Abstract
In this report, we have defined the stage at which Scl functions in the establishment of the hematopoietic system and provide evidence that its primary role is in the generation of the hematopoietic lineages from a progenitor called the blast colony-forming cell (BL-CFC), a cell considered to be the in vitro equivalent of the hemangioblast. Using an embryonic stem (ES) cell line in which lacZ cDNA has been targeted to the Scl locus, we show that most of the BL-CFCs are detected in the SCL/lacZ- population, indicating that this progenitor does not express Scl. In the blast colony assay, Scl-/- cells initiate colony growth but are unable to generate endothelial and hematopoietic progeny and thus form colonies consisting of vascular smooth muscle cells only. The capacity to give rise to blast colonies can be rescued by retroviral transduction of a wild-type Scl gene into Scl-/- FLK-1+ cells, suggesting that the BL-CFC is generated in this population. Finally, we show that Scl-/- endothelial cells display a growth deficiency in monolayer cultures that can be partially overcome by maintaining this population as 3-dimensional aggregates indicating that specific cellular interactions are required for maintenance of the Scl-/- endothelial lineage in vitro.
Introduction
The hematopoietic system has been hypothesized to originate from a bipotential progenitor known as the hemangioblast, a cell with both hematopoietic and endothelial potential.1 Support for the existence of the hemangioblast in the early embryo has been provided by studies demonstrating that the hematopoietic and endothelial lineages share the expression of a large number of genes2-9 and that some of these genes are essential for the proper development of both lineages.10-14 Although these findings are consistent with the notion of a hemangioblast, direct proof that such a progenitor exists was first provided by studies using the developmental model based on the in vitro differentiation potential of embryonic stem (ES) cells. These studies demonstrated that early-stage embryoid bodies (EBs) contain progenitors, known as blast colony-forming cells (BL-CFCs), that are able to generate blast cell colonies with hematopoietic and endothelial potential.15,16 Further analysis revealed that the BL-CFC expresses the vascular endothelial growth factor (VEGF) receptor tyrosine kinase, Flk-117 and the mesodermal gene Brachyury18 and is able to generate vascular smooth muscle (VSM) cells19 in addition to hematopoietic and endothelial progeny. Together, these findings strongly suggest that the BL-CFC is the equivalent of the yolk sac hemangioblast and indicate that it represents a subset of mesoderm, undergoing commitment to the hematopoietic and vascular (endothelial and VSM) lineages.
Molecular studies in the early embryo and developing EBs have provided further insights into the establishment of the hematopoietic system. Expression patterns of Brachyury,20,21 Flk-1,3,22 Scl,23-25 and Gata-12,26,27 have provided the outline of a molecular pathway from mesoderm, to the hemangioblast, to committed hematopoietic progenitors, both in vivo and in vitro.18,28-31 By targeting selectable markers to each of these transcription factors, it has been possible to validate this developmental progression. For instance Brachyury-expressing cells isolated from pre-BL-CFC stage EBs will generate a population that coexpresses Flk-1 and Brachyury and displays BL-CFC potential following a short period in culture.18 Populations that express Flk-1 and Scl contain both BL-CFCs and committed hematopoietic progenitors,31 suggesting that they represent a transition from the hemangioblast to the hematopoietic stage of EB development. Finally the expression of Gata-1 appears to define the hematopoietic commitment stage because Gata-1+ cells display hematopoietic but no endothelial potential.32
The developmental sequence established by the described expression and cell fractionation experiments are supported by gene-targeting studies in vivo. Flk-1-/-embryos do not develop yolk sac blood islands and die around day 9.5.13 The primary defect appears to be one of migration because Flk-1-/- mesodermal cells fail to move to the yolk sac and instead accumulate in the amniotic region of the embryo.14 Scl-/-embryos show a complete absence of blood formation, indicating a pivotal role for this transcription factor in the differentiation of the hematopoietic system.11,12,33,34 In addition to hematopoietic deficiencies, these embryos also display defects in yolk sac angiogenesis, indicating that Scl also functions in the establishment of the vascular system.11,12,35 Findings from in vitro studies are consistent with those on the embryo and demonstrate that the hematopoietic defect is early, at the level of the BL-CFC, because Scl-/- EB-derived cells do not generate typical blast colonies.17,30 The primary defect in GATA-1–deficient embryos and EBs is at the late stages of primitive and definitive erythropoiesis.36,37 Endothelial development, however, appears to be normal,36 indicating that GATA-1 functions at a stage beyond the hemangioblast.
Although the defects observed in Scl-/-embryos and EBs clearly indicated that this transcription factor is essential for hematopoietic differentiation and vascular remodeling, the precise stage at which it functions remains unclear. Several lines of evidence from gain-of-function studies suggest that SCL plays a role early, possibly in the development of the putative hemangioblast.19,38,39 In the zebrafish embryo, forced Scl expression resulted in the expansion of the hematopoietic and endothelial lineages at the expense of somitic and pronephric duct tissue, indicating that this transcription factor can respecify mesoderm to a hemangioblast fate.38 Studies using the ES cell model have shown that expression of Scl from the Flk-1 locus leads to an increase in the number of BL-CFCs in EBs, suggesting that it can function at the level of induction of these progenitors.19 Although these findings indicate that SCL can function early, they do not necessarily demonstrate that it is required at these stages. The observation that hematopoiesis can be rescued in Scl-/-embryos by expression of Scl from the Gata-1 promoter35 is inconsistent with the notion that SCL functions to specify the hemangioblast. Rather, these findings support the interpretation that the primary function of SCL is in the differentiation of this progenitor to a hematopoietic fate.
To further define the role of SCL in early hematopoietic and vascular commitment, we have investigated its expression patterns during BL-CFC development and analyzed the potential of Scl-/- ES cells in vitro. In this report, we provide evidence that Scl is not expressed in BL-CFCs and demonstrate that blast colonies can be rescued from Scl-/- EB-derived cells by retroviral transduction of a wild-type Scl gene. We also show that Scl-/-endothelial cells display a growth deficiency in monolayer cultures that can be partially overcome by maintaining this population as 3-dimensional aggregates. Together, these findings indicate that SCL is not required for BL-CFC development but is essential for the hematopoietic differentiation of this progenitor as well as for the proliferation and maturation of cells of the endothelial lineage in vitro.
Materials and methods
ES cell maintenance and differentiation
Wild-type J+/+ (Scl+/+), Scl-/- (a gift from Dr Stuart Orkin, Harvard Medical School, Cambridge, MA), and Scl+/lacZ40 ES cells were maintained and differentiated as reported.18,41 For reaggregation cultures, 3 to 4 × 105 FLK-1+ cells/mL were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 2 mM l-glutamine (Gibco/BRL, Grand Island, NY), 200 μg/mL transferrin (Boehringer Mannheim, Indianapolis, IN), 4 × 10-4 M MTG (hereafter referred to as supplemented IMDM) plus 10% fetal calf serum (FCS), 20% D4T conditioned media (CM),16 25 ng/mL VEGF, and 20 ng/mL basic fibroblast growth factor (bFGF). Cells were aggregated for 1 day in ultra-low attachment 24-well plates (Corning Costar, Corning, NY) and then transferred to ultra-low attachment 6-well plates for an additional 2 to 3 days of culture. Cultures were maintained in a 37°C humidified chamber in 5% CO2/5% O2/90% N2 mixture (low oxygen conditions).
Colony assays
Blast and hematopoietic colonies were generated as reported.18 For the growth of Scl-/-core colonies, the hemangioblast media was supplemented with 0.5 ng/mL bFGF. Individual blast colonies were expanded as previously described.15 Kit ligand (KL) and interleukin 3 (IL-3) CM were prepared from cytokine-producing cell lines as described.18 VEGF, IL-6, bFGF, granulocyte-macrophage colony-stimulating factor (GM-CSF), thrombopoietin (TPO), and IL-11 were purchased from R&D Systems (Minneapolis, MN).
FACS analyses and cell sorting
EB-derived cells were stained on ice for 20 minutes with biotinylated antibodies against FLK-1,42 platelet endothelial cell adhesion molecule 1 (PECAM-1)/CD31 (BD Pharmingen, San Diego, CA), or vascular endothelial cadherin43 (VECAD) in phosphate-buffered saline (PBS) supplemented with 10% FCS and 0.02% NaN3. Labeled cells were washed twice in PBS and incubated with streptavidin-PE-Cy5 (BD Pharmingen). Fluorescence-activated cell sorting (FACS) analysis was done using a LSRII flow cytometer (Becton Dickinson, San Jose, CA) and the data were analyzed by CellQuest or FlowJo software. Fluorescein di-β-D-galactopyranoside (FDG; Sigma, St Louis, MO) staining was performed as reported.17 When cells were labeled with antibodies against FLK-1 and stained for FDG, the FLK-1 antibody staining was carried out first. For smooth muscle actin (SMA) and Hoechst 33342 (Sigma) staining, cells from adherent and aggregate cultures were labeled with antibodies against FLK-1, CD31, and VECAD. The labeled cells were fixed in 4% paraformaldehyde (PFA) and then resuspended in 1 × PBS supplemented with 1% glucose (Sigma), 0.2% bovine serum albumin (BSA; Sigma), 0.2% NaN3, 5 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma), 0.1% saponin (Sigma), 4 μg/mL Hoechst 33342, and SMA-Cy3 (Sigma) at a 1:100 dilution. Labeling was carried out on a rotating chamber in the dark at 4°C. Cells were washed and resuspended in the supplemented PBS minus the saponin, Hoechst 33342, and SMA-Cy3. Analyses were carried out as described.
Retroviral transduction of SCL
The MSCV-SCL retroviral vector was obtained from Dr Catherine Porcher (University of Oxford and Oxford Radcliffe Hospitals, Oxford, United Kingdom) and the MSCV-PURO retroviral vector was purchased from Clontech (Palo Alto, CA). The virus-producing cell lines, GP + E86/MSCV-SCL and GP + E86/MSCV-PURO were generated as described44 and maintained in supplemented IMDM plus 10% FCS. For infection either 1 × 105 unfractionated EB cells or isolated FLK-1+ cells were incubated on confluent irradiated monolayers of either SCL virus-producing cells (SCLVPCs) or control virus-producing cells (MSCVPCs) in supplemented IMDM containing 10% FCS, 20% D4T CM, KL (2% CM), IL-3 (1% CM), and 2 U/mL erythropoietin (EPO). Following 1 or 3 days of incubation, the EB-derived population together with the virus-producing cells were harvested by trypsinization and assayed for BL-CFCs and hematopoietic progenitors, respectively.
Expansion of FLK-1+ cells for immunostaining
FLK-1+ cells were plated onto fibronectin-coated glass coverslips in 200 μL supplemented IMDM with 10% FCS, 20% D4T CM, 25 ng/mL VEGF, and 20 ng/mL bFGF. Cultures were maintained under low oxygen conditions for 24 hours to allow for cell attachment. The following day the cultures were fed with 1 mL of the above media and then incubated for an additional 2 to 3 days.
Immunohistochemistry
Cells were expanded on glass coverslips as described (see “Expansion of FLK-1+ cells for immunostaining”). The media was aspirated after 3 days and the cells were fixed with 4% PFA for 15 to 30 minutes at room temperature. The PFA was removed and the coverslips gently washed and stored with 1 × PBS at 4°C. The fixed cells were incubated for 1 hour with biotinylated antimouse CD31 antibody at a dilution of 1:50 and antimouse SMA antibody (NeoMarkers, Fremont, CA) at a dilution of 1:100 in staining buffer containing 1 × PBS supplemented with 10% FCS, 2% BSA, 0.2% Tween (Sigma), and 0.02% NaN3. The coverslips were washed 5 times and then incubated with streptavidin-Cy3 conjugate (Sigma) at a dilution of 1:800 and antimouse fluorescein isothiocyanate (FITC; Biosource, Camarillo, CA) at a dilution of 1:200 in staining buffer for 1 hour in the dark at room temperature. The coverslips were washed 5 times and then inverted onto a drop of DAPI (4,6 diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). Fluorescence was visualized using a Leica DMRA2 fluorescence microscope (Wetzlar, Germany) and images were recorded using a digital Hamamatsu CCD camera (Hamamatsu City, Japan), objective × 20 with a numerical aperture of 0.70. OpenLab software (Scientific Software, Pleasanton, CA) was used to analyze the images captured.
Gene expression analyses
RNA was extracted from different cell populations using the Qiagen RNeasy kit (Qiagen, Valencia, CA). The 3′ untranslated region (UTR) cDNA was prepared, amplified, and probed for various genes as previously described.30,45 Alternatively reverse transcription-polymerase chain reaction (RT-PCR) was used to convert the RNA into cDNA and gene-specific PCR was carried out using primers listed in Table 1.
Genes . | Sense . | Antisense . | Annealing Temperature . | Insert Size . |
|---|---|---|---|---|
| c-fms | 5′-GCGATGTGTGAGCAATGGCAGT-3′ | 5′-AGACCGTTTTGCGTAAGACCTG-3′ | 60°C | 341 |
| f4/80 | 5′-GCCTCCCTGACTTTCAAATGG-3′ | 5′-TGGATGTCTCCTCCTTGCTGAG-3′ | 60°C | 312 |
| CD11b | 5′-AAACCACAGTCCCGCAGAGAGC-3′ | 5′-GCCAGGTCCATCAAGCCATCCA-3′ | 60°C | 928 |
| νWF | 5′-GTGGTGGGCATGATGGAGAGGTTA-3′ | 5′-GCAAGGTCACAGAGGTAGCTGACT-3′ | 60°C | 485 |
| β-major | 5′-CTGACAGATGCTCTCTTGGG-3′ | 5′-CACAACCCCAGAAACAGACA-3′ | 60°C | 578 |
| Endogenous-scl | 5′-ATTGCACACACGGGATTCTG-3′ | 5′-GAATTCAGGGTCTTCCTTAG-3′ | 50°C | 321 |
| Viral-scl | 5′-ACTCAATGACCAGGAGGAGGAAG-3′ | 5′-TGGATGTGGAATGTGTGCGAG-3′ | 60°C | 292 |
| β-actin | 5′-ATGAAGATCCTGACCGAGCG-3′ | 5′-TACTTGCGCTCAGGAGGAGC-3′ | 60°C | 443 |
Genes . | Sense . | Antisense . | Annealing Temperature . | Insert Size . |
|---|---|---|---|---|
| c-fms | 5′-GCGATGTGTGAGCAATGGCAGT-3′ | 5′-AGACCGTTTTGCGTAAGACCTG-3′ | 60°C | 341 |
| f4/80 | 5′-GCCTCCCTGACTTTCAAATGG-3′ | 5′-TGGATGTCTCCTCCTTGCTGAG-3′ | 60°C | 312 |
| CD11b | 5′-AAACCACAGTCCCGCAGAGAGC-3′ | 5′-GCCAGGTCCATCAAGCCATCCA-3′ | 60°C | 928 |
| νWF | 5′-GTGGTGGGCATGATGGAGAGGTTA-3′ | 5′-GCAAGGTCACAGAGGTAGCTGACT-3′ | 60°C | 485 |
| β-major | 5′-CTGACAGATGCTCTCTTGGG-3′ | 5′-CACAACCCCAGAAACAGACA-3′ | 60°C | 578 |
| Endogenous-scl | 5′-ATTGCACACACGGGATTCTG-3′ | 5′-GAATTCAGGGTCTTCCTTAG-3′ | 50°C | 321 |
| Viral-scl | 5′-ACTCAATGACCAGGAGGAGGAAG-3′ | 5′-TGGATGTGGAATGTGTGCGAG-3′ | 60°C | 292 |
| β-actin | 5′-ATGAAGATCCTGACCGAGCG-3′ | 5′-TACTTGCGCTCAGGAGGAGC-3′ | 60°C | 443 |
Results
The majority of the BL-CFCs do not express Scl
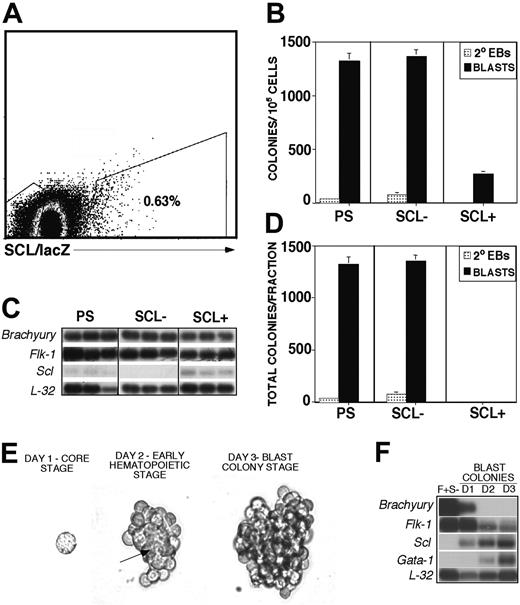
To characterize Scl expression during the formation and differentiation of the BL-CFC, we used ES cells in which the lacZ cDNA was targeted to one of the Scl alleles.40 This targeting enables one to track and isolate SCL/lacZ+ and SCL/lacZ- populations. Kinetic analysis revealed that SCL/lacZ expression was first detectable at 3 days of EB differentiation, at which stage between 0.5% to 1% of the total population was positive (Figure 1A). Analyses of the isolated SCL/lacZ+ and SCL/lacZ- fractions revealed that the frequency of the BL-CFC was 5-fold higher (1368 versus 273/105 cells) in the latter (Figure 1B). Given that the SCL/lacZ+ fraction accounts for less than 1% of the EB population at day 3, the vast majority (99%) of the total BL-CFC pool at this stage of development originates from the SCL/lacZ- fraction (Figure 1D). Secondary (2°) EBs that develop from residual undifferentiated ES cells segregated to the SCL/lacZ- fraction as well. Molecular analyses confirmed the purity of the sort revealing that Scl was only expressed in the SCL/lacZ+ fraction (Figure 1C). Brachyury and Flk-1 were expressed in both fractions. To ascertain the potential of the BL-CFC, blast colonies from both populations were expanded on matrigel and analyzed for hematopoietic and vascular potential. The majority of colonies (77%) from the SCL/lacZ- fraction gave rise to both vascular (adherent) and hematopoietic (nonadherent) cells, whereas only 30% of the blast colonies from the SCL/lacZ+ fraction expanded into both lineages. Most of the blast colonies from the SCL/lacZ+ fraction generated hematopoietic progeny only, suggesting that these colonies developed from a hematopoietic-restricted progenitor.
Previous studies have indicated that Scl is expressed in the blast colony and is required for its formation.17,30 To determine when Scl is up-regulated during blast colony development, colonies were harvested daily and analyzed for Scl expression. Blast colony development is a sequential process that can be divided into discrete stages. The BL-CFC proliferates to initially form a tight core of cells that can be detected 1 to 2 days following replating. Within 48 hours, hematopoietic cells develop from the core and appear on the periphery (G.K. et al, unpublished data, July 2004). Between day 3 and 4, the loosely attached hematopoietic cells proliferate and cover the core, giving rise to the mature blast colony (Figure 1E). These stages will be referred to as the core, the early hematopoietic, and the blast colony stages, respectively. Analyses of the FLK-1+SCL/lacZ- (F+S-) population isolated from day 3 EBs and the developing blast colonies generated from it revealed that Brachyury expression was rapidly down-regulated during colony growth and was no longer detectable by day 2 of growth. Flk-1 expression gradually decreased during blast colony development, whereas Scl expression was up-regulated at the day 1 core stage and gradually increased thereafter. Gata-1 expression followed Scl and correlated with the appearance of early hematopoietic cells at day 2 of colony growth (Figure 1F). Taken together these findings indicate that the majority of BL-CFCs in day 3 EBs do not express Scl. Expression of Scl commences within the first 24 hours of blast colony development from a SCL- BL-CFC.
BL-CFC potential of Scl-expressing EB populations and Scl expression during blast colony development. (A) Percentage of cells expressing SCL in day-3 EBs. Boxes indicate the populations isolated by cell sorting. (B) Frequency (colonies/105 cells) of blast colonies in isolated populations; PS indicates presort; SCL-, SCL/lacZ-; SCL+, SCL/lacZ+ fractions. Blast colonies (BLASTS; ▪) and secondary EBs (2° EBs; ▦) were scored after 4 days in culture. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of the PS, SCL-, and SCL+ fractions. Each lane represents the amplified products from 1000 single cells deposited directly into lysis buffer. The 3′ cDNA was prepared and analyzed as described.30,45 (D) Total blast colonies in each fraction. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (E) FLK-1+ SCL/lacZ- cells were isolated from day 3 EBs and replated into hemangioblast cultures. Shown is colony morphology at day 1, the core stage; day 2, the early hematopoietic stage; and day 3, the blast colony stage. Arrow indicates the developing vascular core at the day 2 early hematopoietic stage of blast colony development. Original magnification, × 400. Objective × 40, numerical aperture 0.50. MagnaFire software (Optronics, Goleta, CA) was used to analyze the images. (F) Expression analyses of the starting population and of colonies at the 3 different developmental stages; 105 FLK-1+ SCL/lacZ- (F+ S-) cells, 1000 day 1 and day 2 pooled colonies and 500 day 3 pooled colonies were used for this analysis. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. Hybridization to the ribosomal gene L-32 was included to control for the level of cDNA in each lane.
BL-CFC potential of Scl-expressing EB populations and Scl expression during blast colony development. (A) Percentage of cells expressing SCL in day-3 EBs. Boxes indicate the populations isolated by cell sorting. (B) Frequency (colonies/105 cells) of blast colonies in isolated populations; PS indicates presort; SCL-, SCL/lacZ-; SCL+, SCL/lacZ+ fractions. Blast colonies (BLASTS; ▪) and secondary EBs (2° EBs; ▦) were scored after 4 days in culture. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of the PS, SCL-, and SCL+ fractions. Each lane represents the amplified products from 1000 single cells deposited directly into lysis buffer. The 3′ cDNA was prepared and analyzed as described.30,45 (D) Total blast colonies in each fraction. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (E) FLK-1+ SCL/lacZ- cells were isolated from day 3 EBs and replated into hemangioblast cultures. Shown is colony morphology at day 1, the core stage; day 2, the early hematopoietic stage; and day 3, the blast colony stage. Arrow indicates the developing vascular core at the day 2 early hematopoietic stage of blast colony development. Original magnification, × 400. Objective × 40, numerical aperture 0.50. MagnaFire software (Optronics, Goleta, CA) was used to analyze the images. (F) Expression analyses of the starting population and of colonies at the 3 different developmental stages; 105 FLK-1+ SCL/lacZ- (F+ S-) cells, 1000 day 1 and day 2 pooled colonies and 500 day 3 pooled colonies were used for this analysis. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. Hybridization to the ribosomal gene L-32 was included to control for the level of cDNA in each lane.
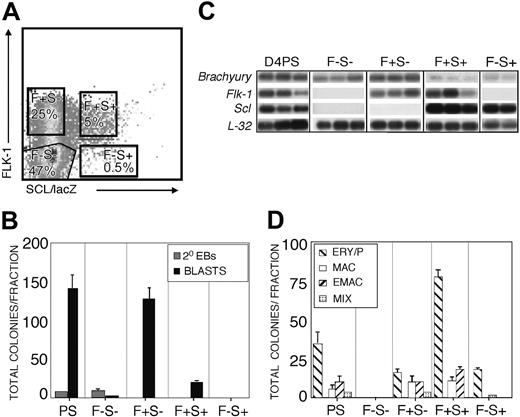
To further evaluate the expression of Scl during the progression of the BL-CFC to the hematopoietic lineage, we analyzed Scl+ and Flk-1+ fractions from day 4 EBs, which represent the transition from the hemangioblast to the hematopoietic stage of development. Four different gated populations, FLK-1-/SCL/lacZ- (F-S-), FLK-1+/SCL/lacZ- (F+S-), FLK-1+/SCL/lacZ+ (F+S+), and FLK-1-/SCL/lacZ+ (F-S+) representing 47%, 25%, 5%, and 0.5% of the total population, respectively, were isolated by cell sorting (Figure 2A) and analyzed for BL-CFCs as well as hematopoietic potential. Of the total number of blast colonies obtained at day 4, 86% segregated to the FLK-1+SCL/lacZ- fraction (Figure 2B). In contrast to the BL-CFCs, the majority (75%) of the hematopoietic progenitors were present in the FLK-1+SCL/lacZ+ fraction (Figure 2D). Gene expression analyses revealed higher levels of Brachyury expression in the SCL/lacZ- fractions compared to the SCL/lacZ+ fractions (Figure 2C). Flk-1 and Scl expression segregated to appropriate populations confirming the purity of the sort. These findings support those from day 3 EBs and demonstrate that most of the BL-CFCs in day 4 EBs express little, if any, Scl. Segregation of the hematopoietic progenitors to the FLK-1+SCL/lacZ+ population suggests that the up-regulation of Scl expression coincides with the transition from the hemangioblast stage into the earliest hematopoietic stage of development.
Rescue of blast colonies from Scl-/- FLK-1+ cells
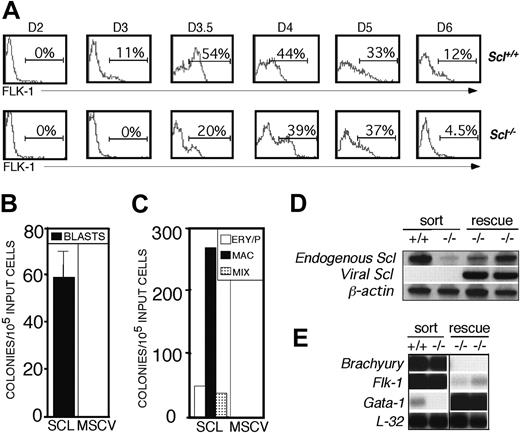
Based on these findings, we hypothesized that SCL is not required for BL-CFC development but is crucial for the generation of the typical morphology of the blast colony (Figure 1E). If this is true, then it should be possible to rescue blast colony development by introduction of SCL into the putative FLK-1+Scl-/- BL-CFC. The kinetics of expression of FLK-1 in Scl-/- EBs is similar to that found in wild-type EBs, with the exception of a slight delay in the onset between days 3.0 and 3.5 of differentiation (Figure 3A). In the first analysis, FLK-1+ cells from day 4 Scl-/- EBs were isolated and cocultured on SCLVPCs or control MSCVPCs. To assay for BL-CFC potential, cells were harvested 24 hours later and replated into hemangioblast conditions. For hematopoietic potential, cells were incubated for an additional 2 days on the virus-producing cells and then replated into methylcellulose containing hematopoietic cytokines. Blast (Figure 3B) and hematopoietic colonies (Figure 3C) were generated from day 4, Scl-/- FLK-1+ EB-derived cells that had been cocultured with SCLVPCs. No colonies appeared when these cells were incubated on MSCVPCs. When rescued blast colonies were picked and expanded, approximately 30% gave rise to both hematopoietic and vascular cells. As expected, the rescued hematopoietic cells did contain viral Scl transcripts (Figure 3D). When the PCR product was sequenced, we discovered that the transduced gene contained a 94-bp deletion at the extreme 3′ end. Such deletions are not uncommon when generating recombinant retroviruses. We do not feel that this poses a significant problem because it has been demonstrated that deletions in this region of Scl do not affect its ability to rescue hematopoietic potential in Scl-/- ES cells.46 Endogenous nonfunctional Scl transcripts were also detected in these cells, when using primers designed against the 3′ UTR of the gene. The targeting removed most of the translatable portion of the Scl gene but not its 3′ UTR region. The up-regulation of endogenous Scl expression in the rescued cells compared to the uninfected population likely reflects expression in the developing hematopoietic cells. Brachyury and Flk-1 were down-regulated in the rescued cells, whereas the hematopoietic transcription factor Gata-1 was strongly expressed in these populations (Figure 3E). These patterns were expected because it has been previously shown that expression of Brachyury and Flk-1 declines as the wild-type hemangioblast population differentiates to the hematopoietic lineages.18
BL-CFC and hematopoietic progenitor content of FLK-1/SCL populations from day 4 EBs. (A) FLK-1-SCL/lacZ- (F-S-), FLK-1+ SCL/lacZ- (F+ S-), FLK-1+ SCL/lacZ+ (F+ S+) and FLK-1-SCL/lacZ+ (F-S+) populations were isolated by cell sorting. Boxes indicate the percentages of different fractions. (B) Total number of blast colonies (BLASTS; ▪) and secondary EBs (2° EBs; ▦) were scored from the unfractionated (PS indicates presort), F-S-, F+ S-, F+ S+, and F-S+ populations. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of isolated fractions. Each lane represents the amplified products from 1000 single cells. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Total number of hematopoietic colonies in each of the indicated fractions. Colonies were categorized as follows: ERY/P (▧), primitive erythroid; MAC (□), macrophages; E/MAC (▨), erythroid-macrophage; and MIX (▦), multilineage. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM.
BL-CFC and hematopoietic progenitor content of FLK-1/SCL populations from day 4 EBs. (A) FLK-1-SCL/lacZ- (F-S-), FLK-1+ SCL/lacZ- (F+ S-), FLK-1+ SCL/lacZ+ (F+ S+) and FLK-1-SCL/lacZ+ (F-S+) populations were isolated by cell sorting. Boxes indicate the percentages of different fractions. (B) Total number of blast colonies (BLASTS; ▪) and secondary EBs (2° EBs; ▦) were scored from the unfractionated (PS indicates presort), F-S-, F+ S-, F+ S+, and F-S+ populations. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of isolated fractions. Each lane represents the amplified products from 1000 single cells. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Total number of hematopoietic colonies in each of the indicated fractions. Colonies were categorized as follows: ERY/P (▧), primitive erythroid; MAC (□), macrophages; E/MAC (▨), erythroid-macrophage; and MIX (▦), multilineage. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM.
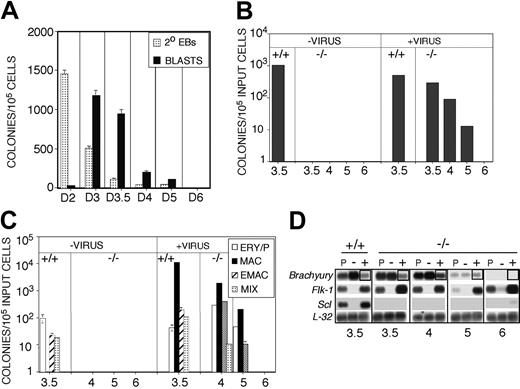
If the rescuable cell is the BL-CFC, it should display a temporal developmental pattern similar to that of the BL-CFC in Scl+/+ EBs. Kinetic analysis revealed that Scl+/+ BL-CFCs were detected as early as day 2.5 of differentiation, reached maximum numbers at day 3, and declined to undetectable levels by day 6 (Figure 4A). To define the kinetics of the cell that can be rescued in Scl-/- EBs, FLK-1+ cells isolated at days 3.5, 4, 5, and 6 of differentiation were cocultured with SCLVPCs for 1 and 3 days and then replated into methylcellulose containing hemangioblast and hematopoietic cytokines. Scl+/+ FLK-1+ cells isolated from day 3.5 EBs were used as a positive control in these rescue assays. Recovery of significant numbers of BL-CFCs and hematopoietic progenitors from Scl+/+ cells cocultured with the virus-producing cells indicated that the conditions were not detrimental to the survival of these progenitors. The highest number of rescued blast colonies was obtained from day 3.5 Scl-/-FLK-1+ cells. The number of blast colonies that could be rescued declined dramatically after day 4 (Figure 4B). No blast colonies were rescued from the day 6 population. This kinetics of rescue is similar to the kinetics of BL-CFC development observed in Scl+/+ EBs. Rescue of the hematopoietic progenitors showed a kinetic pattern identical to that of the BL-CFC (Figure 4C). We have recently demonstrated that the BL-CFC expresses both Flk-1 and Brachyury, suggesting that this coexpression pattern may be the signature of this progenitor.18 To determine if expression of Brachyury correlated with rescue of the blast colony, we analyzed its expression in the Scl-/- FLK-1+ fractions from the different days of differentiation. As shown in Figure 4D, all FLK-1+ populations from which blast colonies could be rescued did express Brachyury. The day 6 FLK-1+ population, which contained no rescuable cells, expressed no Brachyury. Taken together with the previous SCL/lacZ sorting experiments, the findings from the rescue strongly support the interpretation that the BL-CFC is generated in the Scl-/- EBs.
Rescue of blast and hematopoietic colonies from Scl-/- FLK-1+ precursors. (A) Kinetics of FLK-1 expression in Scl+/+ and Scl-/- EBs. Numbers above the graphs indicate the day of differentiation. Percentages of FLK-1+ cells are indicated above the gates. (B) Blast colony potential of day 4 Scl-/- FLK-1+ EB-derived cells following 24 hours of coculture with either SCL or control (MSCV) virus-producing cells. Colony numbers are presented per 105 FLK-1+ cells seeded onto virus-producing cells. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of the infected populations described in panel B. □ indicates ERY/P; ▪, MAC; and ▦, MIX. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression of the endogenous and viral Scl transcripts in day 3.5 Scl+/+ FLK-1+ (sort, +/+), day 4 Scl-/- FLK-1+ (sort, -/-), and 2 pools of rescued Scl-/- FLK-1+ cells (rescue, -/-) harvested following 3 days of coculture with SCL virus-producing cells (SCLVPCs) and 5 days of expansion in KL, IL-3, and EPO. To facilitate the detection of endogenous Scl, primers were designed against the 3′ UTR of the Scl gene. Detection of viral Scl was carried out using a 5′ primer designed against the 3′ translated region of the Scl gene and a 3′ primer designed against the PGK promoter present only in the retroviral transcript. (E) Expression analyses of the populations described in panel D were performed using probes designed against the 3′ UTR of the indicated genes.
Rescue of blast and hematopoietic colonies from Scl-/- FLK-1+ precursors. (A) Kinetics of FLK-1 expression in Scl+/+ and Scl-/- EBs. Numbers above the graphs indicate the day of differentiation. Percentages of FLK-1+ cells are indicated above the gates. (B) Blast colony potential of day 4 Scl-/- FLK-1+ EB-derived cells following 24 hours of coculture with either SCL or control (MSCV) virus-producing cells. Colony numbers are presented per 105 FLK-1+ cells seeded onto virus-producing cells. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of the infected populations described in panel B. □ indicates ERY/P; ▪, MAC; and ▦, MIX. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression of the endogenous and viral Scl transcripts in day 3.5 Scl+/+ FLK-1+ (sort, +/+), day 4 Scl-/- FLK-1+ (sort, -/-), and 2 pools of rescued Scl-/- FLK-1+ cells (rescue, -/-) harvested following 3 days of coculture with SCL virus-producing cells (SCLVPCs) and 5 days of expansion in KL, IL-3, and EPO. To facilitate the detection of endogenous Scl, primers were designed against the 3′ UTR of the Scl gene. Detection of viral Scl was carried out using a 5′ primer designed against the 3′ translated region of the Scl gene and a 3′ primer designed against the PGK promoter present only in the retroviral transcript. (E) Expression analyses of the populations described in panel D were performed using probes designed against the 3′ UTR of the indicated genes.
Colony-forming potential of Scl-/- FLK-1+ EB cells
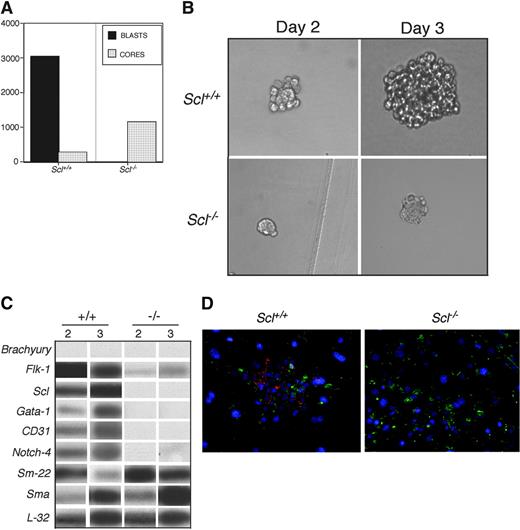
Day 3.5 Scl-/- FLK-1+ cells generated blast colony cores within 24 to 36 hours of growth in hemangioblast conditions. The Scl-/- cores were smaller than those that developed from the wild-type BL-CFCs, suggesting a growth defect at this stage of development. Over the next 2 days of culture, the Scl+/+ cores grew and matured into typical blast colonies. Some, but not all, of the Scl-/- colonies increased in size and retained the morphology of the core (Figure 5B). The number of blast colonies generated by the Scl+/+FLK-1+ cells was approximately 3-fold higher than the number of core colonies generated from the Scl-/- FLK-1+ cells (Figure 5A). This difference in colony number may be due to the fact that many of the Scl-/- cores remained small and were not scored as colonies. Gene expression analyses of developing Scl+/+ and Scl-/- colonies revealed an absence of Brachyury indicating that these colonies had exited the mesodermal stage (Figure 5C). There was also a lack of expression of the endothelial markers Flk-1,3 CD31,5 and Notch-447 as well as the hematopoietic marker Gata-1 in the Scl-/- cores at day 2 and 3 of development. These genes were expressed in the wild-type colonies on both days. Both Scl+/+ and Scl-/- colonies expressed the smooth muscle genes, Sm-2248 and Sma.49 These profiles indicate that the lack of Scl expression led to a loss of endothelial as well as hematopoietic potential in the Scl-/- cores. To further evaluate the vascular potential of Scl+/+ blast colonies and Scl-/- cores, they were expanded on fibronectin-coated glass coverslips and the cells stained with antibodies against CD31 and SMA to analyze for endothelial and smooth muscle potential, respectively. As predicted from expression studies, Scl-/- cores generated only SMA+ cells, whereas Scl+/+ blast colonies gave rise to both CD31+ and SMA+ cells (Figure 5D).
Vascular potential of FLK-1+ population from Scl+/+ and Scl-/- EBs
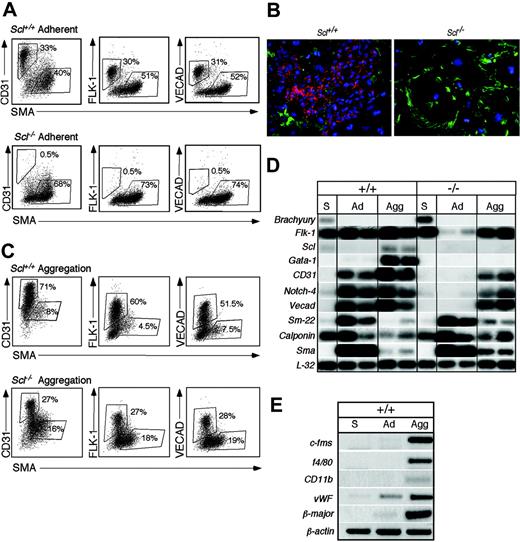
To further investigate the vascular potential of the Scl-/- ES cells, FLK-1+ cells were isolated from day 3.5 EBs and plated directly onto gelatin-coated wells in the presence of bFGF and VEGF, conditions that support the proliferation of both endothelial and VSM lineages from Scl+/+ EBs. To quantitate the percentage of endothelial and smooth muscle cells generated following 3 days of culture, cells were labeled with antibodies against CD31, FLK-1, VECAD,50 and SMA. As shown in Figure 6A, cultures from Scl+/+ FLK-1+ cells contained approximately 30% endothelial cells and 50% smooth muscle cells. In contrast, cultures generated from Scl-/- FLK-1+ cells consisted predominantly of SMA+ cells (70%). Cell cycle analyses demonstrated that the SMA+Scl+/+ and Scl-/- populations were proliferating at the same rate (data not shown), indicating that the difference in proportion of vascular lineage cells was probably due to a defect in Scl-/- endothelial cells. Immunohistochemistry using antibodies against CD31 and SMA on cells from 3-day-old adherent cultures confirmed the FACS analyses (Figure 6B).
Kinetics of blast and hematopoietic colony rescue from Scl-/- derived populations. (A) Kinetics of blast colony development in Scl+/+ EBs (▦ , 2° EBs; ▪, BLASTS). Numbers below the graph indicate the day of differentiation. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (B) Blast colony potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 3.5, 4, 5, and 6 Scl-/- EBs (-/-, 3.5/4/5/6). Colony-forming potential was measured in the various FLK-1+ populations prior to (- virus) and following infection with SCLVPCs (+ virus). Colony numbers are presented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 4, 5, and 6 Scl-/- EBs (-/-, 4/5/6). Colony-forming potential was measured prior to (- virus) and following infection with SCLVPCs (+ virus). □ indicates ERY/P; ▪, MAC; ▨, E/MAC; and ▦, MIX. Colony numbers are represented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression analyses of the presorted (P), FLK-1- (-) and FLK-1+ (+) fractions from day 3.5 Scl+/+ EBs (+/+) and from the different stage Scl-/- EBs (-/-). Boxes highlight Brachyury expression in FLK-1+ fractions from Scl+/+ and Scl-/- EBs at the indicated time points (in days) shown below the figure.
Kinetics of blast and hematopoietic colony rescue from Scl-/- derived populations. (A) Kinetics of blast colony development in Scl+/+ EBs (▦ , 2° EBs; ▪, BLASTS). Numbers below the graph indicate the day of differentiation. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (B) Blast colony potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 3.5, 4, 5, and 6 Scl-/- EBs (-/-, 3.5/4/5/6). Colony-forming potential was measured in the various FLK-1+ populations prior to (- virus) and following infection with SCLVPCs (+ virus). Colony numbers are presented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 4, 5, and 6 Scl-/- EBs (-/-, 4/5/6). Colony-forming potential was measured prior to (- virus) and following infection with SCLVPCs (+ virus). □ indicates ERY/P; ▪, MAC; ▨, E/MAC; and ▦, MIX. Colony numbers are represented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression analyses of the presorted (P), FLK-1- (-) and FLK-1+ (+) fractions from day 3.5 Scl+/+ EBs (+/+) and from the different stage Scl-/- EBs (-/-). Boxes highlight Brachyury expression in FLK-1+ fractions from Scl+/+ and Scl-/- EBs at the indicated time points (in days) shown below the figure.
Colony-forming potential of Scl-/- FLK-1+ EB-derived cells. (A) Blast colony (□) and core colony (▦) development from FLK-1+ day 3 Scl+/+ and day 3.5 Scl-/- EBs. Data are presented as mean number of blast colonies and cores per 105 input cells from 3 dishes. (B) Photographs of Scl+/+ blast colonies and Scl-/- cores at day 2 and 3 of development. Original magnification, × 200. Objective × 20, numerical aperture 0.30. MagnaFire software was used to analyze the images. (C) RT-PCR analysis of Scl+/+ (+/+) and Scl-/- (-/-) colonies at days 2 and 3 of growth. Each lane represents a pool of 500 colonies. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Immunohistochemistry of adhesive cells generated from Scl+/+ blast colonies (Scl+/+) and Scl-/- cores (Scl-/-). CD31+ (red) and SMA+ (green) populations are indicated. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images.
Colony-forming potential of Scl-/- FLK-1+ EB-derived cells. (A) Blast colony (□) and core colony (▦) development from FLK-1+ day 3 Scl+/+ and day 3.5 Scl-/- EBs. Data are presented as mean number of blast colonies and cores per 105 input cells from 3 dishes. (B) Photographs of Scl+/+ blast colonies and Scl-/- cores at day 2 and 3 of development. Original magnification, × 200. Objective × 20, numerical aperture 0.30. MagnaFire software was used to analyze the images. (C) RT-PCR analysis of Scl+/+ (+/+) and Scl-/- (-/-) colonies at days 2 and 3 of growth. Each lane represents a pool of 500 colonies. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Immunohistochemistry of adhesive cells generated from Scl+/+ blast colonies (Scl+/+) and Scl-/- cores (Scl-/-). CD31+ (red) and SMA+ (green) populations are indicated. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images.
One difference between the in vitro cultures and the early embryo is that the cultures are 2-dimensional, thus minimizing cell interactions, whereas the yolk sac vasculature is established in a 3-dimensional environment that enhances cell-to-cell contact. To enable the cells to develop in a 3-dimensional structure in culture, FLK-1+ cells were allowed to reaggregate following sorting. The aggregates were maintained in suspension for 3 days in the presence of VEGF and bFGF, then dissociated and analyzed for CD31, FLK-1, VECAD, and SMA. As shown in Figure 6C, growing the cells as aggregates significantly affected the differentiation program observed in both the Scl+/+ and Scl-/- cultures. More than 50% of the aggregated cells in the Scl+/+ cultures expressed endothelial markers (FLK-1, CD31, and VECAD), whereas less than 10% were positive for the VSM marker, SMA. Interestingly, a significant portion (27%) of Scl-/- aggregated cells also expressed the set of endothelial markers (FLK-1, CD31, and VECAD). As observed in the Scl+/+ cultures, significantly fewer Scl-/- aggregated cells expressed SMA compared to the 2-dimensional adherent cultures. Gene expression analyses (Figure 6D) revealed a strong up-regulation of both endothelial and VSM markers in the Scl+/+ cells after either adherent or aggregate cultures. The adherent cells did not express detectable levels of Scl, Gata-1, β-globin, c-fms, f4/80, or CD11b genes indicative of hematopoietic and macrophage development51-53 (Figure 6D-E). These observations demonstrate that the adherent vascular cultures do not support hematopoiesis. The hematopoietic genes were expressed in the Scl+/+ aggregates, indicating that this population does maintain some hematopoietic potential. The adherent Scl-/- cells expressed only the smooth muscle genes Sma, Sm-22, and Calponin.54 Expression of the endothelial genes, CD31, Vecad, and Notch-4, was detected in the aggregate Scl-/- cultures, consistent with the findings from the flow cytometric analyses (Figure 6C). Taken together these findings indicate that in the absence of SCL, cell-to-cell interactions play an important role in the generation and maintenance of endothelial cells in vitro.
Discussion
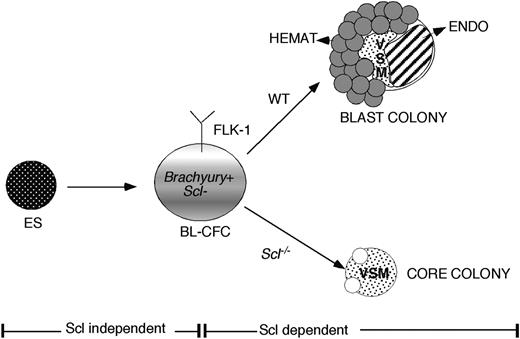
Although numerous studies have shown that SCL is indispensable for the establishment of the hematopoietic system,11,12,33,34 the precise stage of the developmental block in Scl null embryos and EBs has not yet been defined. The following observations from this study support the interpretation that SCL is not required for the development of the hemangioblast/BL-CFC but rather for its differentiation to the hematopoietic and endothelial lineages. First, the majority of BL-CFCs were found in the SCL/lacZ- fraction of both day 3 and 4 EBs, indicating that this progenitor does not express Scl. Expression of Scl was up-regulated within 24 hours of initiation of blast colony development from the BL-CFC. Second, it was possible to rescue blast colony and hematopoietic potential from Scl-/- EBs by retroviral transduction of a wild-type Scl gene. The kinetics of development of the progenitor that could be rescued and its correlation with Brachyury expression mimic that of the wild-type BL-CFC. Third, Scl-/- FLK-1+ cells generate colonies that resemble the early stages of blast colony development. Taken together, our findings support a model in which the BL-CFC is represented as a Brachyury+Flk-1+Scl- progenitor. In this model, the BL-CFC is generated in the absence of SCL, but requires this transcription factor to generate hematopoietic and endothelial progeny in vitro (Figure 7).
Vascular potential of FLK-1+ cells from Scl+/+ and Scl-/- EBs. (A) FLK-1+ cells from day 3.0 Scl+/+ or day 3.5 Scl-/- EBs were cultured on gelatin-coated wells in the presence of VEGF and bFGF for 3 days. Following culture the cells were harvested and stained for CD31, FLK-1, VECAD, and SMA. Numbers indicate the percentage of the respective populations. (B) Immunohistochemistry of day 3 Scl+/+ FLK-1+ cells (Scl+/+) and day 3.5 Scl-/- FLK-1+ cells (Scl-/-) cultured on fibronectin-coated glass coverslips for 3 days. CD31+ cells are shown in red, whereas the SMA+ cells are indicated in green. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images. (C) FLK-1+ cells from day 3 Scl+/+ or day 3.5 Scl-/- EBs were allowed to aggregate by culturing at high density in low-adherence plates. The aggregates were cultured for 3 days in VEGF and bFGF and then processed for CD31, FLK-1, VECAD, and SMA as described in panel A. (D) RT-PCR analyses of day 3.0 Scl+/+ FLK-1+ sorted cells (+/+, S), Scl+/+ adherent cells (+/+, Ad), Scl+/+ aggregated cells (+/+, Agg), Scl-/- day 3.5 FLK-1+ sorted cells (-/-, S), Scl-/- adherent cells (-/-, Ad) and Scl-/- aggregated cells (-/-, Agg). Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (E) RT-PCR analysis of day 3.0 Scl+/+ FLK-1+ sorted cells (+/+, S), Scl+/+ adherent cells (+/+, Ad), and Scl+/+ aggregated cells (+/+, Agg) for the expression of the macrophage-specific genes c-fms, f4/80, CD11b, for the erythroid-specific gene, β-major, and for the endothelial-specific gene, VWF. β-Actin was used to normalize the content of cDNA in these populations.
Vascular potential of FLK-1+ cells from Scl+/+ and Scl-/- EBs. (A) FLK-1+ cells from day 3.0 Scl+/+ or day 3.5 Scl-/- EBs were cultured on gelatin-coated wells in the presence of VEGF and bFGF for 3 days. Following culture the cells were harvested and stained for CD31, FLK-1, VECAD, and SMA. Numbers indicate the percentage of the respective populations. (B) Immunohistochemistry of day 3 Scl+/+ FLK-1+ cells (Scl+/+) and day 3.5 Scl-/- FLK-1+ cells (Scl-/-) cultured on fibronectin-coated glass coverslips for 3 days. CD31+ cells are shown in red, whereas the SMA+ cells are indicated in green. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images. (C) FLK-1+ cells from day 3 Scl+/+ or day 3.5 Scl-/- EBs were allowed to aggregate by culturing at high density in low-adherence plates. The aggregates were cultured for 3 days in VEGF and bFGF and then processed for CD31, FLK-1, VECAD, and SMA as described in panel A. (D) RT-PCR analyses of day 3.0 Scl+/+ FLK-1+ sorted cells (+/+, S), Scl+/+ adherent cells (+/+, Ad), Scl+/+ aggregated cells (+/+, Agg), Scl-/- day 3.5 FLK-1+ sorted cells (-/-, S), Scl-/- adherent cells (-/-, Ad) and Scl-/- aggregated cells (-/-, Agg). Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (E) RT-PCR analysis of day 3.0 Scl+/+ FLK-1+ sorted cells (+/+, S), Scl+/+ adherent cells (+/+, Ad), and Scl+/+ aggregated cells (+/+, Agg) for the expression of the macrophage-specific genes c-fms, f4/80, CD11b, for the erythroid-specific gene, β-major, and for the endothelial-specific gene, VWF. β-Actin was used to normalize the content of cDNA in these populations.
Model of early hemangioblast commitment. The SCL-independent and -dependent phases are indicated. The BL-CFC is represented as a Flk-1+/Brachyury+/Scl- progenitor. In appropriate culture conditions, wild-type (WT) progenitors generate blast colonies composed of the hematopoietic (Hemat), vascular smooth muscle (VSM), and endothelial (endo) lineages. In the absence of SCL, these progenitors fail to generate the hematopoietic and endothelial lineages and give rise to colonies of VSM cells only.
Model of early hemangioblast commitment. The SCL-independent and -dependent phases are indicated. The BL-CFC is represented as a Flk-1+/Brachyury+/Scl- progenitor. In appropriate culture conditions, wild-type (WT) progenitors generate blast colonies composed of the hematopoietic (Hemat), vascular smooth muscle (VSM), and endothelial (endo) lineages. In the absence of SCL, these progenitors fail to generate the hematopoietic and endothelial lineages and give rise to colonies of VSM cells only.
Our cell-sorting studies and gene expression analyses suggest that the onset of Scl expression uniquely defines the transition from the hemangioblast stage to the hematopoietic and vascular stages of EB development. This is best demonstrated by the findings that the day 4 SCL/lacZ+ fractions contain relatively few BL-CFCs but are enriched for hematopoietic progenitors. Of significance is the observation that most colonies generated from SCL/lacZ+ BL-CFCs did not give rise to adherent cells, suggesting that they represent the earliest hematopoietic-restricted progenitors. The fact that our hemangioblast cultures also support the growth of such progenitors is not surprising because they likely represent the immediate descendants of the BL-CFC. This observation does, however, demonstrate the need to analyze blast colonies for vascular potential to ensure that they are derived from a tripotential BL-CFC. Recently, Chung et al31 generated an ES cell line in which human CD4 was targeted as a selectable marker to the Scl locus. Analysis of FLK-1+CD4- and FLK-1+CD4+ populations revealed the presence of BL-CFCs in both, although the frequency was approximately 3-fold higher in the latter. Given the fact that the FLK-1+CD4- population at the stage of analysis was 10-fold larger than the FLK-1+CD4+ population, the majority of BL-CFCs were actually present in the CD4- (SCL-) fraction, as observed in our study.
The ability to rescue blast colony-forming potential from the Scl-/- FLK-1+ population and the kinetics of development of the cell that is rescued strongly suggest that the BL-CFC is generated in the absence of SCL. This interpretation is supported by the observation that Scl-/- FLK-1+ cells initiate blast colony growth when cultured in hemangioblast conditions (Figure 5B). Collectively, these observations position the SCL defect at the level of hematopoietic and endothelial development from the BL-CFC. Findings from the studies of Visvader et al35 are consistent with a role for Scl beyond the induction of the hemangioblast. They demonstrated that ectopic expression of Scl under the Gata-1 promoter could rescue all hematopoietic lineages in Scl-/- embryos. The observation that Gata-1 expression is up-regulated following that of Flk-1 and Scl and beyond the BL-CFC stage of development,29,30 indicates that the putative hemangioblast is generated in the embryo in the absence of SCL. Expression of Scl from the Gata-1 promoter could rescue the capacity of this progenitor to generate the hematopoietic lineages.35
The lack of endothelial potential in the Scl-/- blast core colonies and in the 2-dimensional expansion cultures clearly indicates a defect in the development of this lineage at an early stage. Ema et al19 also noted a defect in endothelial development in Scl-/- EB-derived cells and observed that the majority of FLK-1+ cells isolated at day 2.75 of differentiation were committed to the VSM lineage. The lack of endothelial cells in these cultures is inconsistent with observations in vivo that show that Scl-/- embryos are able to undergo vasculogenesis and generate CD31+, CD34+ endothelial cells.35 The finding that cells that express endothelial markers could be generated when Scl-/- FLK-1+ cells were allowed to develop as aggregates (Figure 6C) indicates that specific cellular interactions are able to overcome, to some extent, the deficiency in endothelial growth observed in the monolayer cultures. Such aggregate cultures may more accurately reflect the 3-dimensional conditions in vivo and more precisely mimic the SCL defect in the yolk sac.
Findings from studies involving the ectopic expression of Scl have been interpreted as evidence that SCL can function at the induction or expansion stage of hemangioblast development, at a point earlier than suggested by our findings. Gering et al38 observed an expansion of the hematopoietic and endothelial lineages and a decrease in somitic and pronephric duct progenitors in zebrafish embryos injected with Scl mRNA. One interpretation of these findings is that expression of Scl in nonhematopoietic mesoderm redirects the fate of these cells to the hematopoietic and vascular lineages. Alternatively, expression of Scl in hematopoietic and vascular progenitors may have increased their proliferation potential, resulting in expansion of the derivative lineages to regions where they are not normally found. Ema et al19 noted increased numbers of BL-CFCs in the FLK-1+ EB population generated from ES cells in which Scl was expressed from the Flk-1 locus. These findings suggest that the earlier than normal expression of Scl induced higher numbers of BL-CFCs. It is also possible that this expression expanded the number of hematopoietic-restricted progenitors within the FLK-1 population because expansion of the hematopoietic lineages was noted in later stage EBs in their study. The notion that altered expression of Scl can influence hematopoietic development is also supported by the study of Endoh et al55 that showed a 3- to 9-fold increase in hematopoietic progenitors following forced Scl expression in day 3.5 FLK-1+ cells. Expansion of hematopoietic potential as a result of forced Scl expression observed in these different studies is in line with the interpretation that the primary function of SCL is in lineage development from the BL-CFC.
In summary, the findings in this study provide strong evidence that Scl is not essential for the development of the BL-CFC, the in vitro equivalent of the hemangioblast, but plays a pivotal role in its commitment to the hematopoietic and endothelial lineages. Although Scl is absolutely required for hematopoietic development, its role in the establishment of the endothelial lineage is less clear and appears to be at the level of regulation of proliferation of cells within the lineage, rather than in the initial commitment steps. Access to the earliest stages of hematopoietic and endothelial development at the level of the BL-CFC and to Scl-/-endothelial cells from early stage EBs will provide appropriate populations with which to further elucidate the role of Scl in the establishment of the hematopoietic and vascular systems.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-09-3611.
Supported by the National Institutes of Health grants, HL 48834, ROI HL65169, and ROI HL71800-01. Presented in abstract form at the 2nd Annual Meeting of the International Society for Stem Cell Research, Boston, MA, June 10, 2004.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank all the members of the Keller laboratory and Dr Deborah French for critically reading this manuscript and providing many useful suggestions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal