Abstract
Thrombopoietin (TPO), the primary regulator of thrombopoiesis, is also an important, nonredundant mediator of hematopoietic stem cell (HSC) development. For example, following transplantation, HSC expansion is approximately 15-fold more robust in normal than in Tpo-/- mice. Vascular endothelial growth factor (VEGF) also plays an important role in HSC development, where it acts in an intracellular autocrine fashion to promote cell survival. Thus, we tested the hypothesis that TPO affects the autocrine production of VEGF to account for its favorable effects on HSCs. We found that VEGF transcripts are reduced in purified sca-1+/c-kit+/Gr-1- marrow cells derived from Tpo-/- mice and that TPO induces VEGF transcripts in these primitive hematopoietic cells. Additional studies determined that TPO induces VEGF expression by increasing the level of its primary transcription factor, hypoxia-inducible factor 1α (HIF-1α), by enhancing its protein stability. Moreover, VEGF expression is important for the TPO effect on primitive hematopoietic cells because blockade of the VEGF receptor with a specific inhibitor substantially blunts TPO-induced growth of single sca-1+/c-kit+/Gr-1- marrow cells in serum-free cultures. Along with previous findings that TPO affects Hox transcription factors that regulate HSC proliferation, these data contribute to our growing understanding of the mechanisms by which a hormone can influence stem cell development.
Introduction
A growing body of evidence has shown that vascular endothelial cell growth factor (VEGF), a principal regulator of angiogenesis, is also crucial for the survival and repopulation of hematopoietic stem cells (HSCs).1,2 For example, the VEGF receptors, VEGF-R1 and VEGF-R2, are expressed in immature hematopoietic cells that display repopulating activity, and inhibition of VEGF-R1 activation leads to reduced hematopoietic recovery following bone marrow suppression.
VEGF is produced by a variety of cells including cells of the bone marrow stroma. The secreted cytokine supports the survival of endothelial cells, increases vascular permeability, and induces chemotaxis in monocytes.1 Interestingly, in contrast to these paracrine effects, recent studies suggest that VEGF-A controls HSC survival and repopulation through an intracellular autocrine loop,3 because treatment of HSCs with a cell-permeable VEGF-R inhibitor, but not a function-blocking VEGF-R antibody, reduced the survival of cultured HSCs. Consistent with this mechanism, HSCs have been shown to express VEGF mRNA, and hypoxia, which induces VEGF-A expression in lin-CD34+ human progenitor cells, also enhances HSC numbers.4 However, whether VEGF-A expression in HSCs is regulated by other extracellular stimuli remains unknown.
Thrombopoietin (TPO), initially identified as the primary regulator of platelet production,5 plays an important and nonredundant role in the self-renewal and expansion of HSCs.6-8 Several studies using hematopoietic cell lines and primary megakaryocytes have revealed that TPO induces the production of VEGF-A.9,10 TPO also enhances the production of VEGF-A in human cord blood-derived CD34+ progenitor cells and augments their differentiation into megakaryocytes.11 These data led us to hypothesize that TPO might control VEGF-A expression in HSCs and thereby account, at least in part, for the capacity of the hormone to promote the survival and proliferation of HSCs. Using both a model cell line and primary, primitive murine sca-1+/c-kit+/Gr-1- marrow cells, we found that TPO increases VEGF mRNA and protein expression in vitro and that the absence of TPO reduces VEGF expression in these cells in vivo, and does so by reducing the ubiquitination and stabilizing the primary transcriptional regulator of the cytokine, hypoxia-inducible factor 1α (HIF-1α).12 To determine the physiologic significance of VEGF production in these cells, we tested the effects of the VEGF-R kinase inhibitor SU5416 on TPO-dependent growth and survival of sca-1+/c-kit+/Gr-1- hematopoietic cells; treatment with the inhibitor reduced TPO-induced cell division and survival of single-cell cultures, yet failed to directly inhibit TPO signaling. Taken together, our data indicate that TPO is an important and nonredundant regulator of VEGF-A production in HSCs and activates a VEGF-A internal autocrine loop, contributing to the favorable TPO effects on HSC self-renewal and expansion.
Materials and methods
Cell culture
UT-7/TPO cells were a gift of Dr Norio Komatsu (Jichi Medical School, Tochigi, Japan) and were maintained in liquid culture with Iscove modified Dulbecco medium containing 10% fetal calf serum (FCS) and 10 ng/mL human TPO (hTPO), a gift of Dr Don Foster (Zymogenetics, Seattle, WA). UT-7/TPO is an immature, factor-dependent, human hematopoietic cell line that responds to multiple cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and TPO.13 In selected experiments spent tissue culture medium was assessed for oxygen content; culture medium was collected in blood gas syringes and PO2 levels were immediately analyzed by a Chiron (Ratigen, Germany) model 840 blood gas analyzer.
Reagents
MG-132 and SU5416 were purchased from Calbiochem (La Jolla, CA). Cobalt chloride, cycloheximide (CHX), and actinomycin D were purchased from Sigma (St Louis, MO). An antibody to human HIF-1α was obtained from BD Biosciences/PharMingen (San Diego, CA). An antibody to murine HIF-1α was purchased from Novus Biologicals (Littleton, CO). Antibodies for phospho-p44/42 mitogen-activated protein kinase (MAPK), p44/p42 MAPK, phospho-AKT, and AKT were purchased from Cell Signaling Technology (Beverly, MA). Anti-VEGF and anti–transcription factor class II H (TFIIH) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for Jak2 and phospho-Jak2 were obtained from Upstate Biotechnology (Lake Placid, NY).
RNA preparation and real-time RT-PCR
Total cellular RNA was extracted from cells using Rneasy Midi Kit (Qiagen, Valencia, CA). To analyze the expression of splice variant forms of VEGF-A, primers spanning exon 3 and exon 8 of VEGF-A were used based on those previously published.14 Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) assays for human VEGF,15 murine VEGF,15 and human HIF-1α16 transcripts were developed using the iCycler (Bio-Rad Laboratories, Hercules, CA). As previously reported, the primer pairs for both the human and murine VEGF-A real-time PCR assays were designed to amplify conserved parts of splice variant forms of each species of VEGF-A transcripts (for primer sequences, see Table 1 in Gerber et al15 ).
All primers were synthesized by Invitrogen Life Technologies (Carlsbad, CA). For analysis of human and murine VEGF expression, 50 ng total RNA was amplified and quantified in a 40-μL reaction mixture containing 2.5 mM MgCl2, 300 μM dNTPs, 0.2 μL AmpliTaq Gold (Applied Biosystems, Foster City, CA), 0.05 μL M-MLV reverse transcriptase (Invitrogen Life Technologies), 0.4 μL Rnasin ribonuclease inhibitor (Promega, Madison, WI), 100 nM forward primer, and 100 nM reverse primer. For analysis of HIF-1α expression, forward and reverse primers were used at 300 nM. All reactions were performed in triplicate. For quantification of gene expression we used a relative standard curve method; as an internal control we used glyceraldehyde phosphate dehydrogenase (GAPDH) forward and reverse primers included in the GAPDH control reagents kit (Applied Biosystems) or a human 18s RNA kit (Applied Biosystems).
Preparation of cell lysates and Western blotting
Nuclear and cytoplasmic extracts were prepared from UT-7/TPO cells according to methods previously described.17 Lysate proteins were size-fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride (PVDF) membranes. The blots were incubated with primary antibodies and visualized using a chemiluminescence detection kit (LumiGLO, Cell Signaling Technology).
Isolation of immature hematopoietic cells
Sca-1+/c-kit+/Gr-1- fraction cells were prepared from C57BL/6, BDF-1, and Tpo-/- mice as previously described.18 The Tpo-/- mice have been back-crossed more than 10 times onto a C57BL/6 background.19 Mice were housed in a specific pathogen-free environment, and the Animal Care Program of the University of California–San Diego approved all protocols involving mice.
Single-cell cultures
Round-bottom 96-well tissue culture plates were used for cell cultures. Each well contained 200 μL STEMPRO-34 serum-free complete medium (Invitrogen Life Technologies) supplemented with 100 ng/mL hTPO. In some experiments SU5416 was added at the concentrations indicated. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. The number of cells per well was monitored by inverted microscopy daily for 7 days.
Luciferase assay
A luciferase reporter plasmid pHRE, containing the HIF-1–binding site of the human VEGF promoter region, was a kind gift of Dr Hiroyasu Esumi (National Cancer Center Research Institute East, Chiba, Japan).20 The reporter plasmid was introduced into UT-7/TPO cells by lipofection with the internal control plasmid pRL-TKLuc (Promega). Twenty-four hours after transfection, the cells were starved for TPO and then stimulated with 100 ng/mL TPO. After 24 hours of stimulation, the cells were harvested for dual luciferase assay according to the manufacturer's instructions (Promega).
Statistical analysis
Statistical analysis was performed by using the Student t test; P < .05 was considered statistically significant.
Results
TPO induces VEGF gene transcription in UT-7/TPO cells
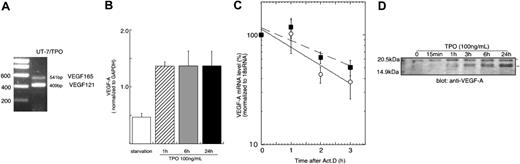
Previous studies have shown that TPO can induce VEGF production in mature hematopoietic cells.9,10 We sought to extend these findings to immature hematopoietic cells, and if present, determine the mechanism of the effect. As shown in Figure 1A, the immature hematopoietic cell line UT-7/TPO expresses a 2-splice form of VEGF-A, VEGF121 and VEGF165. Treatment with TPO induced a 3-fold increase in VEGF mRNA within 1 hour of exposure, which remained elevated for 24 hours (Figure 1B). As determined by Western blotting, both forms of VEGF-A protein levels were also increased after exposure of UT-7/TPO cells to TPO (Figure 1D). To investigate whether the elevation of VEGF-A mRNA was due to an increase in gene transcription or to a prolonged mRNA half-life, we used actinomycin D to study mRNA decay rates; the presence of TPO did not increase the half-life of VEGF-A mRNA (Figure 1C).
Immature sca-1+/c-kit+/Gr-1- hematopoietic cells from Tpo-/- mice display reduced VEGF-A mRNA levels
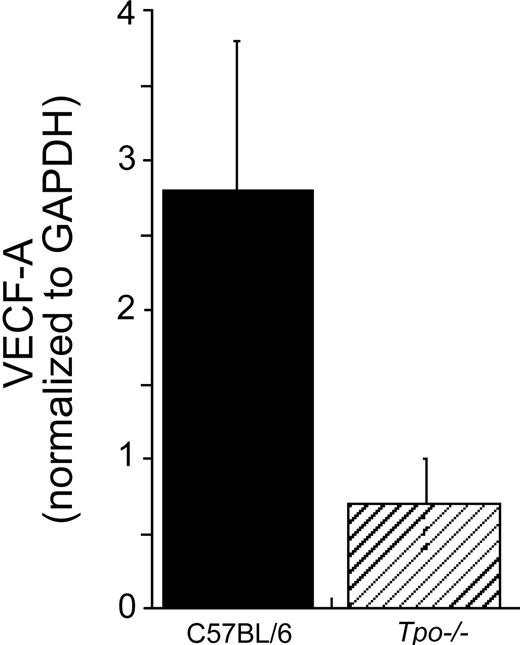
To confirm whether VEGF levels in primary marrow hematopoietic progenitor and stem cells are also affected by TPO, we quantitated VEGF-A–specific mRNA levels in immature hematopoietic cell fractions from Tpo-/- and control mice by real-time RT-PCR. As shown in Figure 2, VEGF-A expression levels were 4 to 5 times lower in the Tpo-/- mice.
HIF-1α expression is increased by TPO
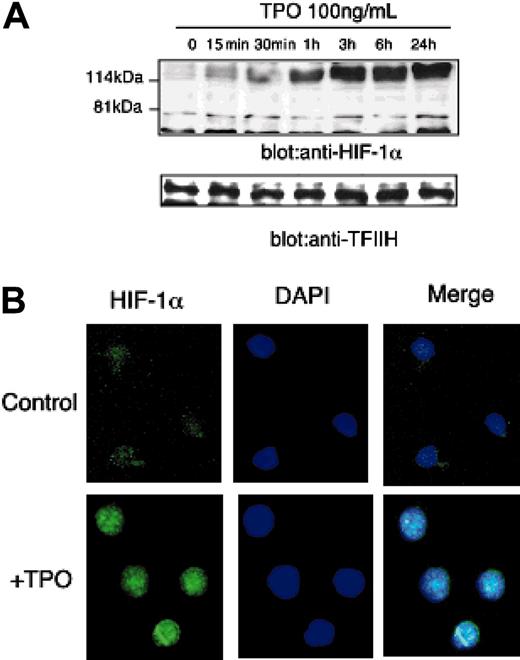
Because VEGF-A mRNA levels are controlled primarily at the level of transcriptional enhancement, we studied whether TPO affects the expression of HIF-1, the primary transcriptional regulator of VEGF-A expression. HIF-1 is composed of 2 subunits, HIF-1α and HIF-1β. Of the 2 subunits, HIF-1α levels are responsive to external stimuli such as hypoxia or cytokines; in contrast, HIF-1β expression is stable.12 As shown in Figure 3A, the addition of TPO dramatically induced HIF-1α protein levels in UT-7/TPO cells. Importantly, because TPO could have affected the metabolism of the culture during the time that we monitored HIF-1α levels, we assessed pO2 levels in the culture medium; the addition of TPO did not affect pO2 levels in the first 60 minutes (starvation: pO2 = 115.7 ± 3.3 mm Hg; TPO 15 minutes: pO2 = 116 ± 1.5 mm Hg; and TPO 60 minutes: pO2 = 118 ± 0.9 mm Hg).
TPO controls VEGF-A at a transcriptional level. (A) To analyze the expression of VEGF-A variant forms, RNA was prepared from UT-7/TPO cells cultured with 10 ng/mL hTPO and RT-PCR was performed with primers spanning exon 3 and exon 8. (B) UT-7/TPO cells were deprived of growth factors for 24 hours (□) and then stimulated with 100 ng/mL hTPO for various time periods (▨, 1 hour; ▦, 6 hours; ▪, 24 hours). RNA was prepared and VEGF-A mRNA levels were analyzed by real-time RT-PCR with primers that recognize all variants of VEGF-A transcripts. Each column represents the average VEGF-A level normalized to a GAPDH internal control ± SD of 3 independent experiments. (C) After 24 hours growth factor deprivation, UT-7/TPO cells were stimulated with 100 ng/mL hTPO for 3 hours, after which 10 μg/mL actinomycin D was added and the cells cultured for the additional indicated time periods with (○ and solid line) or without (▪ and broken line) hTPO. RNA was prepared and VEGF-A levels were monitored by the real-time RT-PCR assay. Levels of 18s RNA were used as an internal control. The graph represents the result of 3 independent experiments ± SD. The difference between the 2 curves does not reach statistical significance. (D) UT-7/TPO cells were deprived of growth factors for 24 hours and then treated with 100 ng/mL hTPO. The cytoplasmic protein fraction was prepared and VEGF-A expression levels were analyzed by Western blotting. *VEGF165; **VEGF121.
TPO controls VEGF-A at a transcriptional level. (A) To analyze the expression of VEGF-A variant forms, RNA was prepared from UT-7/TPO cells cultured with 10 ng/mL hTPO and RT-PCR was performed with primers spanning exon 3 and exon 8. (B) UT-7/TPO cells were deprived of growth factors for 24 hours (□) and then stimulated with 100 ng/mL hTPO for various time periods (▨, 1 hour; ▦, 6 hours; ▪, 24 hours). RNA was prepared and VEGF-A mRNA levels were analyzed by real-time RT-PCR with primers that recognize all variants of VEGF-A transcripts. Each column represents the average VEGF-A level normalized to a GAPDH internal control ± SD of 3 independent experiments. (C) After 24 hours growth factor deprivation, UT-7/TPO cells were stimulated with 100 ng/mL hTPO for 3 hours, after which 10 μg/mL actinomycin D was added and the cells cultured for the additional indicated time periods with (○ and solid line) or without (▪ and broken line) hTPO. RNA was prepared and VEGF-A levels were monitored by the real-time RT-PCR assay. Levels of 18s RNA were used as an internal control. The graph represents the result of 3 independent experiments ± SD. The difference between the 2 curves does not reach statistical significance. (D) UT-7/TPO cells were deprived of growth factors for 24 hours and then treated with 100 ng/mL hTPO. The cytoplasmic protein fraction was prepared and VEGF-A expression levels were analyzed by Western blotting. *VEGF165; **VEGF121.
Immature primary hematopoietic cells cultured with TPO express HIF-1α
To analyze the effects of TPO on HIF-1α expression in a more physiologic setting, we sorted and cultured sca-1+/c-kit+/Gr-1- cells with TPO for 24 hours and stained the cells with an anti-HIF-1α antibody to evaluate the expression and subcellular distribution of the transcription factor by immunofluorescence microscopy. After 24 hours of culture with TPO, almost all cells expressed HIF-1α and the protein was distributed primarily in the nucleus (Figure 3B).
TPO activates the human VEGF promoter containing a HIF-1α–binding site
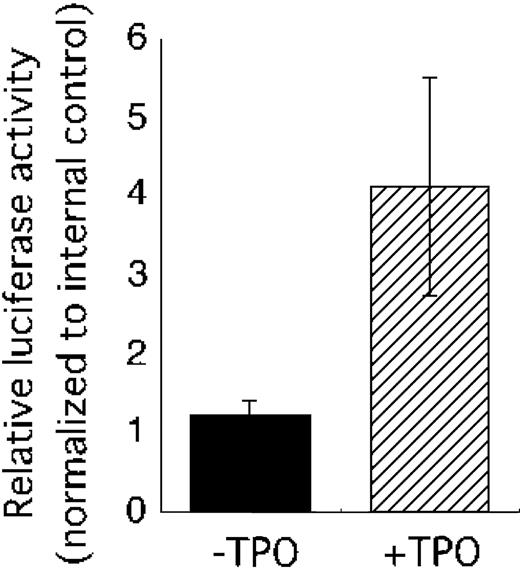
Our observations suggested that TPO might control VEGF-A mRNA expression through induction of HIF-1α. To confirm this notion, we performed a reporter analysis using a plasmid construct containing the HIF-1–binding region of the human VEGF gene. This reporter has been shown to be activated in response to hypoxia.20 As shown in Figure 4, TPO clearly enhanced reporter activity.
TPO controls the stability of HIF-1α protein
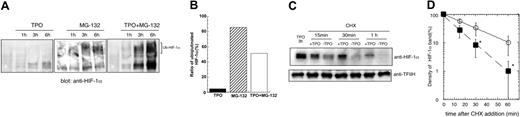
We next studied the mechanism by which TPO regulates the level of HIF-1α expression in hematopoietic cells. Under normoxic conditions HIF-1α is hydroxylated and undergoes degradation by the ubiquitin-proteasome pathway. However, different cytokines and growth factors can induce HIF-1α elevation under normoxic conditions, albeit by several distinct mechanisms. For example, interleukin 3 (IL-3) increases HIF-1α mRNA,21 insulin or insulin-like growth factor augments the translation of HIF-1α mRNA22 and IL-1 enhances the stability of HIF-1α protein.23 To investigate whether any of these mechanisms are responsible for the TPO effect on HIF-1α expression we first conducted real-time RT-PCR experiments. TPO only very minimally affected HIF-1 mRNA expression, increasing it to levels that did not reach a statistically significant difference from nonstimulated cells (data not shown). Next, we analyzed whether TPO up-regulates HIF-1α protein stability. Toward this end we treated UT-7/TPO cells with the proteasome inhibitor MG132 to block degradation of HIF-1α. In the absence of TPO, MG132 induced only a modest increase in the level of HIF-1α, and more than 80% of the protein was ubiquitinated (Figure 5A middle panel and Figure 5B middle column). Because blockade of HIF-1α degradation only modestly increased the level of HIF-1α, this result suggests that increased protein synthesis was required for the TPO-induced HIF-1α elevation. When the MG-132–treated cells were also treated with TPO, substantially greater levels of HIF-1α were present (Figure 5A right panel). However, compared with cells treated with MG132 without TPO, proteasome-blocked cells also displayed higher levels of HIF-1α and only 50% of the protein was ubiquitinated (Figure 5B right column). These results indicate that TPO likely stabilizes HIF-1α protein because ubiquitinated forms are rapidly degraded in the presence of an active proteasome. Consistent with these conclusions, using the protein synthesis inhibitor CHX we found that HIF-1α protein displayed a half-life of 7 minutes in the absence of TPO and 14 minutes when the hormone was added to the cultures (Figure 5C-D).
Immature sca-1+/c-kit+/Gr-1- hematopoietic cells from Tpo-/- mice display reduced VEGF-A–specific mRNA levels. Whole bone marrow cells were prepared from control C57BL/6 and Tpo-/-mice. The cells were pooled (experiment 1: n = 4 C57BL/6, n = 4 Tpo-/- mice; experiment 2: n = 4 C57BL/6, n = 5 Tpo-/-mice) and Sca-1+/c-kit+/Gr-1- cells were collected. Immediately after the collection, mRNA was prepared for real-time PCR analysis. Each column represents an average of VEGF-A mRNA levels normalized to a GAPDH internal control ± SD of 2 experiments (▪, C57BL/6; ▨, Tpo-/-).
Immature sca-1+/c-kit+/Gr-1- hematopoietic cells from Tpo-/- mice display reduced VEGF-A–specific mRNA levels. Whole bone marrow cells were prepared from control C57BL/6 and Tpo-/-mice. The cells were pooled (experiment 1: n = 4 C57BL/6, n = 4 Tpo-/- mice; experiment 2: n = 4 C57BL/6, n = 5 Tpo-/-mice) and Sca-1+/c-kit+/Gr-1- cells were collected. Immediately after the collection, mRNA was prepared for real-time PCR analysis. Each column represents an average of VEGF-A mRNA levels normalized to a GAPDH internal control ± SD of 2 experiments (▪, C57BL/6; ▨, Tpo-/-).
TPO increases HIF-1α expression. (A) After 24 hours of growth factor deprivation, UT-7/TPO cells were stimulated with 100 ng/mL hTPO for various time periods, nuclear extracts were prepared, and HIF-1α levels were analyzed by Western blotting. For an internal control, the membranes were reprobed with an anti-TFIIH antibody. (B) Bone marrow cells were prepared from BDF-1 mice and sca-1+/c-kit+/Gr-1- cells were selected by cell sorting. The cells were cultured with or without 100 ng/mL hTPO for 24 hours; the cells were then fixed and stained with an anti-HIF-1α primary antibody and an Alexa488-conjugated secondary antibody (green). Cells were counterstained with DAPI (4,6 diamidino-2-phenylindole) to visualize nuclear DNA (blue). An example of the predominant staining pattern is shown. Images were obtained with a Leica DMLS microscope system (Wetzlar, Germany). Immunofluorescent images were obtained using SPOT RT software (Diagnostic Instruments, Sterling Heights, MI). The objective lens used was a Leica N plan, 40×/0.65; the camera used was Diagnostic Instruments, model 2.2.1 (Diagnostic Instruments).
TPO increases HIF-1α expression. (A) After 24 hours of growth factor deprivation, UT-7/TPO cells were stimulated with 100 ng/mL hTPO for various time periods, nuclear extracts were prepared, and HIF-1α levels were analyzed by Western blotting. For an internal control, the membranes were reprobed with an anti-TFIIH antibody. (B) Bone marrow cells were prepared from BDF-1 mice and sca-1+/c-kit+/Gr-1- cells were selected by cell sorting. The cells were cultured with or without 100 ng/mL hTPO for 24 hours; the cells were then fixed and stained with an anti-HIF-1α primary antibody and an Alexa488-conjugated secondary antibody (green). Cells were counterstained with DAPI (4,6 diamidino-2-phenylindole) to visualize nuclear DNA (blue). An example of the predominant staining pattern is shown. Images were obtained with a Leica DMLS microscope system (Wetzlar, Germany). Immunofluorescent images were obtained using SPOT RT software (Diagnostic Instruments, Sterling Heights, MI). The objective lens used was a Leica N plan, 40×/0.65; the camera used was Diagnostic Instruments, model 2.2.1 (Diagnostic Instruments).
TPO activates a human VEGF promoter fragment containing a HIF-1α–binding site. The pHRE luciferase plasmid was introduced into UT-7/TPO cells with internal control vector pRL-TKLuc. Twenty-four hours after transfection, the cells were starved for 24 hours and then stimulated with 100 ng/mL TPO (▨) or serum (▪). The cells were then harvested for luciferase assay. Luciferase activity was normalized to internal control and an average ± SD of 3 experiments is shown.
TPO activates a human VEGF promoter fragment containing a HIF-1α–binding site. The pHRE luciferase plasmid was introduced into UT-7/TPO cells with internal control vector pRL-TKLuc. Twenty-four hours after transfection, the cells were starved for 24 hours and then stimulated with 100 ng/mL TPO (▨) or serum (▪). The cells were then harvested for luciferase assay. Luciferase activity was normalized to internal control and an average ± SD of 3 experiments is shown.
Inhibition of VEGF signaling by SU5416 reduces TPO-dependent survival and proliferation of UT-7/TPO and sca-1+/c-kit+/Gr-1- hematopoietic cells
Next, we investigated whether TPO-induced induction of VEGF was of physiologic importance, specifically, whether TPO-dependent support of immature hematopoietic cell survival and proliferation is dependent on VEGF-R signaling. SU5416 is a small molecular inhibitor of the kinase activity of both isoforms of the VEGF receptor, VEGF-R1 and VEGF-R2.3 As shown in Figure 6, SU5416 blocked the growth of UT-7/TPO cells at all concentrations above 0.1 μM. To ensure that the inhibitory effect on TPO-induced hematopoietic cell growth was acting through VEGF-R and not the kinases that mediate TPO signaling, we next analyzed whether the inhibitor affects TPO signaling. UT-7/TPO cells were deprived of TPO and then stimulated with the hormone in the presence or absence of SU5416 and the effects on activation of downstream signaling molecules analyzed. Even at concentrations (10 μM) 100-fold higher than that necessary to exert a statistically significant and biologically important effect on TPO-induced growth of UT-7/TPO cells (0.1 μM), SU5416 did not inhibit TPO-induced activation of Jak2, ERK1/2 (extracellular signal-related kinase), or Akt (Figure 6A). In contrast, the VEGF-R inhibitor significantly inhibited TPO-induced cell proliferation (Figure 6B).
TPO controls HIF-1α protein stability. (A) UT-7/TPO cells were starved for 24 hours and then treated with 100 ng/mL hTPO, 5 μM MG-132, or TPO plus MG-132 for the indicated time periods. Nuclear extracts were prepared and HIF-1α expression was analyzed by Western blotting. The position of ubiquitinated forms of HIF-1α is indicated. (B) The film was subjected to densitometric analysis and the proportion of ubiquitinated to total of HIF-1α at 6 hours was calculated. ▪ indicates TPO; ▨, MG-132; and □, both. (C) UT-7/TPO cells were starved for 24 hours and stimulated with 100 ng/mL hTPO for 3 hours. CHX (500 μM) was then added and the cells cultured with or without hTPO for the additional time periods indicated. Nuclear extracts were then prepared and HIF-1α levels were analyzed by Western blotting. (D) The film was subjected to densitometric analysis and HIF-1α levels were plotted. Each point shows an average ± SD of 3 experiments. The half-life of HIF-1α was calculated by Kaleida Graph software (Synergy Software, Reading, PA). *P < .05.
TPO controls HIF-1α protein stability. (A) UT-7/TPO cells were starved for 24 hours and then treated with 100 ng/mL hTPO, 5 μM MG-132, or TPO plus MG-132 for the indicated time periods. Nuclear extracts were prepared and HIF-1α expression was analyzed by Western blotting. The position of ubiquitinated forms of HIF-1α is indicated. (B) The film was subjected to densitometric analysis and the proportion of ubiquitinated to total of HIF-1α at 6 hours was calculated. ▪ indicates TPO; ▨, MG-132; and □, both. (C) UT-7/TPO cells were starved for 24 hours and stimulated with 100 ng/mL hTPO for 3 hours. CHX (500 μM) was then added and the cells cultured with or without hTPO for the additional time periods indicated. Nuclear extracts were then prepared and HIF-1α levels were analyzed by Western blotting. (D) The film was subjected to densitometric analysis and HIF-1α levels were plotted. Each point shows an average ± SD of 3 experiments. The half-life of HIF-1α was calculated by Kaleida Graph software (Synergy Software, Reading, PA). *P < .05.
SU5416 blocks the TPO-dependent growth of UT-7/TPO cells but not TPO signaling. (A) After 24 hours of growth factor deprivation, UT-7/TPO cells were pretreated with the indicated concentration of SU5416 for 1 hour and then stimulated with 100 ng/mL hTPO for 10 minutes. Whole-cell lysates were prepared and activation of ERK, Akt, and Jak2 was analyzed by Western blotting with antibodies specific for the phosphorylated (activated) form of each protein. As controls, the membranes were reprobed with antibodies for the total level of each protein. (B). UT-7/TPO cells were placed in culture with 10 ng/mL hTPO and the indicated concentrations of SU5416. After 72 hours in culture, cell proliferation was assessed by a methylthiotetrazole (MTT) assay; *P < .05, **P < .01 versus no SU5416.
SU5416 blocks the TPO-dependent growth of UT-7/TPO cells but not TPO signaling. (A) After 24 hours of growth factor deprivation, UT-7/TPO cells were pretreated with the indicated concentration of SU5416 for 1 hour and then stimulated with 100 ng/mL hTPO for 10 minutes. Whole-cell lysates were prepared and activation of ERK, Akt, and Jak2 was analyzed by Western blotting with antibodies specific for the phosphorylated (activated) form of each protein. As controls, the membranes were reprobed with antibodies for the total level of each protein. (B). UT-7/TPO cells were placed in culture with 10 ng/mL hTPO and the indicated concentrations of SU5416. After 72 hours in culture, cell proliferation was assessed by a methylthiotetrazole (MTT) assay; *P < .05, **P < .01 versus no SU5416.
We next investigated the effects of the VEGF-R inhibitor on the TPO-dependent proliferation and survival of single, immature sca-1+/c-kit+/Gr-1- marrow cells grown in vitro under serum-free conditions. We found that 10% to 20% of such cells divide in the presence of TPO, as was previously reported by others.24 The addition of 1 μM SU5416 significantly diminished the effects of TPO on cell division (Figure 7 left panel) and reduced the survival of cells at day 7 of culture (Figure 7 right panel), each by about 50%. These results indicate that VEGF signaling accounts for at least a proportion of the TPO-dependent growth and survival of immature HSCs.
SU5416 inhibits TPO-dependent growth and survival of immature HSCs. Sca-1+/c-kit+/Gr-1- cells were purified from murine bone marrow and single-cell cultures were performed in the presence of 100 ng/mL hTPO with (▨) or without (▪)1 μM SU5416. The number of cells in each well was monitored daily. The columns show the percentage of single cells that divided at least once within the 7-day culture period (left panel) and the percentage of wells that contain at least one cell on day 7 (right panel). Each column represents the average ± SD of 3 independent experiments.
SU5416 inhibits TPO-dependent growth and survival of immature HSCs. Sca-1+/c-kit+/Gr-1- cells were purified from murine bone marrow and single-cell cultures were performed in the presence of 100 ng/mL hTPO with (▨) or without (▪)1 μM SU5416. The number of cells in each well was monitored daily. The columns show the percentage of single cells that divided at least once within the 7-day culture period (left panel) and the percentage of wells that contain at least one cell on day 7 (right panel). Each column represents the average ± SD of 3 independent experiments.
Discussion
The development of hematopoietic cells is orchestrated by numerous hematopoietic cytokines, growth factors, and chemokines. For the most part these proteins are secreted from bone marrow microenviromental cells, including fibroblasts, endothelial cells, and stromal cells, or are displayed on their cell surface. Hematopoietic cells have also been shown to produce numerous cytokines and growth factors. For example, human CD34+ cells express mRNA for SCF, IL-1, and VEGF.25 Furthermore, numerous cytokines and growth factors are actually secreted from these cells.26 Differentiated hematopoietic cells also produce some of these and other cytokines.25 Interestingly, secreted cytokines have also been reported to induce the production of additional cytokines from neighboring cells. For example, VEGF-A increases the expression of TPO and FLT-3 ligand in the bone marrow stromal cell line MS-5.27 In addition, SCF, GM-CSF, and IL-3 stimulate the production of VEGF-A from CD34+ progenitor cells.28 These results suggest that cytokines drive networks of other cytokines to support hematopoietic cell development.
VEGF is the term used to denote a family of cytokines that regulate angiogenesis and vasculogenesis.29 Recent studies identified VEGF-A, and its receptor VEGF-R1, as indispensable for normal HSC homeostasis, because the genetic elimination of VEGF genes in HSCs was associated with decreased cell survival, colony formation, and hematopoietic repopulation following transplantation.3 Inhibition of VEGF-R1 by specific antibodies also delayed reconstitution of the marrow in other transplantation studies, by inhibiting the recruitment and mobilization of HSCs,30 suggesting that VEGF regulates HSC development through several different mechanisms. One particularly intriguing mechanism involves an internal autocrine loop, reported by Gerber and colleagues.3 These investigators reported that inhibition of VEGF-R function by cell-permeable, small chemical inhibitors blocked the colony-forming capacity of HSCs in response to several hematopoietic cytokines. In contrast, a neutralizing antibody for VEGF-A did not display these inhibitory effects.
The secretion of VEGF from endothelial or smooth muscle cells is regulated by extracellular stimuli, inflammatory cytokines, and hypoxia.29 In contrast, it is not clear whether synthesis of VEGF in HSCs is regulated by extracellular signals, and thus, whether the activity of this VEGF-A intracellular autocrine loop is controlled by other cytokines. Because several early acting cytokines, including TPO, affect VEGF-A expression in hematopoietic cells,9,28 we hypothesized that TPO could be a regulator of an HSC VEGF autocrine loop. In support of this hypothesis we found that TPO induced VEGF expression in vitro and in an immature hematopoietic cell line and that the absence of TPO in vivo results in reduced levels of VEGF in immature sca-1+/c-kit+/GR-1- marrow hematopoietic cells. We next examined the mechanism by which TPO controls VEGF-A production, using a model immature hematopoietic cell line, UT-7/TPO. We found that TPO rapidly induces the production of 2 isoforms of VEGF-A mRNA. VEGF-A mRNA levels have been reported to be regulated by both transcriptional control and mRNA stabilization.31,32 We found that the mRNA stability of VEGF-A was unaffected by TPO, making it likely that transcriptional mechanisms are operative in our cell systems. However, this conclusion would require nuclear run-on assays, a study not feasible in the limiting number of primitive hematopoietic cells obtainable for study.
HIF-1 is the primary regulator of most hypoxia-inducible genes, including VEGF-A.12 We found that TPO dramatically induced expression of HIF-1α, the subunit that controls activity of the transcription factor. Furthermore, TPO induced activation of a VEGF-A promoter fragment containing its HIF-1 region in UT-7/PO cells. Although we did not experimentally establish a direct link between expression of HIF-1α and up-regulation of VEGF-A mRNA in pure HSCs, because such experiments are not presently feasible, others have shown that hypoxia expands the number of severe combined immune deficiency (SCID)–repopulating cells in human lin-CD34+ cells,4 indirectly implicating an important expansion function for HIF-1 in HSCs.
Cellular levels of HIF-1α are regulated primarily through degradation of the protein. Under normoxic conditions, HIF-1α is hydroxylated on proline residues by prolyl-hydroxylases (PHDs), leading to its association with the von Hippel-Lindau (VHL) protein, which induces ubiquitination of the transcription factor12 and its subsequent proteasomal degradation. Hypoxia inhibits the activity of the PHDs, and thereby stabilizes HIF-1α. In UT-7/TPO cells we found that TPO doubled the stability of HIF-1α under normoxic conditions. In previous studies, several cytokines have been shown to stabilize HIF-1α under normoxic conditions; tumor necrosis factor α (TNF-α) and IL-1 stabilize HIF-1α through activation of nuclear factor κB (NF-κB)33 Interestingly, TPO also activates NF-κB in UT-7/TPO cells.34 Like TPO, IL-1 and TNF-α are expressed in immature hematopoietic cells.26 Thus, it would be interesting to hypothesize that these cytokines might also contribute VEGF-A production in HSCs through up-regulation of HIF-1α. Another possible explanation for TPO-induced HIF-1α stabilization is that TPO might affect PHD function. Several studies support this possibility. Expression of PHD2 is controlled by extracellular stimuli including hypoxia.35 Angiotensin II stabilizes HIF-1α through reduction of SM-20/PHD3.36 To confirm this hypothesis, further studies are needed.
Although the foregoing data establish that TPO enhances VEGF expression in primitive hematopoietic cells by increasing cellular levels of HIF-1α and identifies at least one mechanism through which it acts, they do not speak to the physiologic relevance of the finding. We thus used an intracellular inhibitor of VEGF-R signaling, SU5416, and studied its effect on TPO-induced survival and proliferation of single, primitive primary hematopoietic cells cultured under serum-free conditions. Gerber and colleagues reported that this inhibitor blocked VEGF function in HSCs at 3 μM.3 We found that 1 μM SU5416 reduced both sca-1+/c-kit+/Gr-1- cell survival and proliferation in response to TPO by 50%. Like all studies using chemical inhibitors, specificity is a critical issue, especially because this compound has been reported to also inhibit fms-like tyrosine (FLT3) kinase.37 Because we used serum-free, defined culture medium adding only TPO, blockade of alternate cytokines is an unlikely explanation for the inhibitory effect of SU5416 in our studies. However, it remained possible that SU5416 directly inhibited TPO-induced VEGF expression by affecting the downstream signaling kinases used by the TPO receptor. To eliminate this possibility, we treated UT-7/TPO cells with TPO and SU5416 and directly assessed TPO signaling, by Western blotting for activated signaling mediators known to be stimulated by TPO. We found that TPO activated all of the major signaling pathways previously found to be activated by TPO, including ERK,Akt, and Jak2, even at very high concentrations (10 μM) of the inhibitor. Thus the effects of SU5416 on HSCs are not likely mediated by cytokines other than VEGF.
In summary, our results indicate that TPO enhances the expression of VEGF through an HIF-1–dependent induction of VEGF-A expression. Activation of this cascade is important for TPO-dependent growth and survival of HSCs, although blockade does not completely eliminate the favorable effects of TPO on primitive hematopoietic cells. Several questions remain unanswered, however: What are the immediate signaling events downstream of the TPO receptor that mediate HIF-1α stabilization; do other cytokines function in a similar pathway downstream of TPO and its receptor; and do the direct effects of TPO act in synergy with those derived from VEGF-R? Previously, we reported that the homeodomain proteins HOXB418 and HOXA938 contribute to the favorable effects of TPO on HSC development. Taken together, our current results contribute to our understanding of how TPO orchestrates intracellular mechanisms that enhance the survival, self-renewal, and growth of HSCs.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-07-2712.
Supported by National Institutes of Health grants R01 49855 and R01 31615.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal