Abstract
It has recently been shown that the iron isotopic composition of blood differs between individuals and sexes, which is supposed to reflect individual differences in iron metabolism. We hypothesized that patients suffering from hereditary hemochromatosis would demonstrate alterations in the iron isotopic composition of blood due to persistent up-regulation of intestinal iron absorption. Blood from 30 patients with homozygous C282Y hemochromatosis was analyzed for iron isotopic composition by a newly developed technique using multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS). Blood of patients with hemochromatosis is characterized by a higher 56Fe/54Fe isotope ratio than blood of healthy individuals, which are either members of an age-matched control group (n = 10; P < .001) or young adults (n = 36; P < .001). In patients with hereditary hemochromatosis, the 56Fe/54Fe isotope ratio of blood significantly correlates with total-body iron accumulation, severity of clinical disease, and the need for regular phlebotomies to prevent iron reaccumulation. We conclude that blood of patients with hereditary hemochromatosis contains more of the heavier iron isotopes than blood of healthy individuals. The primary determinant of the iron isotopic composition of blood appears to be isotope-sensitive iron absorption in the intestine and the efficiency of this process.
Introduction
Hemochromatosis is a frequent genetic disorder in populations of European ancestry that is characterized by progressive iron overload of tissues due to an ineffective control of intestinal iron absorption. Following identification of the HFE gene,1 several studies showed that about 90% of the patients with clinical features of hemochromatosis are homozygous for a C282Y mutation of the HFE gene.2 Estimates of gene frequency range from 5% to 10%, with a homozygote frequency of 0.2 to 0.5% in white populations.3-5 Typical clinical manifestations are hepatic fibrosis, arthropathy, diabetes mellitus, cardiopathy, and hypogonadism. Epidemiologic studies indicate, however, a low clinical penetrance of the homozygous HFE C282Y mutation.3,6,7
Iron homeostasis is maintained by the body by regulating iron absorption in the proximal small intestine.8,9 Heme-bound iron such as myoglobin (and hemoglobin) in foods of animal origin is taken up by an endocytotic process in which the porphyrin ring of the heme molecule is broken up within the epithelial cell.10,11 Uptake of nonheme iron, mostly from plant foods, is mediated by ferric reductase and divalent metal transporter-1 (DMT-1)12-14 or, possibly, by the integrin-mobilferrin-paraferritin pathway.15,16 An iron-regulated transporter 1 (IREG1) is involved in subsequent iron transfer from the epithelial cell to blood circulation.17 There is no satisfactory technique available to date to measure regulation of intestinal iron absorption. Measurements by conventional tracer techniques using stable or radioactive isotopes are jeopardized by physiologic day-to-day variations of iron absorption.
All iron in nature consists of 4 stable isotopes that differ in their masses and natural abundances: 54Fe (5.8%), 56Fe (91.8%), 57Fe (2.1%), and 58Fe (0.3%).18 In general, the iron isotopic composition of an element can be altered by physical processes such as diffusion or evaporation if the transfer is mass dependent or by chemical processes if breaking/formation of chemical bonds depends on the masses of the isotopes involved. Mass balance principles dictate, however, that element transfer between compartments must be incomplete to generate an isotope effect.
Substantial isotope effects are known in nature for a number of light elements such as hydrogen, oxygen, and carbon. Until recently, isotope effects were considered to be too small to be measurable for most of the heavier elements such as iron. Using multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS), Walczyk and von Blanckenburg demonstrated that human tissues are depleted in heavy iron isotopes compared with the diet and the geosphere.19 Each individual bears a distinct 56Fe/54Fe isotope ratio in the blood that is, on average, lower in males than in females.19,20 It has been hypothesized that the iron isotopic composition is altered during intestinal absorption. Stability of the iron isotopic signature in blood over one year was recently shown in a healthy subject.20
In the present study, we show that patients with hemochromatosis have an altered iron isotopic composition of blood when compared with healthy controls. This strengthens the hypothesis that the iron isotopic composition of blood is primarily determined by the efficiency at which iron is absorbed from the diet.
Patients, materials, and methods
Study design
Iron isotopic composition of blood was compared among 30 patients with homozygous C282Y hemochromatosis and 2 control groups, that is, an age-matched control group of 10 elderly subjects, and 36 young adults included in a previous study.19
Study patients
The study protocol was approved by the ethical committee of the University Hospital of Zurich, Switzerland. All patients gave written informed consent to participate in the study. There were 30 patients with established diagnosis of hemochromatosis enrolled, showing a homozygous C282Y mutation of the HFE gene and serum ferritin concentration above the reference range at time of diagnosis. Clinical manifestations, histologic findings, laboratory results, and amounts of iron removed by phlebotomy were assessed by patients' history and from their medical records. As an age-matched control group, 10 patients without history of blood donation or disorders of iron metabolism were recruited. They were not tested for HFE genotype as they all had transferrin saturations less than 45%. Of 5 females included in this control group, 4 did not have menstrual blood loss for 10 years or more.
Assessment of biochemical variables and clinical manifestations
When included in this study, 22 of 30 patients with hemochromatosis had completed iron depletion therapy and continued regular maintenance therapy (referred to as treated patients). In 8 patients, either no phlebotomy therapy ever (2 patients), no phlebotomies during the last 2 years (5 patients), or inappropriate phlebotomy therapy (1 patient) had been carried out (referred to as untreated patients). Iron removal by phlebotomy (500 mL blood ≈ 250 mg iron) was assessed as (1) total amount of iron removed to achieve serum ferritin within the reference range (iron depletion therapy) and (2) iron removed per year to maintain ferritin within the reference range (maintenance therapy). Maintenance therapy was assessed in 20 patients having continued this therapy for at least 2 years.
Liver disease was assessed using as criterion either substantial fibrosis (either substantial portal fibrosis, or septal/bridging fibrosis or cirrhosis) in histologic examination of liver tissue or elevated serum alanine aminotransferase concentration (ALT) at time of diagnosis. Assessing total number of clinical manifestations, histologic examination of liver tissue was used as primary determinant; elevated ALT was considered in patients without biopsy. All patients with liver disease were serologically tested for chronic hepatitis B and C virus infection. Habitual alcohol intake was estimated by interviewing the patients. Diabetes mellitus, cardiopathy, and hypogonadism were taken over as established diagnosis from medical records; arthropathy of metacarpophalangeal joints was taken over as established diagnosis or assessed by clinical investigation.
Iron isotope analysis
High-precision iron isotope ratios were measured at the University of Hanover, Germany, by multicollector ICP-MS (Neptune; ThermoFinnigan, Bremen, Germany).21 Venous blood samples (0.5 mL) were mineralized by microwave digestion and iron was purified by anion exchange. The isotopes 54Fe, 56Fe, 57Fe, and 58Fe were all measured simultaneously on Faraday collectors. Any residual isobaric interference of 54Cr+ was corrected for by monitoring 52Cr+, ArO, and ArN. Interferences were resolved optically by high-mass resolution techniques.22 δ56Fe/54Fe (%thou) was calculated as follows:
and was measured by linear interpolation between alternating standards (IRMM-014) and samples that were matched for iron concentration. The mass spectrometric reproducibility was assessed by repeated runs of an internal iron standard (Fe Puratronic wire, 99.998% purity, lot NM3688; Johnson&Matthey, Karlsruhe, Germany), which gave δ56Fe/54Fe = 0.422 ± 0.044%thou and δ57Fe/54Fe = 0.632 ± 0.070%thou (95% confidence level). To confirm data accuracy, 3 isotope plots were used consistently. The external reproducibility was assessed to be the same by multiple chemical separations of organic samples.
Genetic analysis
HFE gene C282Y (G845A) mutation was determined by LightCycler polymerase chain reaction (Roche Molecular Biochemicals, Rotkreuz, Switzerland) and melting curve analyses using ToolSets (Genes-4U, Zurich, Switzerland) with specific primers and fluorescent probes according to the manufacturers' instructions.
Statistical analysis
Evaluations were performed using Statistica version 6 (StatSoft, Tulsa, OK). All comparisons of this study were done using either Student t test, Chi-square test, or Spearman rank order correlation. Threshold of significance was defined as α = .05.
Results
Iron isotopic composition of blood in patients with hemochromatosis
The iron isotopic composition of blood obtained from patients C282Y hemochromatosis (n = 30) was compared with that of 2 control groups. The first control group consisted of elderly subjects (n = 10; range, 44-72 years) without history of disorders of iron metabolism or blood donation who were not significantly different from the patient group regarding age (P = .95) and sex (P = .86). The second control group consisted of young adults (n = 36; range, 20-32 years) included in a previous study.19 Blood of patients with hereditary hemochromatosis contains more of the heavier iron isotopes than blood of healthy individuals (ie, it is characterized by a higher 56Fe/54Fe isotope ratio). Table 1 shows that the δ56Fe/54Fe value (relative deviation of the 56Fe/54Fe isotope ratio of blood from the isotope ratio of the iron isotopic reference material IRM-014) was -2.11 ± 0.47%thou in patients with hemochromatosis compared with -2.72 ± 0.27%thou in the age-matched control group (P < .001) and -2.58 ± 0.25%thou in healthy young adults (P < .001).
Iron isotopic composition of blood in patients with homozygous C282Y hemochromatosis and control subjects, presented as relative deviation of the 56Fe/54Fe isotope ratio of blood from the isotope ratio of the isotopic reference material IRM-014 (means ± 1 SD)
. | . | Control groups . | . | |
|---|---|---|---|---|
. | Patients with hemochromatosis . | Age-matched controls . | Young adults . | |
| Age (years) | 61.8 ± 10.7 | 61.6 ± 9.8 | 23.6 ± 3.2 | |
| Male gender (%) | 53 | 50 | 47 | |
| δ 56Fe/54Fe (‰), all patients | -2.11 ± 0.47 (n = 30) | -2.72 ± 0.27 (n = 10, P < .001*) | -2.58 ± 0.25 (n = 36, P < .001*) | |
| Male subjects | NA | -2.92 ± 0.22 (n = 5, P < .001†) | -2.75 ± 0.17 (n = 17, P < .001†) | |
| Treated | -1.95 ± 0.40 (n = 12) | NA | NA | |
| Untreated | -2.56 ± 0.37 (n = 4) | NA | NA | |
| Female subjects | NA | -2.52 ± 0.10 (n = 5, P = .025†) | -2.43 ± 0.21 (n = 19, P = .002†) | |
| Treated | -2.02 ± 0.43 (n = 10) | NA | NA | |
| Untreated | -2.35 ± 0.59 (n = 4) | NA | NA | |
. | . | Control groups . | . | |
|---|---|---|---|---|
. | Patients with hemochromatosis . | Age-matched controls . | Young adults . | |
| Age (years) | 61.8 ± 10.7 | 61.6 ± 9.8 | 23.6 ± 3.2 | |
| Male gender (%) | 53 | 50 | 47 | |
| δ 56Fe/54Fe (‰), all patients | -2.11 ± 0.47 (n = 30) | -2.72 ± 0.27 (n = 10, P < .001*) | -2.58 ± 0.25 (n = 36, P < .001*) | |
| Male subjects | NA | -2.92 ± 0.22 (n = 5, P < .001†) | -2.75 ± 0.17 (n = 17, P < .001†) | |
| Treated | -1.95 ± 0.40 (n = 12) | NA | NA | |
| Untreated | -2.56 ± 0.37 (n = 4) | NA | NA | |
| Female subjects | NA | -2.52 ± 0.10 (n = 5, P = .025†) | -2.43 ± 0.21 (n = 19, P = .002†) | |
| Treated | -2.02 ± 0.43 (n = 10) | NA | NA | |
| Untreated | -2.35 ± 0.59 (n = 4) | NA | NA | |
NA indicates either not applicable or not shown for clarity.
when compared to all patients with hemochromatosis
when compared to treated (either male or female) patients with hemochromatosis
Influence of sex, age, and phlebotomy therapy
The difference in the iron isotopic composition of blood (δ56Fe/54Fe) among treated patients with hemochromatosis and the 2 control groups was found to be more accentuated in males than in females (Table 1).An effect of sex on the iron isotopic composition of blood was observed in a previous study (ie, blood of young males was found to be characterized by a significantly lower 56Fe/54Fe isotopic ratio than blood of young females).19 Table 1 shows a similar effect of sex for healthy elderly subjects included in the present study; δ56Fe/54Fe was -2.92 ± 0.22%thou in males and -2.52 ± 0.10%thou in females (P = .006). In contrast, no difference in the iron isotopic composition between sexes could be identified in treated patients with hemochromatosis; δ56Fe/54Fe was -1.95 ± 0.40%thou in males and -2.02 ± 0.43%thou in females (P = .94). This might be explained by inappropriate up-regulation of intestinal iron absorption in both males and females suffering from hereditary hemochromatosis.8
No significant effect of age on 56Fe/54Fe isotopic ratio of blood was detected either in patients with hemochromatosis or healthy individuals. This refers in particular to the subgroup of healthy adult females; Table 1 shows that δ56Fe/54Fe did not differ significantly between young (-2.43 ± 0.21%thou) and elderly (-2.52 ± 0.10%thou) healthy females (P = .35), whereas 4 of 5 elderly females had not had menstrual blood losses for about 10 years. This points to absence or only marginal influence of menstrual blood loss on the iron isotopic composition of blood.
Differences in the iron isotopic composition (δ56Fe/54Fe) between untreated patients with hemochromatosis and control subjects did not reach statistical significance. It is worth noting, however, that the group of untreated patients with hemochromatosis was small and heterogenous. Nevertheless, in untreated patients with hemochromatosis, the 56Fe/54Fe isotope ratio of blood was found to correlate with biochemical and clinical manifestations of the disease (see next paragraph). Hemochromatosis appears to be generally associated with an increased 56Fe/54Fe isotope ratio of blood, with a stronger association, however, in patients undergoing phlebotomy therapy.
Correlation with iron parameters
The iron isotopic composition of blood (δ56Fe/54Fe) significantly correlates with variables reflecting total body iron accumulation, that is, serum ferritin concentration measured at time of diagnosis (P = .017) and total amount of iron removed during subsequent iron depletion therapy (P = .025; Figure 1A-C). Linear regression analyses indicate that the association between δ56Fe/54Fe and the serum ferritin concentration is similar in treated and untreated patients with hemochromatosis. In contrast to variables reflecting iron accumulation by the total body, no relevant association was found between hepatic iron concentration and the 56Fe/54Fe isotope ratio of blood. This might be explained either by the limited number of patients evaluated (n = 8) or by previously reported absence of an accurate association between total body iron stores and hepatic iron content.23
Moreover, δ56Fe/54Fe was found to significantly correlate with annual amounts of iron removed by phlebotomy to maintain the serum ferritin concentration within the reference range (P = .029; Figure 1D). Measurements were made during maintenance therapy, that is, in a steady state of continuous intestinal iron (hyper)absorption and regular phlebotomies to prevent iron reaccumulation.
Association of iron isotopic composition of blood and iron parameters. Correlation of the iron isotopic composition of blood (δ56Fe/54Fe) with serum ferritin concentration at time of diagnosis in treated (A) and untreated (B) patients with hemochromatosis and with the amount of iron removed by phlebotomy during iron depletion therapy (C) and maintenance therapy (D). Solid lines represent linear regression analyses; bars in panel D represent means ± 1 SD.
Association of iron isotopic composition of blood and iron parameters. Correlation of the iron isotopic composition of blood (δ56Fe/54Fe) with serum ferritin concentration at time of diagnosis in treated (A) and untreated (B) patients with hemochromatosis and with the amount of iron removed by phlebotomy during iron depletion therapy (C) and maintenance therapy (D). Solid lines represent linear regression analyses; bars in panel D represent means ± 1 SD.
Correlation with clinical manifestations
Iron isotopic composition of blood (δ56Fe/54Fe) is significantly associated with liver disease assessed either as substantial fibrosis (substantial portal fibrosis, or septal/bridging fibrosis or cirrhosis) in histologic examination24 (P = .003) or, alternatively, as elevated serum alanine aminotransferase concentration (ALT) at the time of diagnosis (P < .001; Table 2). Moreover, δ56Fe/54Fe was found to significantly correlate with ALT, in treated (P = .020) as well as in untreated (P = .036) patients with hemochromatosis. Considering that the iron isotopic composition of blood and parameters of liver disease are associated, correlation between δ56Fe/54Fe and the iron content of liver tissue would be expected. However, hepatic iron concentration was available only in a limited number of patients with a similar extent of liver disease. Chronic hepatitis B and C virus infection, tested in all patients with liver disease, was never diagnosed. Estimated habitual alcohol intake was 7.4 ± 7.6 g/d in patients with liver disease and 14.3 ± 14.9 g/d in patients without liver disease. Hence, a confounding effect of alcohol intake on liver disease is improbable because of the higher mean alcohol intake in the group without liver disease.
Association of the iron isotopic composition of blood (δ56Fe/54Fe; means ± 1 SD) with clinical manifestations in patients with hemochromatosis homozygous for the HFE G845A (C282Y) mutation
Clinical manifestations of hemochromatosis . | δ56Fe/54Fe, ‰ . | P . |
|---|---|---|
| Liver disease | ||
| Histologic examination | ||
| Substantial fibrosis,* n = 15 | - 1.90 ± 0.34 | .003 |
| No substantial fibrosis* in histologic examination or no biopsy carried out in the presence of normal ALT, n = 10 | - 2.43 ± 0.47 | |
| Serum alanine aminotransferase concentration measured at the time of diagnosis | ||
| ALT above the reference range, n = 17 | - 1.89 ± 0.37 | < .001 |
| ALT within the reference range, n = 10 | - 2.48 ± 0.42 | |
| Arthropathy of metacarpophalangeal joints | ||
| Present, n = 17 | - 1.93 ± 0.40 | .019 |
| Absent, n = 13 | - 2.33 ± 0.47 | |
| Diabetes mellitus | ||
| Present, n = 3 | - 1.88 ± 0.35 | NS |
| Absent, n = 27 | - 2.13 ± 0.48 | |
| Cardiopathy, n = 1 | - 1.59 | |
| Hypogonadism, n = 1 | - 2.02 | |
| Total no. of clinical manifestations† | ||
| 2 or more, n = 14 | - 1.89 ± 0.37 | .003 |
| 1, n = 9 | - 2.09 ± 0.42 | |
| 0, n = 7 | - 2.56 ± 0.41 |
Clinical manifestations of hemochromatosis . | δ56Fe/54Fe, ‰ . | P . |
|---|---|---|
| Liver disease | ||
| Histologic examination | ||
| Substantial fibrosis,* n = 15 | - 1.90 ± 0.34 | .003 |
| No substantial fibrosis* in histologic examination or no biopsy carried out in the presence of normal ALT, n = 10 | - 2.43 ± 0.47 | |
| Serum alanine aminotransferase concentration measured at the time of diagnosis | ||
| ALT above the reference range, n = 17 | - 1.89 ± 0.37 | < .001 |
| ALT within the reference range, n = 10 | - 2.48 ± 0.42 | |
| Arthropathy of metacarpophalangeal joints | ||
| Present, n = 17 | - 1.93 ± 0.40 | .019 |
| Absent, n = 13 | - 2.33 ± 0.47 | |
| Diabetes mellitus | ||
| Present, n = 3 | - 1.88 ± 0.35 | NS |
| Absent, n = 27 | - 2.13 ± 0.48 | |
| Cardiopathy, n = 1 | - 1.59 | |
| Hypogonadism, n = 1 | - 2.02 | |
| Total no. of clinical manifestations† | ||
| 2 or more, n = 14 | - 1.89 ± 0.37 | .003 |
| 1, n = 9 | - 2.09 ± 0.42 | |
| 0, n = 7 | - 2.56 ± 0.41 |
NS indicates not significant.
Either substantial portal fibrosis, or septal/bridging fibrosis or cirrhosis of liver tissue
Assessing total number of clinical manifestations, liver disease was defined as substantial fibrosis in histologic examination or, in patients with biopsy, elevated ALT at the time of diagnosis
A significant association was also found between the iron isotopic composition of blood (δ56Fe/54Fe) and arthropathy of the metacarpophalangeal joints (P = .019; Table 2). Less common manifestations of hemochromatosis such as diabetes mellitus, cardiopathy, or hypogonadism were also observed among the study patients, but prevalence was too low for proper statistical evaluation. Table 2 moreover shows that the 56Fe/54Fe isotope ratio of blood significantly correlates with the number of clinical manifestations of hemochromatosis (P = .003). The presented data provide strong evidence that the iron isotopic composition of blood is associated with the severity of clinical disease, in treated as well as in untreated patients with hemochromatosis.
Discussion
Blood of patients with hereditary hemochromatosis contains more of the heavier iron isotopes than blood of healthy individuals, that is, it is characterized by a higher 56Fe/54Fe isotope ratio resembling more the value of dietary iron. This can be explained by iron absorption from the diet being less selective (less preferential for lighter iron isotopes) in patients with hemochromatosis than in healthy individuals (see next paragraph). Moreover, the 56Fe/54Fe ratio is shown to correlate significantly with phenotypic expression of hemochromatosis, that is, total body iron accumulation (serum ferritin concentration at time of diagnosis, phlebotomy requirements during iron depletion therapy), severity of clinical disease (prevalence of liver disease, arthropathy of metacarpophalangeal joints, total number of clinical manifestations), and the need for regular phlebotomies to prevent iron reaccumulation during maintenance therapy.
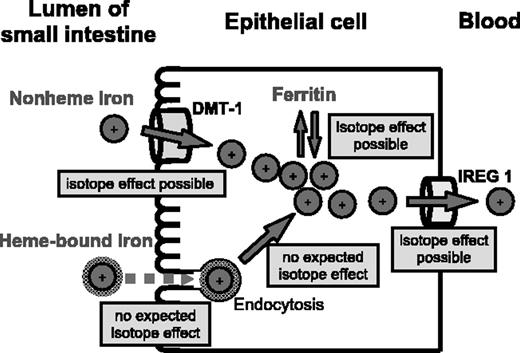
Iron of human blood contains more of the lighter iron isotopes, that is, it is characterized by a lower 56Fe/54Fe isotope ratio than dietary iron.19 Findings can be explained by iron absorption from the diet being a mass-dependent process with preference for the lighter iron isotopes. This is true for healthy subjects and—though less accentuated—patients with hemochromatosis. Mass balance principles dictate that iron isotope fractionation during absorption is less accentuated the more completely iron is absorbed, that is, the 56Fe/54Fe isotope ratio of absorbed iron increases and approaches the value of dietary iron. Figure 2 illustrates how isotope fractionation of dietary iron may occur during the absorption process. Iron is absorbed from 2 major dietary pools, that is, foods of animal origin containing primarily heme-bound iron10,11 and plant foods containing nonheme iron.13,14 Isotope effects (isotope fractionation) may be induced by preferential uptake of lighter isotopes of nonheme iron by DMT-1 and/or iron release from the epithelial cell into the circulatory system by IREG1 and/or preferential deposition of heavier iron isotopes into ferritin (which is removed from the body by regular apoptosis and shedding of epithelial cells into the lumen of the small intestine). In contrast to nonheme iron, it is unlikely that the iron isotopic composition of heme-bound iron is affected during uptake by the intestinal epithelial cell. Relative mass differences of the heme isotopomers, which are taken up intact, are too small to cause a significant isotope effect during endocytosis. Subsequent release of iron from the heme molecule by heme oxygenase is quantitative within epithelial cells and, therefore, cannot cause an isotope effect for reasons of mass balance. Besides isotope effects during absorption, the ratio between heme-bound iron and nonheme iron absorbed from the diet may influence the iron isotope signature of blood. In an earlier survey, foods of animal origin were found to be more depleted in the heavier iron isotopes than plant foods.19 However, results of more than 70 individuals (including unpublished data [T.W. and F.v.B., August 2004]) do not indicate a significant difference in the iron isotopic composition of blood between vegetarians and omnivores.
Transfer of heme iron (foods of animal origin) and nonheme iron (plant foods) through the intestinal mucosa into blood. Nonheme iron is taken up by divalent metal transporter-1 (DMT-1), while heme iron enters the epithelial cell by endocytosis. Nonheme iron as well as iron released from heme is either transported by iron regulatory protein 1 (IREG 1) from the epithelial cell to the blood or deposited in ferritin. Isotope effects (isotope fractionation) may occur during transport of preferentially lighter isotopes by DMT-1 or IREG 1 and/or during deposition of heavier iron isotopes into ferritin. In contrast, endocytosis of heme isotopomers (small relative mass differences) and release of iron from the heme molecule (quantitative process) are not expected to be isotope selective.
Transfer of heme iron (foods of animal origin) and nonheme iron (plant foods) through the intestinal mucosa into blood. Nonheme iron is taken up by divalent metal transporter-1 (DMT-1), while heme iron enters the epithelial cell by endocytosis. Nonheme iron as well as iron released from heme is either transported by iron regulatory protein 1 (IREG 1) from the epithelial cell to the blood or deposited in ferritin. Isotope effects (isotope fractionation) may occur during transport of preferentially lighter isotopes by DMT-1 or IREG 1 and/or during deposition of heavier iron isotopes into ferritin. In contrast, endocytosis of heme isotopomers (small relative mass differences) and release of iron from the heme molecule (quantitative process) are not expected to be isotope selective.
Our recent findings support the hypothesis that the iron isotopic composition of blood is primarily determined by the efficiency at which iron is absorbed from the diet, that is, an increased 56Fe/54Fe isotope ratio of blood reflects up-regulated intestinal iron absorption:
Iron homeostasis is maintained by the body by regulating intestinal iron absorption, however, control of intestinal absorption is ineffective in hereditary hemochromatosis. Inappropriately enhanced intestinal absorption of iron, more accentuated for nonheme iron than heme-bound iron,25 and inappropriately up-regulated intestinal expression of DMT-126 have previously been reported in patients suffering from hereditary hemochromatosis. An increased 56Fe/54Fe isotope ratio of blood as observed in patients with hemochromatosis is expected to be related with the underlying mechanism of the disorder.
We found a higher 56Fe/54Fe isotope ratio in treated than in untreated patients with hemochromatosis. According to our hypothesis, this finding would indicate that iron absorption is up-regulated more distinctly in patients with hemochromatosis undergoing phlebotomy therapy than in untreated patients. Prior studies showed that intestinal expression of DMT-1 is increased in treated26 but not in untreated27 patients with hemochromatosis. Other studies have shown that phlebotomy therapy per se causes a significant increase of intestinal iron absorption in patients with hemochromatosis.28,29
After initial iron depletion therapy, patients with hemochromatosis reach a steady state of continuous intestinal iron (hyper)absorption, which is balanced by iron depletion (phlebotomy) therapy in regular intervals. Phlebotomy requirements for maintaining steady-state conditions are supposed to be higher in patients who absorb iron more efficiently. Hence, we would expect a more pronounced increase in the 56Fe/54Fe ratio of blood in patients phlebotomized more frequently. In the present study, we have found a significant correlation between the 56Fe/54Fe isotope ratio of blood and the annual amount of iron removed by phlebotomy to maintain body iron stores in steady state.
Blood of patients with hemochromatosis contains more of the heavier iron isotopes, that is, it is characterized by a higher 56Fe/54Fe isotope ratio than blood of healthy individuals because intestinal iron absorption is up-regulated and, therefore, the isotope effect (ie, preferential transfer of lighter isotopes) is less accentuated. However, processes related to inhomogenous distribution of iron isotopes between body compartments such as blood, liver, and muscle may also contribute to our findings in patients with hemochromatosis. Earlier investigations have shown that liver tissue is characterized by a higher 56Fe/54Fe isotope ratio than blood in humans.19 If this is also the case in patients with hemochromatosis, release of isotopically heavier liver iron during initial phlebotomy therapy and its subsequent use for erythropoiesis should result in an increase in the 56Fe/54Fe isotope ratio of blood until liver stores are emptied. However, it appears that the iron isotopic signature of blood is not primarily determined by the release of isotopically heavier liver iron since no correlation between the iron isotope composition of blood and total duration of phlebotomy therapy (continued up to 18 years) was observed. Once inappropriate iron stores of the liver are emptied, the iron isotopic composition of blood should be determined mainly by continuously absorbed dietary iron, which is presumed to reflect the efficiency at which iron is absorbed from the diet. In principle, hemoglobin concentration might be associated with the iron isotopic composition of blood, too; this however has not been systematically investigated in the present study.
Considering our recent results, it appears that we now have, basically, a method at our hands to determine the efficiency of intestinal (nonheme) iron absorption. The finding that the iron isotopic composition of blood is significantly associated with phenotypic expression of hereditary hemochromatosis can be considered as an early proof of concept. The method bears potential relevance as an indicator for disorders affecting regulatory mechanisms of iron metabolism.8,9,25-28 Conventional tracer techniques are insensitive since intestinal iron absorption is subject to strong day-to-day variations. This is not the case for iron isotope effects in the blood, which—if not perturbed—are stable during months or even years as recently shown.20
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-07-2807.
Supported by the Lixmar Foundation, Switzerland, and through funding of the mass spectrometry laboratory at the University of Hannover, Germany, by the Volkswagen Stiftung and Deutsche Forschungsgemeinschaft grant BL562-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank R. F. Hurrell, W. Vetter, and F. E. Maly for supporting the study and providing infrastructure; M. H. Balsat, M. Mamberti, P. Greminger, and A. Himmelmann for assistance; and all acknowledged persons for valuable discussions. We thank G. Hinz, MEDIDATA, for help in statistical analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal