/M colonies formed. These data provide the first independent evidence that the bias toward myelopoiesis observed in motheaten mice is likely the result of a cell autonomous effect. Additionally, we present novel evidence that SHP-1 can act at multiple stages of hematopoiesis, including early mesodermal differentiation. We propose that events regulated by SHP-1 during early hematopoiesis contribute to the development of the phenotype of the motheaten mice.
One of the key issues regarding the phenotype of me and mev mice has been to identify which effects are the result of primary cell autonomous defects and which arise as a secondary consequence. Defects in myelopoiesis, resulting in increased neutrophil and monocyte numbers in peripheral blood4 and increased proliferation of macrophages,14,15 contribute to the development of the fatal pneumonitis experienced by me and mev mice.4,5 However, no independent experimental models have examined the role of SHP-1 in myeloid development. Importantly in this regard, we have now shown that the expression of dominant-negative SHP-1 throughout either OP-9 or embryoid body–facilitated ES cell differentiation leads to enhanced myeloid colony formation. Significantly, the expression of WT SHP-1, which elevates levels of SHP-1 activity, results in the opposite effect, and myeloid development is decreased. WT SHP-1 expression increased the numbers of secondary embryoid bodies and mixed-lineage colonies formed in HCAs, indicating that more pluripotent progenitors were present in the cells induced to express WT SHP-1 at the time they were plated into the HCA, consistent with a slowing in progression of differentiation. However, the expression of flk-1, brachyury, and c-kit indicate no gross alterations in differentiation. Overall, these data are consistent with the propensity for myeloid differentiation in the presence of reduced SHP-1 activity resulting from a cell autonomous effect.
Three lines of evidence suggest that SHP-1 can act at much earlier stages of hematopoietic development than previously thought. First, the expression of dominant-negative R459M SHP-1 during phase 1 of OP-9–facilitated differentiation, when hemangioblasts/blast colony-forming cells (BL-CFCs) form,37,40 led to an increase in cell number, whereas the expression of WT SHP-1 reduced proliferation. Second, the expression of R459M SHP-1 during the first 3 days of EB development, when hemangioblasts develop,41 led to a significant acceleration in the appearance of hematopoietic colonies. When the potential of these cells was measured in a second round of embryoid body formation followed by the HCA, there was again a significant bias toward myeloid development. Third, the expression of WT SHP-1 during the first phase of OP-9 coculture reduced GM/M colony formation while leading to increased formation of secondary EBs. Taken together, these results suggest that SHP-1 can act during early mesodermal specification and that, even at this early stage, the ultimate hematopoietic differentiation capacity of the progenitors becomes biased. These effects may be accounted for by the altered proliferation of progenitors resulting from perturbation of SHP-1 activity, or SHP-1 may influence lineage determination. However, we cannot distinguish between these possibilities.
The ES model most closely reflects developmental hematopoiesis rather than adult hematopoiesis, and it has proven difficult to generate HSCs from ES cells that can reconstitute a lethally irradiated mouse. However, our results raise the possibility that HSC function may be affected in motheaten mice, in contrast to previous biologic analyses suggesting that me hematopoietic stem cell function was normal,16 although of course we cannot formally exclude the possibility that background strain differences—strain 129 for E14tg2a ES cells compared with the C57BL/6 background of motheaten mice—may underlie these effects. Earlier analyses were based on bone marrow reconstitution experiments before the ability to select HSCs for transplantation. The extensive proliferation of myeloid progenitors derived from me donor bone marrow could have masked underlying effects on the HSCs themselves. In light of our data suggesting a role for SHP-1 at earlier stages of hematopoiesis, it would be interesting to reevaluate the function of HSCs derived from me and mev mice.
Our data are also consistent with SHP-1 acting during the generation of hematopoietic progenitors. Manipulation of levels of active SHP-1 during phase 2 of OP-9 coculture, when hematopoietic progenitors arise,34,35,37 alters colony formation, consistent with SHP-1 affecting lineage balance in the progenitor cell population. In this case, based on GM/M colony formation, WT SHP-1 reduced and R459M SHP-1 increased myelopoiesis. These results reflect the data demonstrating hyperproliferation of Kit+ Linbone marrow progenitors derived from motheaten mice in response to stem cell factor45 and the proposal of Shultz et al16 that it is the hemopoietic progenitor population of me mice that is defective.
The function of many terminally differentiated cells derived from me mice is perturbed,4-9 as is cell function on ectopic expression of dominant-negative forms of SHP-1 in mature hematopoietic cells.10-13 Interestingly, we have found that the expression of R459M SHP-1 during the latter stages of hematopoietic development increases colony size, perhaps reflecting the hyperresponsiveness to cytokines reported previously.18,19 Interestingly, the expression of WT SHP-1 during only the HCA phase did not perturb the numbers of colonies formed, indicating that, by this stage, elevating SHP-1 activity further has little physiological effect.
Using 2 differentiation protocols, we failed to detect significant consistent effects on erythroid colony formation on expression of either WT or R459M SHP-1. These results contrast to the reported effects on erythropoiesis in mev mice, where older mice exhibit increases in CFU-E in the spleen and increased sensitivity to erythropoietin, although BFU-E frequency is reduced in bone marrow and spleen.17 It may be that the times we chose to assay for colony-forming cells was after the peak of BFU-E formation and before CFU-E formation or that the cytokine cocktail within the HCA, while including EPO, favored the development of myeloid colonies. Further analyses, closely tailored to addressing the role of SHP-1 in erythroid development, are required to address these apparent discrepancies.
In addition to demonstrating the effects of SHP-1 during early stages of hematopoiesis, our study has confirmed a previous report42 that SHP-1 is expressed in undifferentiated ES cells and is consistent with the recent report demonstrating SHP-1 expression as early as the 4-cell stage of the preimplantation mouse embryo and the blastocyst,46 from which ES cells are derived. We further show that SHP-1 is located in the cytosol and nucleus of ES cells. In mature hemopoietic cells, SHP-1 localization is mainly limited to the cytosol, though at later times after stimulation with cytokines, SHP-1 can be detected in the nucleus of hematopoietic cells by virtue of a bipartite nuclear localization signal in the carboxy-terminus.47 In nonhemopoietic cells, SHP-1 has been reported to locate within the nucleus.48 The significance of SHP-1 within the nucleus of undifferentiated ES cells is unclear, especially given that it appears that only SHP-1 present in the NP40-soluble fraction (cytosol/membrane) is associated with phosphotyrosine-containing proteins and so is likely to be active by virtue of its SH2-domains being engaged.49 The lack of phosphotyrosine-containing proteins coprecipitating with nuclear SHP-1 is surprising, but it suggests that perhaps this pool of SHP-1 is bound in an inactive complex.
Based on our study and on the fact that SHP-1 is expressed at the very earliest stages of development, we propose that the abnormalities in the motheaten mouse may arise far earlier during development than previously thought. The model system we have developed will allow detailed examination of the precise molecular mechanisms regulated by SHP-1 during early development to be conducted.
Prepublished online as Blood First Edition Paper, February 8, 2005; DOI 10.1182/blood-2004-08-3271.
Supported by a grant from the Biology and Biotechnology Research Council, United Kingdom.
N.R.D.P. and M.J.W. both contributed to the design and performance of the experiments, to the data analysis, and to the writing of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Owen Witte for the E14-tTA ES cells, Dr Lesley Forrester for advice on hematopoietic differentiation protocols, and Drs Helen Wheadon and Heather Bone for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal