Abstract
Primary cutaneous lymphomas are currently classified by the European Organization for Research and Treatment of Cancer (EORTC) classification or the World Health Organization (WHO) classification, but both systems have shortcomings. In particular, differences in the classification of cutaneous T-cell lymphomas other than mycosis fungoides, Sézary syndrome, and the group of primary cutaneous CD30+ lymphoproliferative disorders and the classification and terminology of different types of cutaneous B-cell lymphomas have resulted in considerable debate and confusion. During recent consensus meetings representatives of both systems reached agreement on a new classification, which is now called the WHO-EORTC classification. In this paper we describe the characteristic features of the different primary cutaneous lymphomas and other hematologic neoplasms frequently presenting in the skin, and discuss differences with the previous classification schemes. In addition, the relative frequency and survival data of 1905 patients with primary cutaneous lymphomas derived from Dutch and Austrian registries for primary cutaneous lymphomas are presented to illustrate the clinical significance of this new classification.
Introduction
A variety of T- and B-cell neoplasms can involve the skin, either primarily or secondarily. The term “primary cutaneous lymphoma” refers to cutaneous T-cell lymphomas (CTCLs) and cutaneous B-cell lymphomas (CBCLs) that present in the skin with no evidence of extracutaneous disease at the time of diagnosis. After the gastrointestinal tract, the skin is the second most common site of extranodal non-Hodgkin lymphoma, with an estimated annual incidence of 1:100,000.1
Primary cutaneous lymphomas often have a completely different clinical behavior and prognosis from histologically similar systemic lymphomas, which may involve the skin secondarily, and therefore require different types of treatment. For that reason, recent classification systems for non-Hodgkin lymphomas such as the European Organization for Research and Treatment of Cancer (EORTC) classification for primary cutaneous lymphomas and the World Health Organization (WHO) classification for tumors of hematopoietic and lymphoid tissues included primary cutaneous lymphomas as separate entities.2,3 In the EORTC classification, distinction was made between primary cutaneous lymphomas with an indolent, intermediate, or aggressive clinical behavior. The clinical validity of this classification has been validated by several large studies, including follow-up data of more than 1300 patients with a primary cutaneous lymphoma.2,4,5 Although there was consensus between the EORTC and WHO classifications on the classification of most types of CTCLs, remaining differences between the 2 classification systems, in particular the controversy on the definition and terminology of the different types of CBCLs, has resulted in considerable debate and confusion.6-9
During consensus meetings in Lyon, France (September 2003) and Zurich, Switzerland (January 2004), these differences were resolved by representatives of both classification systems, and a consensus classification was developed (Table 1). In this report we present the new WHO-EORTC classification for cutaneous lymphomas. This review will focus on primary cutaneous lymphomas and a few other conditions that frequently first present in the skin, such as CD4+/CD56+ hematodermic neoplasm (formerly also known as blastic natural killer [NK] cell lymphoma) and adult T-cell leukemia/lymphoma. Other neoplasms that may also first present in the skin in a minority of cases, such as precursor B-lymphoblastic leukemia/lymphoma and acute myeloid leukemia, and secondary cutaneous manifestations of systemic lymphomas, are not discussed, but will be included in the monograph to be published in the WHO Blue Book series in 2005. After a discussion of the 2 most controversial groups of cutaneous lymphomas that were defined differently in the original EORTC and WHO classification schemes, the main features of the different types of primary cutaneous lymphoma are presented. In Table 2 the relative frequency and survival of 1905 patients with primary cutaneous lymphomas derived from Dutch and Austrian cutaneous lymphoma registries are presented, to illustrate the clinical significance of the WHO-EORTC classification.
WHO-EORTC classification of cutaneous lymphomas with primary cutaneous manifestations
Cutaneous T-cell and NK-cell lymphomas |
| Mycosis fungoides |
| MF variants and subtypes |
| Folliculotropic MF |
| Pagetoid reticulosis |
| Granulomatous slack skin |
| Sézary syndrome |
| Adult T-cell leukemia/lymphoma |
| Primary cutaneous CD30+ lymphoproliferative disorders |
| Primary cutaneous anaplastic large cell lymphoma |
| Lymphomatoid papulosis |
| Subcutaneous panniculitis-like T-cell lymphoma* |
| Extranodal NK/T-cell lymphoma, nasal type |
| Primary cutaneous peripheral T-cell lymphoma, unspecified |
| Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma (provisional) |
| Cutaneous γ/δ T-cell lymphoma (provisional) |
| Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma (provisional) |
| Cutaneous B-cell lymphomas |
| Primary cutaneous marginal zone B-cell lymphoma |
| Primary cutaneous follicle center lymphoma |
| Primary cutaneous diffuse large B-cell lymphoma, leg type |
| Primary cutaneous diffuse large B-cell lymphoma, other Intravascular large B-cell lymphoma |
| Precursor hematologic neoplasm |
| CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma)† |
Cutaneous T-cell and NK-cell lymphomas |
| Mycosis fungoides |
| MF variants and subtypes |
| Folliculotropic MF |
| Pagetoid reticulosis |
| Granulomatous slack skin |
| Sézary syndrome |
| Adult T-cell leukemia/lymphoma |
| Primary cutaneous CD30+ lymphoproliferative disorders |
| Primary cutaneous anaplastic large cell lymphoma |
| Lymphomatoid papulosis |
| Subcutaneous panniculitis-like T-cell lymphoma* |
| Extranodal NK/T-cell lymphoma, nasal type |
| Primary cutaneous peripheral T-cell lymphoma, unspecified |
| Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma (provisional) |
| Cutaneous γ/δ T-cell lymphoma (provisional) |
| Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma (provisional) |
| Cutaneous B-cell lymphomas |
| Primary cutaneous marginal zone B-cell lymphoma |
| Primary cutaneous follicle center lymphoma |
| Primary cutaneous diffuse large B-cell lymphoma, leg type |
| Primary cutaneous diffuse large B-cell lymphoma, other Intravascular large B-cell lymphoma |
| Precursor hematologic neoplasm |
| CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma)† |
Relative frequency and disease-specific 5-year survival of 1905 primary cutaneous lymphomas classified according to the WHO-EORTC classification
WHO-EORTC classification . | No. . | Frequency, %* . | Disease-specific 5-year survival, % . |
|---|---|---|---|
| Cutaneous T-cell lymphoma | |||
| Indolent clinical behavior | |||
| Mycosis fungoides | 800 | 44 | 88 |
| Folliculotropic MF | 86 | 4 | 80 |
| Pagetoid reticulosis | 14 | < 1 | 100 |
| Granulomatous slack skin | 4 | < 1 | 100 |
| Primary cutaneous anaplastic large cell lymphoma | 146 | 8 | 95 |
| Lymphomatoid papulosis | 236 | 12 | 100 |
| Subcutaneous panniculitis-like T-cell lymphoma | 18 | 1 | 82 |
| Primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma† | 39 | 2 | 75 |
| Aggressive clinical behavior | |||
| Sézary syndrome | 52 | 3 | 24 |
| Primary cutaneous NK/T-cell lymphoma, nasal-type | 7 | < 1 | NR |
| Primary cutaneous aggressive CD8+ T-cell lymphoma† | 14 | < 1 | 18 |
| Primary cutaneous γ/δ T-cell lymphoma† | 13 | < 1 | NR |
| Primary cutaneous peripheral T-cell lymphoma, unspecified‡ | 47 | 2 | 16 |
| Cutaneous B-cell lymphoma | |||
| Indolent clinical behavior | |||
| Primary cutaneous marginal zone B-cell lymphoma | 127 | 7 | 99 |
| Primary cutaneous follicle center lymphoma | 207 | 11 | 95 |
| Intermediate clinical behavior | |||
| Primary cutaneous diffuse large B-cell lymphoma, leg type | 85 | 4 | 55 |
| Primary cutaneous diffuse large B-cell lymphoma, other | 4 | < 1 | 50 |
| Primary cutaneous intravascular large B-cell lymphoma | 6 | < 1 | 65 |
WHO-EORTC classification . | No. . | Frequency, %* . | Disease-specific 5-year survival, % . |
|---|---|---|---|
| Cutaneous T-cell lymphoma | |||
| Indolent clinical behavior | |||
| Mycosis fungoides | 800 | 44 | 88 |
| Folliculotropic MF | 86 | 4 | 80 |
| Pagetoid reticulosis | 14 | < 1 | 100 |
| Granulomatous slack skin | 4 | < 1 | 100 |
| Primary cutaneous anaplastic large cell lymphoma | 146 | 8 | 95 |
| Lymphomatoid papulosis | 236 | 12 | 100 |
| Subcutaneous panniculitis-like T-cell lymphoma | 18 | 1 | 82 |
| Primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma† | 39 | 2 | 75 |
| Aggressive clinical behavior | |||
| Sézary syndrome | 52 | 3 | 24 |
| Primary cutaneous NK/T-cell lymphoma, nasal-type | 7 | < 1 | NR |
| Primary cutaneous aggressive CD8+ T-cell lymphoma† | 14 | < 1 | 18 |
| Primary cutaneous γ/δ T-cell lymphoma† | 13 | < 1 | NR |
| Primary cutaneous peripheral T-cell lymphoma, unspecified‡ | 47 | 2 | 16 |
| Cutaneous B-cell lymphoma | |||
| Indolent clinical behavior | |||
| Primary cutaneous marginal zone B-cell lymphoma | 127 | 7 | 99 |
| Primary cutaneous follicle center lymphoma | 207 | 11 | 95 |
| Intermediate clinical behavior | |||
| Primary cutaneous diffuse large B-cell lymphoma, leg type | 85 | 4 | 55 |
| Primary cutaneous diffuse large B-cell lymphoma, other | 4 | < 1 | 50 |
| Primary cutaneous intravascular large B-cell lymphoma | 6 | < 1 | 65 |
NR indicates not reached.
Data are based on 1905 patients with a primary cutaneous lymphoma registered at the Dutch and Austrian Cutaneous Lymphoma Group between 1986 and 2002
Primary cutaneous peripheral T-cell lymphoma, unspecified excluding the three provisional entities indicated with a double dagger (‡)
Classification of CTCLs other than mycosis fungoides, Sézary syndrome, and primary cutaneous CD30+ lymphoproliferations
The classification of this remaining group of CTCLs is difficult and confusing, which is not surprising given the heterogeneity and rarity of these tumors. Together, they constitute less than 10% of all CTCLs.2,5 With few exceptions, these lymphomas are clinically aggressive and in most cases systemic chemotherapy is required. In the EORTC classification, most of these lymphomas were grouped as primary cutaneous CD30- large T-cell lymphoma or in the provisional group of primary cutaneous small/medium-sized pleomorphic CTCLs. Distinction between these 2 categories, which is based on the presence of more or less than 30% large neoplastic T cells, was considered useful because several studies demonstrated a significant difference in survival between these 2 groups.2,4,5,10 Recent studies suggest, however, that this favorable prognosis is restricted to small/medium-sized pleomorphic CTCLs with a CD4+ T-cell phenotype, in particular those that present with localized disease, in contrast to those with a CD8+ T-cell phenotype.11 Moreover, the EORTC category of CD30- large-cell CTCLs has become quite heterogeneous through the recognition of new diagnostic categories, such as subcutaneous panniculitis-like T-cell lymphoma (SPTL),12-17 extranodal NK/T-cell lymphoma, nasal type,18-20 CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma),21-24 aggressive epidermotropic CD8+ CTCL,16,25,26 and cutaneous gamma/delta T-cell lymphoma.27-31 In the WHO classification, SPTL, nasal-type NK/T-cell lymphoma, and blastic NK-cell lymphoma were included as separate entities, whereas the other entities were part of the broad category of peripheral T-cell lymphoma (PTL), unspecified.
In the WHO-EORTC classification, extranodal NK/T-cell lymphoma, nasal type, and CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma), are defined as separate entities. Recent studies showed both clinical, histologic, and immunophenotypical differences between cases of SPTL with an α/β T-cell phenotype and those with a γ/δ T-cell phenotype, suggesting that these may represent different entities. Whereas SPTLs with an α/β T-cell phenotype are homogeneous with a rather indolent clinical behavior in many patients, SPTLs with a γ/δ T-cell phenotype overlap with other types of γ/δ+ T/NK-cell lymphoma and invariably run a very aggressive clinical course.14,16,17,31,32
We therefore suggest that the term “SPTL” be restricted for SPTLs with an α/β T-cell phenotype.32 Recent studies have suggested that some disorders can be separated out as provisional entities from the broad group of “PTL, unspecified,” in the WHO classification. These include aggressive epidermotropic CD8+ CTCL, cutaneous gamma-delta T-cell lymphoma (including cases formerly diagnosed as SPTL with a gamma/delta phenotype), and primary cutaneous small-medium CD4+ T-cell lymphoma. In the WHO-EORTC classification the term “PTL, unspecified,” is maintained for remaining cases that do not fit into either of these provisional entities.
Primary cutaneous follicle-center cell lymphoma and primary cutaneous diffuse large B-cell lymphoma
In recent years, the EORTC categories of primary cutaneous follicle center cell lymphoma (PCFCCL) and primary cutaneous large B-cell lymphoma of the leg (PCLBCL-leg) have been the subject of much debate. The term PCFCCL was introduced in 1987 as a term encompassing cutaneous lymphomas that were composed of cells with the morphology of follicle center cells (ie, centroblasts and [large] centrocytes), and that were classified as either centroblastic/centrocytic or centroblastic lymphoma according to criteria of the Kiel classification.33 Whereas small and early lesions may show both small and large neoplastic B cells and many admixed T cells, and may have a partly follicular growth pattern, tumorous lesions generally show a predominance of large B cells, particularly large cleaved or multilobated cells, and less frequently a predominance of typical centroblasts and immunoblasts.33-35 In contrast to nodal follicular lymphomas, PCFCCLs do generally not express bcl-2 and are not typically associated with the t(14;18) translocation.36,37 Clinically, most patients present with localized skin lesions on the head or trunk and, regardless of the histologic subclassification on the basis of growth pattern or number of blast cells, are highly responsive to radiotherapy and have an excellent prognosis.2,4,5,33-35,38
The WHO classification approached these lesions from a different perspective. PCFCCLs with a partly follicular growth pattern were included as a variant of follicular lymphoma and designated cutaneous follicle center lymphoma, while cases with a diffuse growth pattern and a predominance of large centrocytes or centroblasts were generally classified as diffuse large B-cell lymphoma. The designation of this latter group as diffuse large B-cell lymphoma was controversial, since it could lead to overtreatment with muliagent chemotherapy rather than radiotherapy.
PCLBCL-leg was initially recognized as a subgroup of PCFCCL with a somewhat different histology and a more unfavorable prognosis.39 In the EORTC classification it was included as a separate subgroup. PCLBCL-leg particularly affects elderly people and has a higher relapse rate and a more unfavorable prognosis than PCFCCL with a diffuse large B-cell morphology.40 Histologically, they have a predominance of centroblasts and immunoblasts rather than large centrocytes, and consistently strongly express bcl-2 protein.41 Although delineation of this subgroup based on site has been criticized,6,8 recent clinicopathologic and genetic studies further support that PCFCCL and PCLBCL-leg are distinct groups of CBCL.42-46
During the consensus meeting in Zurich, histologic slides of a large number of PCFCCLs and PCLBCL-leg's were reviewed, together with the immunophenotype and clinical data. It was recognized that PCFCCLs as defined in the EORTC classification indeed form a spectrum of disease that includes cases with a follicular, a follicular and diffuse, and a diffuse growth pattern, and a range of cellular composition from predominantly small centrocytes to a predominance of large centrocytes with variable numbers of admixed centroblasts and immunoblasts. This entity will further be referred to as “primary cutaneous follicle center lymphoma” (PCFCL). The group of PCLBCL-leg was also recognized as a separate entity. According to the results of a recent multicenter study, it is also clear that cases with a similar morphology (predominance or cohesive sheets of centroblasts and immunoblasts), immunophenotype (strong expression of bcl-2 and Mum-1/IRF4), and prognosis may arise at sites other than the leg.42 In the WHO-EORTC classification the term “PCLBCL, leg type” is proposed for both lesions on the legs and similar lesions at other skin sites. In addition, the term “PCLBCL, other” is introduced for rare cases of PCLBCL not belonging to the group of PCLBCL, leg type, or PCFCL with a diffuse infiltration of large centrocytes.
Cutaneous T-cell lymphomas
Mycosis fungoides
Definition. Mycosis fungoides (MF) is a commonly epidermotropic CTCL characterized by a proliferation of small-to medium-sized T lymphocytes with cerebriform nuclei. The term MF should be used only for the classical “Alibert-Bazin” type characterized by the evolution of patches, plaques, and tumors, or for variants showing a similar clinical course. MF is the most common type of CTCL and accounts for almost 50% of all primary cutaneous lymphomas (Table 2).
Clinical features. MF typically affects older adults (median age at diagnosis: 55-60 years; male-to-female ratio: 1.6-2.0:1), but may occur in children and adolescents.47-50 MF has an indolent clinical course with slow progression over years or sometimes decades, from patches to more infiltrated plaques and eventually to tumors (Figure 1A). In some patients, lymph nodes and visceral organs may become involved in the later stages of the disease. The initial skin lesions have a predilection for the buttocks and other sun-protected areas. Patients with tumor-stage MF characteristically show a combination of patches, plaques, and tumors, which often show ulceration. If only tumors are present, without preceding or concurrent patches or plaques, a diagnosis of MF is highly unlikely and another type of CTCL should be considered.
Histopathology. Early patch lesions in MF show superficial bandlike or lichenoid infiltrates, mainly consisting of lymphocytes and histiocytes. Atypical cells with small-to medium-sized, highly indented (cerebriform), and sometimes hyperchromatic nuclei are few, and mostly confined to the epidermis (epidermotropism). They characteristically colonize the basal layer of the epidermis either as single, often haloed cells, or in a linear configuration.51 In typical plaques, epidermotropism is generally more pronounced than in the patches (Figure 1B). The presence of intraepidermal collections of atypical cells (Pautrier microabscesses) is a highly characteristic feature, but is observed in only a minority of cases.52 With progression to tumor stage, the dermal infiltrates become more diffuse and epidermotropism may be lost. The tumor cells increase in number and size, showing variable proportions of small, medium-sized, to large cerebriform cells, blast cells with prominent nuclei, and intermediate forms. Transformation to a diffuse large-cell lymphoma that may be either CD30- or CD30+ may occur and is often associated with a poor prognosis.53
Mycosis fungoides. (A) Typical patches and plaques on the trunk. (B) Infiltration of atypical T cells into the epidermis with formation of Pautrier microabscess (hematoxylin and eosin [H&E] staining; original magnification, × 200). The image in panel B was obtained through a Leica DM6000B microscope (Leica, Rijswijk, the Netherlands). Image acquisition was performed with a ProgResC10 camera and software (JenaOptik, Jena, Germany). An HC Plan APO 40×/0.85 objective was used.
Mycosis fungoides. (A) Typical patches and plaques on the trunk. (B) Infiltration of atypical T cells into the epidermis with formation of Pautrier microabscess (hematoxylin and eosin [H&E] staining; original magnification, × 200). The image in panel B was obtained through a Leica DM6000B microscope (Leica, Rijswijk, the Netherlands). Image acquisition was performed with a ProgResC10 camera and software (JenaOptik, Jena, Germany). An HC Plan APO 40×/0.85 objective was used.
Immunophenotype. The neoplastic cells in MF have a mature CD3+, CD4+, CD45RO+, CD8- memory T-cell phenotype. In rare cases of otherwise classical, MF a CD4-, CD8+ mature T-cell phenotype may be seen.25,26,54,55 Such cases have the same clinical behavior and prognosis as CD4+ cases, and should not be considered separately. Demonstration of an aberrant phenotype (eg, loss of pan–T-cell antigens such as CD2, CD3, and CD5) is often seen and is an important adjunct in the diagnosis of MF.54 Expression of cytotoxic proteins (T-cell intracellular antigen-1 [TIA-1], granzyme B) by the neoplastic CD4+ T-cells has been detected in 10% of MF plaques, but is much more common in tumors showing blastic transformation.56
Genetic features. Clonal T-cell receptor gene rearrangements are detected in most cases.51 Many structural and numerical chromosomal abnormalities have been described, in particular in the advanced stages of MF, but recurrent, MF-specific chromosomal translocations have not been identified.51,57 Chromosomal loss at 10q and abnormalities in p15, p16, and p53 tumor suppressor genes are commonly found in patients with MF.51
Prognosis and predictive factors. The prognosis of patients with MF is dependent on stage, and in particular the type and extent of skin lesions and the presence of extracutaneous disease.47-49 Patients with limited patch/plaque-stage MF have a similar life expectancy to an age-, sex-, and race-matched control population. In recent studies, 10-year disease-specific survivals were 97%-98% for patients with limited patch/plaque disease (covering less than 10% of the skin surface), 83% for patients with generalized patch/plaque disease (covering more than 10% of the skin surface), 42% for patients with tumor stage disease, and about 20% for patients with histologically documented lymph node involvement.47-49 Patients with effaced lymph nodes, visceral involvement, and transformation into a large T-cell lymphoma have an aggressive clinical course. Patients usually die of systemic involvement or infections.
Therapy. As long as the disease is confined to the skin, skin-targeted therapies as photo (chemo)–therapy (eg, psoralen plus ultraviolet A [PUVA]), topical application of nitrogen mustard or chlormustine (BCNU), or radiotherapy, including total skin electron beam irradiation, are preferred.58-60 In patients with limited patch-stage disease topical steroids or bexarotene gel can be used. Biologicals such as interferon alpha and other cytokines (eg, interleukin-12 [IL-12]), traditional and new retinoids such as bexarotene, and receptor-targeted cytotoxic fusion proteins (eg, DAB389IL-2; denileukin diftitox), are increasingly used in the treatment of MF.58,60-63 However, the exact place of these new treatments, either as single-agent therapy or in combination with other therapies (eg, PUVA) in the treatment of MF remains to be established. Multiagent chemotherapy is generally used in case of unequivocal lymph node or systemic involvement, or in cases with widespread tumor-stage MF refractory to skin-targeted therapies, but should not be considered in early patch/plaque stage disease.64
Variants and subtypes of mycosis fungoides
Apart from the classical Alibert-Bazin type of MF, many clinical and/or histologic variants have been reported. Clinical variants, such as bullous and hyper- or hypopigmented MF, have a clinical behavior similar to that of classical MF, and therefore are not considered separately. In contrast, folliculotropic MF (MF-associated follicular mucinosis), pagetoid reticulosis, and granulomatous slack skin have distinctive clinicopathologic features, and are therefore considered separately.
Folliculotropic MF. Definition. Folliculotropic MF is a variant of MF characterized by the presence of folliculotropic infiltrates, often with sparing of the epidermis, and preferential involvement of the head and neck area. Most cases show mucinous degeneration of the hair follicles (follicular mucinosis) and are traditionally designated as MF-associated follicular mucinosis. Similar cases, but without follicular mucinosis, have been reported as folliculocentric or pilotropic MF.65 Recent studies showed no differences in clinical presentation and clinical behavior between cases of folliculotropic MF with or without associated follicular mucinosis, and suggested that cases with a preferential infiltration of hair follicles with or without the presence of mucin should be termed “follicular MF” or “folliculotropic MF.”66-68 In the WHO-EORTC classification, folliculotropic MF is preferred as the most appropriate term. From a biologic point of view, the most relevant feature in both cases with and without associated follicular mucinosis is the deep, follicular, and perifollicular localization of the neoplastic infiltrates, which makes them less accessible to skin-targeted therapies.
Clinical features. Folliculotropic MF occurs mostly in adults, but may occasionally affect children and adolescents. Males are more often affected than females. Patients may present with grouped follicular papules, acneiform lesions, indurated plaques, and sometimes tumors, which preferentially involve and are most pronounced in the head and neck area.68 The skin lesions are often associated with alopecia, and sometimes with mucinorrhea. Infiltrated plaques in the eyebrows with concurrent alopecia are a common and highly characteristic finding (Figure 2A). Unlike in classical MF, pruritus is often severe, and may represent a good parameter of disease progression. Secondary bacterial infections are frequently observed.
Histopathology. Characteristic findings include the primarily perivascular and periadnexal localization of the dermal infiltrates with variable infiltration of the follicular epithelium by small, medium-sized, or sometimes large hyperchromatic cells with cerebriform nuclei, and sparing of the epidermis (folliculotropism instead of epidermotropism; Figure 2B). Most cases show mucinous degeneration of the follicular epithelium (follicular mucinosis), as assessed with Alcian blue staining. There is often a considerable admixture of eosinophils and sometimes plasma cells. In most cases the neoplastic T cells have a CD3+, CD4+, CD8- phenotype as in classical MF. An admixture of CD30+ blast cells is common.
Follicular mycosis fungoides. (A) Infiltrated plaques on the forehead and right eyebrow showing hair loss. (B) Diffuse dermal infiltrate surrounding follicular structures. Note infiltrate-free zone beneath epidermis (no epidermotropism) (H&E staining; original magnification, × 25). Image acquisition was performed as described for Figure 1B. An HC FLUOTAR 2.5×/0.07 objective was used.
Follicular mycosis fungoides. (A) Infiltrated plaques on the forehead and right eyebrow showing hair loss. (B) Diffuse dermal infiltrate surrounding follicular structures. Note infiltrate-free zone beneath epidermis (no epidermotropism) (H&E staining; original magnification, × 25). Image acquisition was performed as described for Figure 1B. An HC FLUOTAR 2.5×/0.07 objective was used.
In some cases, prominent infiltration of both follicular epithelium and eccrine sweat glands may be observed.68 Similar cases with prominent infiltration of eccrine sweat glands, often associated with alopecia, have been designated as syringotropic MF.69,70
Prognosis. Recent studies described a disease-specific 5-year survival of approximately 70%-80% in patients with folliculotropic MF (Table 2), which is similar to that of classical tumor-stage MF, but significantly worse than that of patients with classical plaque stage MF.68,71
Treatment. Because of the perifollicular localization of the dermal infiltrates, folliculotropic MF is often less responsive to skin-targeted therapies, such as PUVA and topical nitrogen mustard, than classical plaque-stage MF. In such cases total skin electron beam irradiation is an effective treatment, but sustained complete remissions are rarely achieved.68 Alternatively, PUVA combined with retinoids or interferon alpha may be considered, whereas persistent tumors can be effectively treated with local radiotherapy.
Pagetoid reticulosis. Definition. Pagetoid reticulosis is a variant of MF characterized by the presence of localized patches or plaques with an intraepidermal proliferation of neoplastic T cells. The term pagetoid reticulosis should only be used for the localized type (Woringer-Kolopp type) and not for the disseminated type (Ketron-Goodman type). Generalized cases would currently likely be classified as aggressive epidermotropic CD8+ CTCL, cutaneous gamma/delta-positive T-cell lymphoma, or tumor-stage MF.2,25,72
Clinical features. Patients present with a solitary psoriasiform or hyperkeratotic patch or plaque, which is usually localized on the extremities, and is slowly progressive. In contrast to classical MF, extracutaneous dissemination or disease-related deaths have never been reported.
Histopathology. The typical histologic picture shows a hyperplastic epidermis with marked infiltration by atypical pagetoid cells, singly or arranged in nests. The atypical cells have medium-sized or large, sometimes hyperchromatic and cerebriform nuclei, and abundant, vacuolated cytoplasm. The upper dermis may show a mixed infiltrate of lymphocytes or histiocytes, but does not contain neoplastic T cells.
Immunophenotype. The neoplastic T cells may have either a CD3+, CD4+, CD8- or a CD3+, CD4-, CD8+ phenotype. CD30 is often expressed.73,74
Treatment. The preferred mode of treatment is radiotherapy or surgical excision. In some instances topical nitrogen mustard or topical steroids may be an acceptable alternative.
Granulomatous slack skin. Definition. Granulomatous slack skin (GSS) is an extremely rare subtype of CTCL characterized by the slow development of folds of lax skin in the major skin folds and histologically by a granulomatous infiltrate with clonal T cells.75
Clinical features. This condition shows circumscribed areas of pendulous lax skin with a predilection for the axillae and groins. In approximately one-third of the reported patients, an association with Hodgkin lymphoma was observed, and association with classical MF has also been reported.75-77 Most patients have an indolent clinical course (Table 2).
Histopathology. Fully developed lesions show dense granulomatous dermal infiltrates containing atypical T cells with slightly indented to cerebriform nuclei, macrophages and often many multinucleated giant cells, and destruction of elastic tissue. The epidermis may show focal infiltration by small atypical T cells. The atypical T cells have a CD3+, CD4+, CD8- phenotype.
Therapy. Radiotherapy may be effective, but experience is limited. Rapid recurrences after surgical excision have been reported.
Sézary syndrome
Definition. Sézary syndrome (SS) is defined historically by the triad of erythroderma, generalized lymphadenopathy, and the presence of neoplastic T cells (Sézary cells) in skin, lymph nodes, and peripheral blood.78 In a recent report of the International Society for Cutaneous Lymphomas (ISCL), criteria recommended for the diagnosis of SS include one or more of the following: an absolute Sézary cell count of least 1000 cells/mm3; demonstration of immunophenotypical abnormalities (an expanded CD4+ T-cell population resulting in a CD4/CD8 ratio more than 10, loss of any or all of the T-cell antigens CD2, CD3, CD4, and CD5, or both); or the demonstration of a T-cell clone in the peripheral blood by molecular or cytogenetic methods.79 It is acknowledged that SS is part of a broader spectrum of erythrodermic CTCL, and that alternative staging systems for assessment of the degree of peripheral blood involvement in these erythrodermic CTCLs have been proposed.79,80 However, until the results of an ISCL study investigating the clinical validity of these proposals are available, demonstration of a T-cell clone (preferably of the same T-cell clone in skin and peripheral blood) in combination with one of the above-mentioned cytomorphologic or immunophenotypic criteria are suggested as minimal criteria for the diagnosis of SS to exclude patients with benign inflammatory conditions simulating SS.
Clinical features. SS is a rare disease and occurs exclusively in adults. It is characterized by erythroderma, which may be associated with marked exfoliation, edema, and lichenification, and which is intensely pruritic. Lymphadenopathy, alopecia, onychodystrophy, and palmoplantar hyperkeratosis are common findings.78
Histopathology. The histological features in SS may be similar to those in MF. However, the cellular infiltrates in SS are more often monotonous, and epidermotropism may sometimes be absent. In up to one-third of biopsies from patients with otherwise classical SS the histologic picture may be nonspecific.81,82 Involved lymph nodes characteristically show a dense, monotonous infiltrate of Sézary cells with effacement of the normal lymph node architecture.83 Bone marrow may be involved, but the infiltrates are often sparse and mainly interstitial.84
Immunophenotype. The neoplastic T cells have a CD3+, CD4+, CD8- phenotype. In cases with a predominant CD3+, CD4-, CD8+ T-cell population in the skin and peripheral blood, the diagnosis of actinic reticuloid should be considered.85 Circulating Sézary cells often show loss of CD7 and CD26.79
Genetic features. T-cell receptor genes are clonally rearranged. Demonstration of clonal T cells in the peripheral blood is considered as an important diagnostic criterion allowing differentiation between SS and benign forms of erythroderma.2,79,80 Recurrent chromosomal translocations have not been detected in SS, but complex karyotypes are common.86,87 Several studies have identified a consistent pattern of identical chromosomal abnormalities in SS, which was almost identical to that in MF, suggesting that both conditions represent parts of the same spectrum of disease with a similar pathogenesis.88,89 Chromosomal amplification of the JUNB gene, a member of the activator protein-1 (AP-1) transcription factor complex involved in cell proliferation and T helper 2 (Th2) cytokine expression by T cells, has been identified in SS.90,91
Prognosis and predictive factors. The prognosis is generally poor, with a median survival between 2 and 4 years, depending on the exact definition used.2,80 The disease-specific 5-year survival of 52 SS patients included in the Dutch and Austrian registries was 24% (Table 2). Most patients die of opportunistic infections that are due to immunosuppression.
Therapy. Extracorporeal photopheresis (ECP), either alone or in combination with other treatment modalities (eg, interferon alpha), has been reported as an effective treatment in SS and erythrodermic MF, with overall response rates of 30%-80%, and complete response rates of 14%-25%.92,93 This great variation in response rates may reflect differences in patient selection and/or concurrent therapies. The suggested superiority of ECP over the traditional low-dose chemotherapy regimens has not yet been substantiated by controlled randomized trials.93 Beneficial results have also reported of interferon alpha, either alone or in combination with PUVA therapy, prolonged treatment with a combination of low-dose chlorambucil (2-4 mg/d) and prednisone (10-20 mg/d) or with methotrexate (5-25 mg/wk), but complete responses are uncommon. Skin-directed therapies like PUVA or potent topical steroids may be used as adjuvant therapy. Recent studies report beneficial effects of bexarotene and alemtuzumab (anti-CD52) but the long-term effects of these therapies remain to be established.58,62,94
Adult T-cell leukemia/lymphoma
Definition. Adult T-cell leukemia/lymphoma (ATLL) is a T-cell neoplasm etiologically associated with the human T-cell leukemia virus 1 (HTLV-1). Skin lesions are generally a manifestation of widely disseminated disease. However, a slowly progressive form that may have only skin lesions has been described (smoldering variant).95
Clinical features. ATLL is endemic in areas with a high prevalence of HTLV-1 in the population, such as southwest Japan, the Caribbean islands, South America, and parts of Central Africa. ATLL develops in 1% to 5% of seropositive individuals after more than 2 decades of viral persistence. Most patients present with acute ATLL characterized by the presence of leukemia, lymphadenopathy, organomegaly, hypercalcemia, and in about 50% skin lesions, most commonly nodules or tumors (33%), generalized papules (22%), or plaques (19%).96 Chronic and smoldering variants frequently present with skin lesions, which may closely resemble MF, whereas circulating neoplastic T cells are few or absent.
Histopathology. Skin lesions show a superficial or more diffuse infiltration of medium-sized to large T cells with pleomorphic or polylobated nuclei, which often display marked epidermotropism. The histologic picture may be indistinguishable from MF. Skin lesions in the smoldering type may show sparse dermal infiltrates with only slightly atypical cells. The neoplastic T cells express a CD3+, CD4+, CD8- phenotype. CD25 is highly expressed.95,96
Genetic features. T-cell receptor genes are clonally rearranged. Clonally integrated HTLV-1 genes are found in all cases, and are useful in differentiating between chronic or smoldering variants of ATLL and classical MF or SS.97
Prognosis and predictive factors. Clinical subtype is the main prognostic factor. Survival in acute and lymphomatous variants ranges from 2 weeks to more than 1 year. Chronic and smoldering forms have a more protracted clinical course and a longer survival, but transformation into an acute phase with an aggressive course may occur.95,96
Primary cutaneous CD30+ lymphoproliferative disorders
Primary cutaneous CD30+ lymphoproliferative disorders (LPDs) are the second most common group of CTCLs, accounting for approximately 30% of CTCLs (Table 2). This group includes primary cutaneous anaplastic large cell lymphoma (C-ALCL), lymphomatoid papulosis (LyP), and borderline cases. It is now generally accepted that C-ALCL and LyP form a spectrum of disease, and that histologic criteria alone are often insufficient to differentiate between these 2 ends of this spectrum.100 The clinical appearance and course are used as decisive criteria for the definite diagnosis and choice of treatment. The term “borderline case” refers to cases in which, despite careful clinicopathologic correlation, a definite distinction between C-ALCL and LyP cannot be made. Clinical examination during further follow-up will generally disclose whether the patient has C-ALCL or LyP.101
Primary cutaneous anaplastic large-cell lymphoma. Definition. Primary cutaneous anaplastic large cell lymphoma. (C-ALCL) is composed of large cells with an anaplastic, pleomorphic, or immunoblastic cytomorphology and expression of the CD30 antigen by the majority (more than 75%) of tumor cells.100 There is no clinical evidence or history of LyP, MF, or another type of CTCL.
Clinical features. C-ALCL affects mainly adults with a male to female ratio of 2-3:1. Most patients present with solitary or localized nodules or tumors, and sometimes papules, and often show ulceration (Figure 3).101,102 Multifocal lesions are seen in about 20% of the patients. The skin lesions may show partial or complete spontaneous regression, as in LyP. These lymphomas frequently relapse in the skin. Extracutaneous dissemination occurs in approximately 10% of the patients, and mainly involves the regional lymph nodes.
Histopathology. There is a diffuse, nonepidermotropic infiltrate with cohesive sheets of large CD30+ tumor cells. In most cases the tumor cells have the characteristic morphology of anaplastic cells, showing round, oval, or irregularly shaped nuclei, prominent eosinophilic nucleoli, and abundant cytoplasm (Figure 3). Less commonly (20%-25%), they have a nonanaplastic (pleomorphic or immunoblastic) appearance.101,103 Reactive lymphocytes are often present at the periphery of the lesions. Ulcerating lesions may show a LyP-like histology with an abundant inflammatory infiltrate of reactive T cells, histiocytes, eosinophils and/or neutrophils, and relatively few CD30+ cells. In such cases epidermal hyperplasia may be prominent.
Primary cutaneous CD30+lymphoproliferative disease (pcCD30+ LPD). (A) Diffuse dermal infiltrate of large atypical cells admixed with small lymphocytes. (H&E staining; original magnification, × 300). (B) The large atypical cells are strongly positive for CD30. (C-D) The histologic picture in panels A and B can be found both in C-ALC and in LyP. The final diagnosis depends on the clinical presentation. In combination with the solitary tumor of the patient shown in panel C the definite diagnosis will be C-ALC; in combination with recurrent, self-healing papulonecrotic skin lesions (D), the final diagnosis is LyP. Image acquisition for panels A and B was performed as described for Figure 1B. An HC Plan APO 20×/0.70 objective was used.
Primary cutaneous CD30+lymphoproliferative disease (pcCD30+ LPD). (A) Diffuse dermal infiltrate of large atypical cells admixed with small lymphocytes. (H&E staining; original magnification, × 300). (B) The large atypical cells are strongly positive for CD30. (C-D) The histologic picture in panels A and B can be found both in C-ALC and in LyP. The final diagnosis depends on the clinical presentation. In combination with the solitary tumor of the patient shown in panel C the definite diagnosis will be C-ALC; in combination with recurrent, self-healing papulonecrotic skin lesions (D), the final diagnosis is LyP. Image acquisition for panels A and B was performed as described for Figure 1B. An HC Plan APO 20×/0.70 objective was used.
Immunophenotype. The neoplastic cells generally show an activated CD4+ T-cell phenotype with variable loss of CD2, CD5, and/or CD3, and frequent expression of cytotoxic proteins (granzyme B, TIA-1, perforin).104,105 Some cases (less than 5%) have a CD8+ T-cell phenotype. CD30 must be expressed by the majority (more than 75%) of the neoplastic T cells.106 Unlike systemic CD30+ lymphomas, most C-ALCLs express the cutaneous lymphocyte antigen (CLA), but do not express epithelial membrane antigen (EMA) and anaplastic lymphoma kinase (ALK), indicative of the 2;5 chromosomal translocation or its variants.2,107,108 Unlike Hodgkin and Reed-Sternberg cells in Hodgkin disease, staining for CD15 is generally negative. Coexpression of CD56 is observed in rare cases, but does not appear to be associated with an unfavorable prognosis.109
Genetic features. Most cases show clonal rearrangement of T-cell receptor genes. The (2;5)(p23;q35) translocation and its variants, which is a characteristic feature of systemic ALCL, is not or rarely found in C-ALCL.108
Prognosis and predictive factors. The prognosis is usually favorable with a 10-year disease-related survival exceeding 90%.101,102 Patients presenting with multifocal skin lesions and patients with involvement of only regional lymph nodes have a similar prognosis to patients with only skin lesions.101 No difference in clinical presentation, clinical behavior, or prognosis is found between cases with an anaplastic morphology and cases with a nonanaplastic (pleomorphic or immunoblastic) morphology.101,103
Treatment. Radiotherapy or surgical excision is the first choice of treatment in patients presenting with a solitary or few localized nodules or tumors. Patients presenting with multifocal skin lesions can best be treated with radiotherapy in case of only a few lesions, or with low-dose methotrexate, as in LyP.101,110 Patients presenting with or developing extracutaneous disease or rare patients with rapidly progressive skin disease should be treated with doxorubicin-based multiagent chemotherapy.
Lymphomatoid papulosis. Definition. Lymphomatoid papulosis (LyP) is defined as a chronic, recurrent, self-healing papulonecrotic or papulonodular skin disease with histologic features suggestive of a (CD30+) malignant lymphoma.
Clinical features. LyP generally occurs in adults (median age, 45 years; male-to-female ratio, 1.5:1), but may occur in children as well.101,102,111,112 LyP is characterized by the presence of papular, papulonecrotic, and/or nodular skin lesions at different stages of development, predominantly on the trunk and limbs. Individual skin lesions disappear within 3 to 12 weeks, and may leave behind superficial scars (Figure 3). The duration of the disease may vary from several months to more than 40 years. In up to 20% of patients LyP may be preceded by, associated with, or followed by another type of malignant (cutaneous) lymphoma, generally MF, a (C-)ALCL, or Hodgkin lymphoma.101
Histopathology. The histologic picture of LyP is extremely variable, and in part correlates with the age of the biopsied skin lesion. Three histologic subtypes of LyP (types A, B, and C) have been described, which represent a spectrum with overlapping features.100,101,112 In LyP type A lesions, scattered or small clusters of large, sometimes multinucleated or Reed-Sternberg–like, CD30+ cells are intermingled with numerous inflammatory cells, such as histiocytes, small lymphocytes, neutrophils, and/or eosinophils. LyP type C lesions demonstrate a monotonous population or large clusters of large CD30+ T cells with relatively few admixed inflammatory cells. LyP type B is uncommon (less than 10%) and is characterized by an epidermotropic infiltrate of small atypical cells with cerebriform nuclei similar to that observed in MF.
Immunophenotype. The large atypical cells in the LyP type A and type C lesions have the same phenotype as the tumor cells in C-ALCL.113 The atypical cells with cerebriform nuclei in the LyP type B lesions have a CD3+, CD4+, CD8- phenotype and do not express CD30 antigen.
Genetic features. Clonally rearranged T-cell receptor genes have been detected in approximately 60%-70% of LyP lesions.114 Identical rearrangements have been demonstrated in LyP lesions and associated lymphomas.115 The (2;5)(p23;q35) translocation is not detected in LyP.108
Prognosis and predictive factors. LyP has an excellent prognosis. In a recent study of 118 LyP patients only 5 (4%) patients developed a systemic lymphoma, and only 2 (2%) patients died of systemic disease over a median follow-up period of 77 months.101 Risk factors for the development of a systemic lymphoma are unknown.
Treatment. Since a curative therapy is not available and none of the available treatment modalities affects the natural course of the disease, the short-term benefits of active treatment should be balanced carefully against the potential side effects.101 Low-dose oral methotrexate (5-20 mg/wk) is the most effective therapy to suppress the development of new skin lesions.110 Beneficial effects have been reported of PUVA and topical chemotherapy. However, after discontinuation of treatment the disease generally relapses within weeks or months. Therefore, in patients with relatively few and nonscarring lesions, long-term follow-up without active treatment should be considered.
Subcutaneous panniculitis-like T-cell lymphoma
Definition. Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is defined as a cytotoxic T-cell lymphoma characterized by the presence of primarily subcutaneous infiltrates of small, medium-sized, or large pleomorphic T cells and many macrophages, predominantly affecting the legs, and often complicated by a hemophagocytic syndrome.12 Recent studies suggest that at least 2 groups of SPTL with a different histology, phenotype, and prognosis can be distinguished. Cases with an α/β+ T-cell phenotype are usually CD8+, are restricted to the subcutaneous tissue (no dermal and/or epidermal involvement), and often run an indolent clinical course.14,16,17,32 In contrast, SPTL with a γ/δ T-cell phenotype, approximately 25% of all cases, are typically CD4-, CD8-, and often coexpress CD56, the neoplastic infiltrates are not confined to the subcutaneous tissue, but may involve the epidermis and/or dermis as well, and invariably have a very poor prognosis.13,14,16,17,116 In the WHO-EORTC classification the term “SPTL” is only used for cases with an α/β+ T-cell phenotype, whereas cases with a γ/δ+ T-cell phenotype are included in the category of cutaneous γ/δ T-cell lymphomas.32
Clinical features. SPTL occurs in adults as well as in young children, and both sexes are equally affected. Patients generally present with solitary or multiple nodules and plaques, which mainly involve the legs, or may be more generalized (Figure 4A). Ulceration is uncommon. Systemic symptoms such as fever, fatigue, and weight loss may be present. The disease may be complicated by a hemophagocytic syndrome, which is generally associated with a rapidly progressive course.117 However, a hemophagocytic syndrome is probably less common than in cutaneous γ/δ T-cell lymphomas with panniculitis-like lesions. Dissemination to extracutaneous sites is rare. SPTL may be preceded for years or decades by an seemingly benign panniculitis.15,17,117
Histopathology. Histopathology reveals subcutaneous infiltrates simulating a panniculitis showing small, medium-sized, or sometimes large pleomorphic T cells with hyperchromatic nuclei and often many macrophages. The overlying epidermis and dermis are typically uninvolved.16,116 Rimming of individual fat cells by neoplastic T cells is a helpful, though not completely specific, diagnostic feature (Figure 4B,C).32 Necrosis, karyorrhexis, and cytophagocytosis are common findings. In the early stages the neoplastic infiltrates may lack significant atypia and a heavy inflammatory infiltrate may predominate.15,17,117
Immunophenotype. These lymphomas show a α/β+, CD3+, CD4-, CD8+ T-cell phenotype, with expression of cytotoxic proteins (Figure 4D).14,16,17,32 CD30 and CD56 are rarely, if ever, expressed.
Subcutaneous panniculitis-like T-cell lymphoma. (A) Deeply seated nodular skin lesions and residual lipodystrophy after disappearance of the skin lesions. (B) Infiltrates are almost exclusively localized in subcutaneous tissue resembling a lobular panniculitis (H&E staining; original magnification, × 25). (C) Rimming of individual fat cells by neoplastic T cells (H&E staining; original magnification, × 480). (D) Tumor cells show positive staining for CD8. Image acquisition for panels B-D was performed as described for Figure 1B. An HC FLUOTAR 2.5×/0.07 objective was used for panel B; an HC Plan APO 40×/0.85 objective was used for panels C and D.
Subcutaneous panniculitis-like T-cell lymphoma. (A) Deeply seated nodular skin lesions and residual lipodystrophy after disappearance of the skin lesions. (B) Infiltrates are almost exclusively localized in subcutaneous tissue resembling a lobular panniculitis (H&E staining; original magnification, × 25). (C) Rimming of individual fat cells by neoplastic T cells (H&E staining; original magnification, × 480). (D) Tumor cells show positive staining for CD8. Image acquisition for panels B-D was performed as described for Figure 1B. An HC FLUOTAR 2.5×/0.07 objective was used for panel B; an HC Plan APO 40×/0.85 objective was used for panels C and D.
Genetic features. The neoplastic T cells show clonal T-cell receptor (TCR) gene rearrangements. Specific genetic abnormalities have not been identified. Epstein-Barr virus (EBV) is absent.
Prognosis and predictive factors. In contrast to prior reports indicating that patients with a SPTL have a rapidly fatal course, recent studies suggest that many patients with a SPTL (with a CD8+, α/β+ T-cell phenotype) have a protracted clinical course with recurrent subcutaneous lesions but without extracutaneous dissemination or the development of a hemophagocytic syndrome.17,32 Based on the few published reports in which appropriate phenotyping was performed, the 5-year survival of such patients may be more than 80%,32 which is consistent with the data presented in Table 2.
Extranodal NK/T-cell lymphoma, nasal type
Definition. Extranodal NK/T-cell lymphoma, nasal type, is a nearly always EBV+ lymphoma of small, medium, or large cells usually with an NK-cell, or more rarely a cytotoxic T-cell, phenotype. The skin is the second most common site of involvement after the nasal cavity/nasopharynx, and skin involvement may be a primary or secondary manifestation of the disease. Since both groups show an aggressive clinical behavior and require the same type of treatment, distinction between “primary” and “secondary” cutaneous involvement seems not useful for this category.16,19,32,118,119 Therefore, the WHO classification–derived term “extranodal NK/T-cell lymphoma, nasal type,” rather than “(primary) cutaneous NK/T-cell lymphoma, nasal type,” is preferred.
Clinical features. Patients are adults with a predominance of males. This lymphoma is more common in Asia, Central America, and South America. Patients generally present with multiple plaques or tumors preferentially on the trunk and extremities, or in the case of nasal NK/T-cell lymphoma with a midfacial destructive tumor, previously also designated “lethal midline granuloma.”18,19,32,119-122 Ulceration is common. Systemic symptoms such as fever, malaise, and weight loss may be present, and some cases are accompanied by a hemophagocytic syndrome. The disease is closely related to aggressive NK-cell leukemia, which also may have cutaneous manifestations, and is also EBV associated.
Histopathology. These lymphomas show dense infiltrates involving the dermis and often the subcutis. Epidermotropism may be present. Prominent angiocentricity and angiodestruction are often accompanied by extensive necrosis.18,19 NK/T-cell lymphoma has a broad cytologic spectrum ranging from small to large cells, with most cases consisting of medium-sized cells. The cells may have irregular or oval nuclei, moderately dense chromatin, and pale cytoplasm. In some cases a heavy inflammatory infiltrate of small lymphocytes, histiocytes, plasma cells, and eosinophils can be seen.
Immunophenotype. The neoplastic cells express CD2, CD56, cytoplasmic CD3ϵ, and cytotoxic proteins (TIA-1, granzyme B, perforin), but lack surface CD3.122 In rare CD56- cases detection of EBV by in situ hybridization and expression of cytotoxic proteins are required for diagnosis (Figure 5B).3 Latent membrane protein-1 (LMP-1) is inconsistently expressed.
Genetic features. The T-cell receptor is usually in germline configuration, but can be rearranged in rare tumors with a cytotoxic T-cell phenotype. EBV is expressed almost in all cases, suggesting a pathogenetic role of this virus.18
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. (A) Typical presentation with nodules and tumors showing central ulceration. (B-D) Tumor cells show striking epidermotropism (H&E staining; original magnification, × 150), and strongly express CD8 (C) and TIA-1 (D). Image acquisition for panels B-D was performed as described for Figure 1B. An HC Plan APO 10×/0.40 objective lens was used.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. (A) Typical presentation with nodules and tumors showing central ulceration. (B-D) Tumor cells show striking epidermotropism (H&E staining; original magnification, × 150), and strongly express CD8 (C) and TIA-1 (D). Image acquisition for panels B-D was performed as described for Figure 1B. An HC Plan APO 10×/0.40 objective lens was used.
Prognosis and predictive factors. Nasal-type NK/T-cell lymphoma presenting in the skin is a highly aggressive tumor with a median survival of less than 12 months.18,19,120,121 The most important factor predicting poor outcome is the presence of extracutaneous involvement at presentation. In patients presenting with only skin lesions, a median survival of 27 months was reported, compared with 5 months for patients presenting with cutaneous and extracutaneous disease.121 CD30+, CD56+ cases reported to have a better prognosis may have been examples of C-ALCL with coexpression of CD56.20
Therapy. Systemic chemotherapy is the first choice of treatment, but the results are disappointing.120,121
Variant. Hydroa vacciniforme-like CTCL is a rare type of EBV-associated lymphoma of CD8+ cytotoxic T cells, which affects children almost exclusively in Latin America and Asia.123-125 Patients present with a papulovesicular eruption clinically resembling hydroa vacciniforme, particularly on the face and upper extremities (sunexposed areas). The prognosis is poor.
Primary cutaneous peripheral T-cell lymphoma, unspecified
PTL, unspecified, in the WHO classification represent a heterogeneous group which includes all T-cell neoplasms that do not fit into any of the better defined subtypes of T-cell lymphoma/leukemia. Recent studies have suggested that primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, cutaneous gamma-delta T-cell lymphoma, and primary cutaneous small-medium CD4+ T-cell lymphoma can be separated out as provisional entities. For the remaining diseases that do not fit into either of these provisional entities the designation PTL, unspecified, is maintained. In all cases a diagnosis of MF must be ruled out by complete clinical examination and an accurate clinical history.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma (provisional entity). Definition. Primary cutaneous aggressive epidermotropic CD8+ CTCL is characterized by a proliferation of epidermotropic CD8+ cytotoxic T-cells and an aggressive clinical behavior.25,26 Differentiation from other types of CTCL expressing a CD8+ cytotoxic T-cell phenotype, as observed in more than 50% of patients with pagetoid reticulosis, and in rare cases of MF, LyP, and C-ALCL, is based on the clinical presentation and clinical behavior.25 In these latter conditions, no difference in clinical presentation or prognosis between CD4+ and CD8+ cases is found.
Clinical features. Clinically, these lymphomas are characterized by the presence of localized or disseminated eruptive papules, nodules, and tumors showing central ulceration and necrosis or by superficial, hyperkeratotic patches and plaques (Figure 5A).16,25 The clinical features are very similar to those observed in patients with a cutaneous γ/γ T-cell lymphoma and cases described as generalized pagetoid reticulosis (Ketron-Goodman type) in the past.32 These lymphomas may disseminate to other visceral sites (lung, testis, central nervous system, oral mucosa), but lymph nodes are often spared.25
Histopathology. Histologically, these lymphomas show an acanthotic or atrophic epidermis, necrotic keratinocytes, ulceration, and variable spongiosis, sometimes with blister formation.16,25 Epidermotropism is often pronounced ranging from a linear distribution to a pagetoid pattern throughout the epidermis (Figure 5B). Invasion and destruction of adnexal skin structures are commonly seen. Angiocentricity and angioinvasion may be present. Tumor cells are small-medium or medium-large with pleomorphic or blastic nuclei.
Immunophenotype. The tumor cells have a betaF1+, CD3+, CD8+, granzyme B+, perforin+, TIA-1+, CD45RA+, CD45RO-, CD2-, CD4-, CD5-, CD7-/+ phenotype (Figure 5C and 5D).11,16,25,26,32 EBV is generally negative.
Genetic features. The neoplastic T cells show clonal TCR gene rearrangements. Specific genetic abnormalities have not been described.
Prognosis and predictive factors. These lymphomas often have an aggressive clinical course with a median survival of 32 months.25 There is no difference in survival between cases with a small- or large-cell morphology.11
Therapy. Patients are generally treated with doxorubicin-based multiagent chemotherapy.
Cutaneous gamma/delta T-cell lymphoma (provisional entity). Definition. Cutaneous gamma/delta T-cell lymphoma (CGD-TCL) is a lymphoma composed of a clonal proliferation of mature, activated gamma/delta T cells with a cytotoxic phenotype. This group includes cases previously known as SPTL with a gamma/delta phenotype. A similar and possibly related condition may present primarily in mucosal sites.28 Whether cutaneous and mucosal gamma/delta TCL are all part of a single disease (ie, muco-cutaneous gamma/delta TCL) is not yet clear.29,122 Distinction between “primary” and “secondary” cutaneous cases is not useful in this group, since both groups have a very grim prognosis.
Clinical features. Patients with CGD-TCL generally present with disseminated plaques and/or ulceronecrotic nodules or tumors, particularly on the extremities, but other sites may be affected as well.27-31 Involvement of mucosal and other extranodal sites is frequently observed,28 but involvement of lymph nodes, spleen, or bone marrow is uncommon. A hemophagocytic syndrome may occur in patients with panniculitis-like tumors.12,31
Histopathology. Three major histologic patterns of involvement can be present in the skin: epidermotropic, dermal, and subcutaneous. Often more than one histologic pattern is present in the same patient in different biopsy specimens or within a single biopsy specimen.27,31 The neoplastic cells are generally medium to large in size with coarsely clumped chromatin. Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. Apoptosis and necrosis are common, often with angioinvasion. The subcutaneous cases may show rimming of fat cells, similar to SPTL of alpha/beta origin.
Immunophenotype. The tumor cells characteristically have a betaF1-, CD3+, CD2+, CD5-, CD7+/-, CD56+ phenotype with strong expression of cytotoxic proteins. Most cases lack both CD4 and CD8, though CD8 may be expressed in some cases.29,31 In frozen sections the cells are strongly positive for TCR-delta. If only paraffin sections are available, the absence of betaF1 may be used to infer a gamma/delta origin under appropriate circumstances.14,30
Genetic features. The cells show clonal rearrangement of the TCR gamma gene. TCR beta may be rearranged or deleted, but is not expressed. EBV is generally negative.28,31
Prognosis and predictive factors. Most patients have aggressive disease resistant to multiagent chemotherapy and/or radiation.27-32 In a recent series of 33 patients, a median survival of 15 months was noted.31 This study showed a trend for decreased survival for patients who had subcutaneous fat involvement compared with patients who had epidermal or dermal disease only.
Therapy. Patients should be treated with systemic chemotherapy, but the results are often disappointing.
Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma (provisional entity). Definition. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma is a CTCL defined by a predominance of small-to medium-sized CD4+ pleomorphic T cells without (a history of) patches and plaques typical of MF, and in most cases, a favorable clinical course.2 In contrast to the EORTC classification, in the WHO-EORTC classification the term “small/medium-sized pleomorphic CTCL” is restricted to cases with a CD4+ T-cell phenotype. Cases with a CD3+, CD4-, CD8+ phenotype usually have a more aggressive clinical course are included in the group of aggressive epidermotropic CD8+ CTCLs.11
Clinical features. Characteristically, these lymphomas present with a solitary plaque or tumor, generally on the face, the neck, or the upper trunk (Figure 6A). Less commonly, they present with one or several papules, nodules, or tumors.10,11,126-128
Histologic features. These lymphomas show dense, diffuse, or nodular infiltrates within the dermis with a tendency to infiltrate the subcutis. Epidermotropism may be present focally. There is a predominance of small/medium-sized pleomorphic T cells (Figure 6B). A small proportion (less than 30%) of large pleomorphic cells may be present.10 In some cases a considerable admixture with small reactive lymphocytes and histiocytes may be observed.
Immunophenotype. By definition these lymphomas have a CD3+, CD4+, CD8-, CD30- phenotype, sometimes with loss of pan–T-cell markers. Cytotoxic proteins are generally not expressed.11
Genetic features. The TCR genes are clonally rearranged.126,128 No consistent cytogenetic abnormalities have yet been identified. Demonstration of an aberrant T-cell phenotype and clonality are useful criteria to differentiate these small/medium-sized pleomorphic CTCLs from pseudo–T-cell lymphomas, which may also present with a solitary plaque or nodule.129
Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma. (A) Presentation with a solitary tumor on the scalp. (B) Diffuse dermal infiltrate mainly composed of small pleomorphic T cells with few scattered blasts cells (H&E staining; original magnification, × 750). Image acquisition was performed as described for Figure 1B. An HC FLUOTAR 63×/0.90 objective lens was used.
Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma. (A) Presentation with a solitary tumor on the scalp. (B) Diffuse dermal infiltrate mainly composed of small pleomorphic T cells with few scattered blasts cells (H&E staining; original magnification, × 750). Image acquisition was performed as described for Figure 1B. An HC FLUOTAR 63×/0.90 objective lens was used.
Prognosis and predictive factors. These lymphomas have a rather favorable prognosis, with an estimated 5-year survival of approximately 60% to 80% (Table 2).2,4,5,10,11,126-128 Particularly, cases presenting with a solitary or localized skin lesions seem to have an excellent prognosis.11
Therapy. In patients with solitary localized skin lesions, surgical excision or radiotherapy is the preferred mode of treatment. Cyclophosphamide as single-agent therapy and interferon alpha have been reported effective in patients with more generalized skin disease.128 However, the optimal treatment for this group has still to be defined.
Primary cutaneous peripheral T-cell lymphoma, unspecified. Definition. The designation PTL, unspecified, is maintained for cutaneous T-cell lymphomas that do not fit into any of the better defined subtypes of CTCL. Hence, other categories of T-cell lymphoma must be excluded. These include the 3 provisional entities described earlier.
Clinical features. Patients are commonly adults, who present with solitary, localized, or more frequently generalized nodules or tumors.2,4,10,11 No sites of predilection have been recorded.
Histopathology. Skin lesions show nodular or diffuse infiltrates with variable numbers of medium-to large-sized pleomorphic or immunoblast-like T cells. Epidermotropism is generally mild or absent. Large neoplastic cells represent at least 30% of the total tumor cell population.10
Immunophenotype. Most cases show an aberrant CD4+ T-cell phenotype with variable loss of pan–T-cell antigens. CD30 staining is negative or restricted to a few scattered tumor cells. Rare cases may show coexpression of CD56. Expression of cytotoxic proteins is uncommon.11
Prognosis and predictive factors. The prognosis is generally poor, with 5-year survival rates of less than 20% (Table 2).2,4,5,10,11 No statistical differences in survival were found between cases presenting with solitary/localized lesions and cases presenting with generalized skin lesions.11
Treatment. Patients should be treated with multiagent chemotherapy.
Precursor hematologic neoplasm
CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma)
Definition. In the WHO classification, blastic NK-cell lymphoma was included as a clinically aggressive neoplasm with a high incidence of cutaneous involvement and risk of leukemic dissemination. The blastic cytologic appearance and CD56 expression initially suggested an NK-precursor origin.3 More recent studies suggest derivation from a plasmacytoid dendritic cell precursor.23,24 “CD4+/CD56+ hematodermic neoplasm”22 and “early plasmacytoid dendritic cell leukemia/lymphoma”23 have been suggested as more appropriate terms for this condition.
Clinical features. CD4+/CD56+ hematodermic neoplasm commonly presents in the skin with solitary or multiple nodules or tumors with or without concurrent extracutaneous localizations (Figure 7A).21-24,32 About half of the patients have nodal or bone marrow involvement at presentation.23,121 Most patients presenting with only skin lesions rapidly develop involvement of bone marrow, peripheral blood, lymph nodes, and extranodal sites.23,121 CD4+/CD56+ hematodermic neoplasm should be differentiated above all from myelomonocytic leukemia cutis, and are conceptually similar to so-called “aleukemic leukemia cutis.”32
CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma). (A) Presentation with large tumor on the back. (B) Monotonous infiltration of medium-sized tumor cells (H&E staining; original magnification, × 480). (C) The tumor cells strongly express CD56. Image acquisition for panels B and C was performed as described for Figure 1B. An HC Plan APO 40×/0.85 objective lens was used.
CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma). (A) Presentation with large tumor on the back. (B) Monotonous infiltration of medium-sized tumor cells (H&E staining; original magnification, × 480). (C) The tumor cells strongly express CD56. Image acquisition for panels B and C was performed as described for Figure 1B. An HC Plan APO 40×/0.85 objective lens was used.
Histopathology. These lymphomas show nonepidermotropic, monotonous infiltrates of medium-sized cells with finely dispersed chromatin, and absent or indistinct nucleoli resembling lymphoblasts or myeloblasts (Figure 7B).3,21-24 The cells have sparse cytoplasm. Mitotic figures are frequent. Inflammatory cells are absent. There is generally no necrosis or angioinvasion.
Immunophenotype. The tumor cells usually have a CD4+, CD56+, CD8-, CD7+/-, CD2-/+, CD45RA+ phenotype, but do not express surface and cytoplasmic CD3 or cytotoxic proteins (Figure 7C).3,23 TdT and CD68 may be positive. Since lymphoblastic and myeloblastic neoplasms can also be positive for CD56, stains for CD3 and myeloperoxidase should always be performed in order to exclude these entities.3 The cells express CD123 and TCL1, both of which support a relationship to plasmacytoid dendritic cells.23,24,130
Genetic features. T-cell receptor genes are in germline configuration. Tumor cells are negative for EBV.
Prognosis and predictive factors. CD4+/CD56+ hematodermic neoplasm is an aggressive disease with a poor prognosis (median survival, 14 months).21-24,121 Systemic chemotherapy usually results in a complete remission, but quick relapses unresponsive to further chemotherapy are the rule. No significant difference in survival is found between patients presenting with skin lesions with or without concurrent extracutaneous disease.121
Recent studies suggest that patients can best be treated with regimens used in acute leukemias.121
Cutaneous B-cell lymphomas
Primary cutaneous marginal-zone B-cell lymphoma
Definition. Primary cutaneous marginal zone B-cell lymphoma (PCMZL) is an indolent lymphoma composed of small B cells, including marginal zone (centrocyte-like) cells, lymphoplasmacytoid cells, and plasma cells. It includes cases previously designated as primary cutaneous immunocytoma,131 and cases of cutaneous follicular lymphoid hyperplasia with monotypic plasma cells.132 Exceptional cases of primary cutaneous plasmacytoma without underlying multiple myeloma (extramedullary plasmacytoma of the skin) show considerable overlap with PCMZL and are therefore included in this category.133 PCMZL is considered part of the broad group of extranodal marginal zone B-cell lymphomas commonly involving mucosal sites, called MALT (mucosa-associated lymphoid tissue) lymphomas.
Clinical features. In most instances patients with PCMZL present with red to violaceous papules, plaques, or nodules localized preferentially on the trunk or extremities, especially the arms. In contrast to PCFCL, presentation with multifocal skin lesions is frequent (Figure 8). Ulceration is uncommon. PCMZLs have a tendency to recur in the skin, but dissemination to extracutaneous sites is exceedingly rare.131,134-136 In some cases spontaneous resolution of the skin lesions may be observed. The development of anetoderma in spontaneously resolving lesions has been observed.137 An association with Borrelia burgdorferi infection has been reported in a significant minority of European cases of PCMZL, but not in Asian cases or cases from the United States.134,138-140 Associated autoimmune diseases are uncommon in PCMZL, but rather suggest secondary cutaneous involvement of a systemic lymphoma.131
Histopathology. These lymphomas show nodular to diffuse infiltrates with sparing of the epidermis. The infiltrates are composed of small lymphocytes, marginal zone B cells (centrocyte-like cells), lymphoplasmacytoid cells, and plasma cells, admixed with small numbers of centroblast- or immunoblast-like cells and many reactive T cells. Reactive germinal centers are frequently observed. They may be surrounded by a population of small-to medium-sized cells with irregular nuclei, inconspicuous nucleoli, and abundant pale cytoplasm (marginal zone B cells). Monotypic plasma cells are often located at the periphery of the infiltrates and in the superficial dermis beneath the epidermis.131,132,135,136 periodic acid-schiff (PAS)+ intranuclear or intracytoplasmic inclusions may be present in cases with a predominance of lymphoplasmacytoid cells. PCMZLs rarely show transformation into a diffuse large B-cell lymphoma, but a relative increase in large transformed cells can be seen in some cases.
Immunophenotype. The marginal zone B cells express CD20, CD79a, and bcl-2, but are negative for CD5, CD10, and bcl-6, which may be useful in distinction from PCFCL.141,142 Reactive germinal centers are typically bcl-6+, CD10+, and bcl-2-. Plasma cells express CD138 and CD79a, but generally not CD20, and show monotypic cytoplasmic immunoglobulin light chain expression on paraffin sections.
Genetic features. Immunoglobulin heavy chain (IgH) genes are clonally rearranged. Recent studies suggest the presence of the t(14;18)(q32;q21) involving the IGH gene on chromosome 14 and the MLT gene on chromosome 18, and t(3;14)(p14.1;q32) involving IGH and FOXP1 genes, in a proportion of PCMZLs.143,144 However, other translocations observed in gastric MALT lymphomas, such as t(11;18)(q21;q21) and t(1;14)(p22;q32), have not been found in PCMZL.45,145
Prognosis and predictive factors. The prognosis of PCMZL is excellent with a 5-year survival close to 100% (Table 2).131-136
Therapy. Patients with a solitary or a few lesions can be treated with radiotherapy or surgical excision. In patients with associated B burgdorferi infection, systemic antibiotics should be tried first.146 For patients presenting with multifocal skin lesions, chlorambucil or intralesional or subcutaneous administration of interferon alpha may produce complete responses in approximately 50% of patients.146 Very good results have also been obtained with the use of systemic or intralesional anti-CD20 antibody (rituximab).147 In patients showing frequent skin relapses, topical or intralesional steroids may be considered; alternatively, an expectant strategy can be followed, similar to that used in other indolent B-cell lymphomas and leukemias.
Primary cutaneous marginal zone lymphoma. Characteristic clinical presentation with multiple nodules and small tumors on the back and arms.
Primary cutaneous marginal zone lymphoma. Characteristic clinical presentation with multiple nodules and small tumors on the back and arms.
Primary cutaneous follicle-center lymphoma
Definition. Primary cutaneous follicle center lymphoma (PCFCL) is defined as a tumor of neoplastic follicle center cells, usually a mixture of centrocytes (small and large cleaved follicle center cells) and variable numbers of centroblasts (large noncleaved follicle center cells with prominent nucleoli), with a follicular, a follicular and diffuse, or a diffuse growth pattern, which generally present on the head or trunk. Lymphomas with a diffuse growth pattern and a monotonous proliferation of centroblasts and immunoblasts are, irrespective of site, excluded and are classified as PCLBCL (Table 3).
Characteristic features of PCFCL and PCLBCL, leg type
. | PCFCL . | PCLBCL, leg type . |
|---|---|---|
| Morphology | Predominance of centrocytes that are often large, especially in diffuse lesions. | Predominance or confluent sheets of medium-sized to large B cells with round nuclei, prominent nucleoli, and coarse chromatin resembling centroblasts and/or immunoblasts. |
| Centroblasts may be present, but not in confluent sheets. | Diffuse growth pattern. | |
| Growth pattern may be follicular, follicular and diffuse, or diffuse (a continuum without distinct categories or grades). | ||
| Phenotype | Bcl-2: -/+* | Bcl-2: ++‡ |
| Bcl-6: + | Bcl-6: +/- | |
| CD10: -/+† | CD10: - | |
| Mum-1: - | Mum-1: + | |
| Clinical features | Middle-aged adults. | Elderly, especially females, |
| Localized lesions on head or trunk (90%). | Lesions localized on leg(s), most often below the knee. | |
| Multifocal lesions in rare cases. | Rare cases with lesions at other sites than the leg (10%). |
. | PCFCL . | PCLBCL, leg type . |
|---|---|---|
| Morphology | Predominance of centrocytes that are often large, especially in diffuse lesions. | Predominance or confluent sheets of medium-sized to large B cells with round nuclei, prominent nucleoli, and coarse chromatin resembling centroblasts and/or immunoblasts. |
| Centroblasts may be present, but not in confluent sheets. | Diffuse growth pattern. | |
| Growth pattern may be follicular, follicular and diffuse, or diffuse (a continuum without distinct categories or grades). | ||
| Phenotype | Bcl-2: -/+* | Bcl-2: ++‡ |
| Bcl-6: + | Bcl-6: +/- | |
| CD10: -/+† | CD10: - | |
| Mum-1: - | Mum-1: + | |
| Clinical features | Middle-aged adults. | Elderly, especially females, |
| Localized lesions on head or trunk (90%). | Lesions localized on leg(s), most often below the knee. | |
| Multifocal lesions in rare cases. | Rare cases with lesions at other sites than the leg (10%). |
Faint staining, when present; minority of cells (see comments in paragraph on PCFCL)
Diffuse lesions, mostly CD10-
Strong staining; in most neoplastic cells
Clinical features. PCFCL has a characteristic clinical presentation with solitary or grouped plaques and tumors, preferentially located on the scalp or forehead or on the trunk, and rarely on the legs (Figure 9A-B).33-35 Particularly on the trunk, these tumors may be surrounded by erythematous papules and slightly indurated plaques, which may precede the development of tumorous lesions for months or even many years. In the past, PCFCLs with such a typical presentation on the back were referred to as “reticulohistiocytoma of the dorsum” or “Crosti lymphoma (Figure 9A).”34,148 Presentation with multifocal skin lesions is observed in a small minority of patients, but is not associated with a more unfavorable prognosis.42,149 If left untreated, the skin lesions gradually increase in size over years, but dissemination to extracutaneous sites is uncommon.
Primary cutaneous follicle center lymphoma. (A) Typical presentation with tumors on the chest surrounded by less infiltrated erythematous skin lesions. (B) Presentation with multiple tumors confined to the scalp. (C) Diffuse dermal infiltrate mainly consisting of large centrocytes and multilobated cells (H&E staining; original magnification, × 480). (D-E) Serial sections stained for CD20 (D) and bcl-2 (E). Bcl-2 is expressed by perivascular T cells, but not by the neoplastic B cells. Image acquisition for panels A and C-E was performed as described for Figure 1B. An HC Plan APO objective was used (40×/0.85 for panel C; 10×/0.40 for panels D and E).
Primary cutaneous follicle center lymphoma. (A) Typical presentation with tumors on the chest surrounded by less infiltrated erythematous skin lesions. (B) Presentation with multiple tumors confined to the scalp. (C) Diffuse dermal infiltrate mainly consisting of large centrocytes and multilobated cells (H&E staining; original magnification, × 480). (D-E) Serial sections stained for CD20 (D) and bcl-2 (E). Bcl-2 is expressed by perivascular T cells, but not by the neoplastic B cells. Image acquisition for panels A and C-E was performed as described for Figure 1B. An HC Plan APO objective was used (40×/0.85 for panel C; 10×/0.40 for panels D and E).
Histopathology. PCFCLs show nodular to diffuse infiltrates with almost constant sparing of the epidermis. The histologic picture is variable, which relates primarily to the age and the growth rate of the biopsied skin lesion as well as the location.35,39 A clear-cut follicular growth pattern is more commonly observed in lesions arising on the scalp than those presenting on the trunk.150 Small and early lesions contain a mixture of centrocytes, relatively few centroblasts, and many reactive T cells. Large centrocytes, often multilobated, are a common feature of PCFCL (Figure 9C). The large neoplastic B cells may have a fibroblast-like appearance. In small and/or early lesions a clear-cut follicular growth pattern or, more often, remnants of a follicular growth pattern may be observed. If present, the abnormal follicles are composed of malignant bcl-6+ follicle center cells enmeshed in a network of CD21+ or CD35+ follicular dendritic cells. The follicles are ill-defined, lack tingible body macrophages, and generally have a reduced or absent mantle zone.150,151 With progression to tumorous lesions the neoplastic B cells increase both in number and size, whereas the number of reactive T cells steadily decreases.35,39 Follicular structures, if present before, are no longer visible except for occasional scattered CD21+ or CD35+ follicular dendritic cells. Tumorous skin lesions generally show a monotonous population of large follicle center cells, generally large centrocytes and multilobated cells, and in rare cases spindle-shaped cells, with a variable admixture of centroblasts and immunoblasts.34,35,39,42,152 Usually, a prominent stromal component is present.
Immunophenotype. The neoplastic cells express the B-cell–associated antigens CD20 and CD79a, and may show monotypic staining for surface immunoglobulins (sIgs). However, absence of detectable sIg is common in tumorous lesions showing a diffuse population of large follicle center cells. PCFCLs consistently express bcl-6.141,142,151 CD10 expression is particularly observed in cases with a follicular growth pattern, but is uncommon in PCFCLs with a diffuse growth pattern.142,153 Staining for CD5 and CD43 is negative. Unlike nodal and secondary cutaneous follicular lymphomas, PCFCLs do not express bcl-2 protein or show faint bcl-2 staining in a minority of neoplastic B-cells.36,41,150 (Figure 9D,E). Staining for MUM-1/IRF4 is negative.46
Genetic features. Clonally rearranged immunoglobulin genes are present. Somatic hypermutation of variable heavy and light chain genes has been demonstrated, which further supports the follicle center cell origin of these lymphomas.154,155 In most studies PCFCLs, including cases with a follicular growth pattern, do not show the t(14;18), which is characteristically found in systemic follicular lymphomas and a proportion of systemic diffuse large B-cell lymphoma.37,41,151,153 (see “Comment” under “Primary cutaneous follicle-center lymphoma”).
Inactivation of p15 and p16 tumor suppressor genes by promotor hypermethylation has been reported in about 10% and 30% of PCFCLs, respectively.156 Chromosomal imbalances have been identified by comparative genomic hybridization (CGH) analysis in a minority of PCFCLs, but a consistent pattern has not yet emerged.44,157 In a recent study using interphase fluorescence in situ hybridization, no evidence for translocations involving IgH, myc, or bcl-6 loci were found.45 PCFCLs have the gene expression profile of germinal center–like large B-cell lymphomas.46
Prognosis and predictive factors. Regardless of the growth pattern (follicular or diffuse), the number of blast cells, or the presence of either localized or multifocal skin disease, these PCFCLs have an excellent prognosis with a 5-year survival of more than 95% (Table 2).2,4,5,33-35,38,42,150,151 A recent study suggests that strong expression of bcl-2 in PCFCLs with a diffuse large-cell histology is associated with a more unfavorable prognosis.158
Therapy. In patients with localized or few scattered skin lesions, radiotherapy is the preferred mode of treatment, even in cases with a predominance of large “cleaved” cells.38,42,149,159-161 Cutaneous relapses, observed in approximately 20% of patients, do not indicate progressive disease and can be treated with radiotherapy as well. Anthracycline-based chemotherapy is required only in patients with very extensive cutaneous disease and patients developing extracutaneous disease.42,150 Recent studies report beneficial effects of systemic or intralesional administration of anti-CD20 antibody (rituximab) therapy in small series of PCFCLs, but the long-term effects of this approach have yet to be determined.162-164
Comment. Characteristically, PCFCLs do not express bcl-2 protein, or show faint bcl-2 staining in a minority of tumor cells, and do not show the t(14;18).36,37,41 However, recent studies report the presence of t(14;18) and/or bcl-2 expression in a significant minority of PCFCLs.43,153,165-167 Whether these discrepant results are the result of differences in patient selection (eg, incomplete staging) or different definitions for bcl-2 positivity, or represent regional differences is as yet unknown. Importantly, in PCFCLs with a follicular growth pattern there are no differences in clinical presentation and behavior between bcl-2 and/or t(14;18)-positive and -negative cases.36,152,165-167 In contrast, recent studies suggest that expression of bcl-2 protein by more than 50% of the neoplastic B cells in PCFCLs with a diffuse proliferation of large centrocytes, observed in approximately 15% of cases, is associated with a more unfavorable prognosis.158 Further studies are warranted to define the clinical and biological significance of bcl-2 expression and/or the presence of t(14;18) observed in some cases of PCFCL. Notwithstanding, demonstration of bcl-2 expression and/or t(14; 18) should always raise suspicion of a systemic lymphoma involving the skin secondarily.
Primary cutaneous diffuse large B-cell lymphoma, leg type
Definition. Primary cutaneous diffuse large B-cell lymphoma, leg type, is a PCLBCL with a predominance or confluent sheets of centroblasts and immunoblasts, characteristically presenting with skin lesions on the (lower) legs. Uncommonly, skin lesions with a similar morphology and phenotype can arise at sites other than the legs.
Clinical features. PCLBCL, leg type, predominantly affects elderly patients, particularly females.40,42,43 Patients present with generally rapidly growing red or bluish-red tumors on one or both (lower) legs (Figure 10A). In contrast to the group of PCFCLs, these lymphomas more often disseminate to extracutaneous sites and have a more unfavorable prognosis.40,42 Reports on PCLBCL, leg type, arising at sites other than the leg are few. In a recent European multicenter study, 16 of 17 patients presented with solitary or localized skin lesions either on the trunk or head, and 7 of 17 patients developed extracutaneous disease.42
Histopathology. These lymphomas show diffuse infiltrates, which often extend into the subcutaneous tissue. These infiltrates generally show a monotonous population or confluent sheets of centroblasts and immunoblasts (Figure 10B).40,42 Mitotic figures are frequently observed. Small B cells are lacking and reactive T cells are relatively few and often confined to perivascular areas. A prominent stromal reaction as in PCFCLs is not observed.
Immunophenotype. The neoplastic B cells express monotypic sIg and/or cIg and B-cell–associated antigens CD20 and CD79a. In contrast to the group of PCFCLs, PCLBCL, leg type, shows strong bcl-2 expression, also in cases not located on the legs (Figure 10C).41,43,142,158 Bcl-6 is expressed by most cases, whereas CD10 staining is generally absent.142 Unlike PCFCL, most PCLBCL, leg types, express MUM-1/IRF4 protein (Figure 10D).46,168
Genetic features. The t(14;18) is not found in PCLBCLs, although strong bcl-2 expression is common in this group.41,43 In some cases bcl-2 overexpression may result from chromosomal amplification of the bcl-2 gene.157 Inactivation of p15 and p16 tumor suppressor genes by promotor hypermethylation has been detected in 11% and 44% of PCLBCLs, respectively.156 Chromosomal imbalances have been identified in up to 85% of PCLBCLs, with gains in 18q and 7p and loss of 6q as most common findings.44,157 Recent studies demonstrated translocations involving myc, bcl-6, and IgH genes in 11 of 14 PCLBCL-leg cases, but not in patients with a PCFCL with a diffuse infiltration of large centrocytes.45 Recent studies suggest that patients with PCLBCL-leg have an activated B-cell gene expression profile.46
Prognosis and predictive features. The 5-year survival of 78 cases included in the Dutch and Austrian registries was 55% (Table 2). PCLBCLs on the leg have an inferior prognosis compared to PCLBCLs presenting at other sites.42 The presence of multiple skin lesions at diagnosis is a significant adverse risk factor. In a recent study, patients presenting with a single skin tumor on one leg had a disease-related 5-year survival of 100%, whereas patients presenting with multiple skin lesions on one or both legs had a disease-related 5-year survival of 45% and 36%, respectively.42
Primary cutaneous diffuse large B-cell lymphoma, leg type. (A) Clinical presentation with multiple tumors on right lower leg. (B) Monotonous proliferation of centroblasts and immunoblasts (H&E staining; original magnification,× 480). (C-D) Characteristically, the neoplastic B cells strongly express bcl-2 (C) and Mum-1/IRF-4 (D). Image acquisition for panels B-D was performed as described for Figure 1B. An HC Plan APO objective was used (40×/0.85 for panel B; 20×/0.70 for panels C and D).
Primary cutaneous diffuse large B-cell lymphoma, leg type. (A) Clinical presentation with multiple tumors on right lower leg. (B) Monotonous proliferation of centroblasts and immunoblasts (H&E staining; original magnification,× 480). (C-D) Characteristically, the neoplastic B cells strongly express bcl-2 (C) and Mum-1/IRF-4 (D). Image acquisition for panels B-D was performed as described for Figure 1B. An HC Plan APO objective was used (40×/0.85 for panel B; 20×/0.70 for panels C and D).
Therapy. These lymphomas should be treated as systemic diffuse large B-cell lymphomas with anthracycline-based chemotherapy.40,42 In patients presenting with a single small skin tumor, radiotherapy may sometimes be considered.42 Systemic administration of anti-CD20 antibody (rituximab) has proved effective in some patients, but long-term follow-up data are not available and the place of rituximab in the treatment of PCLBCL, either as single agent therapy or in combination with systemic chemotherapy remains to be established.162,169
Primary cutaneous diffuse large B-cell lymphoma, other
The term “PCLBCL, other,” refers to rare cases of large B-cell lymphomas arising in the skin, which do not belong to the group of PCLBCL, leg type, or the group of PCFCLs. These cases include morphologic variants of diffuse large B-cell lymphoma, such as anaplastic or plasmablastic subtypes or T-cell/histiocyte rich large B-cell lymphomas. Such cases are generally a skin manifestation of a systemic lymphoma. Plasmablastic lymphomas are seen almost exclusively in the setting of HIV infection or other immune deficiencies.170-172 Some of these cases had only skin lesions at presentation. Rare cases of primary cutaneous T-cell/histiocyte-rich B-cell lymphoma, characterized by the presence of large scattered B cells in a background of numerous reactive T cells, have been reported.173,174 Clinically, they show similarities with the groups of PCFCL and PCMZL. These lymphomas commonly present with skin lesions on the head, the trunk or the extremities, and may in fact represent an exaggerated T-cell infiltrate in association with other forms of CBCL. Unlike their nodal counterparts, they appear to have an excellent prognosis.173,174 In addition, rare cases of primary cutaneous intravascular large B-cell lymphoma may be included in this category.
Intravascular large B-cell lymphoma. Intravascular large B-cell lymphoma is a well-defined subtype of large B-cell lymphoma, defined by an accumulation of large neoplastic B cells within blood vessels. These lymphomas preferentially affect the central nervous system, lungs, and skin and are generally associated with a poor prognosis.175
Patients often have widely disseminated disease, but cases with only skin involvement may occur. Clinically, intravascular large B-cell lymphoma may present with violaceous patches and plaques or teleangiectatic skin lesions usually on the (lower) legs or the trunk.175,176 Patients presenting with only skin lesions appear to have a significantly better survival than patients with other clinical presentations (3-year overall survival: 56% versus 22%).176 Interestingly, colonization of cutaneous cherry hemangiomas by neoplastic cells as the only presenting sign of intravascular large B-cell lymphoma has been reported.177,178 Histologically, dilated blood vessels in the dermis and subcutis are filled and often extended by a proliferation of large neoplastic B cells. These cells may cause vascular occlusion of venules, capillaries, and arterioles. In some cases small numbers of tumor cells can also be observed around blood vessels. Multiagent chemotherapy is the preferred mode of treatment, also in patients presenting with skin-limited disease.176
Conclusions and future directions
The WHO-EORTC classification for cutaneous lymphomas presented herein may be considered as an important step forward. First, the development of this consensus classification will put an end to the ongoing discussion whether the EORTC or the WHO scheme can best be used, and is expected to contribute to a more uniform diagnosis and hence a more uniform treatment of patients with a cutaneous lymphoma. Second, major progress has been made in a better definition of some controversial groups of cutaneous lymphoma, in particular the group of PCFCL/PCLBCL and the group of CTCL other than MF, SS, and the group of primary cutaneous CD30+ LPD.
The new definitions of the groups of PCFCL, PCLBCL, leg type, and PCLBCL, other, will allow a more reliable distinction between indolent and more aggressive types of CBCL, and facilitate the decision whether radiotherapy or systemic chemotherapy should be selected as first choice of treatment. Large multicenter studies are now required to validate the current proposals, and in particular to investigate the diagnostic and prognostic value of bcl-2 and Mum-1/IRF4 protein expression.
The classification of CTCL other than MF, SS, and the group of primary cutaneous CD30+ LPD is still difficult, as it requires accurate clinicopathologic correlation and a number of complementary techniques to arrive at a definite diagnosis. SPTL (with an alpha/beta phenotype), extranodal NK/T-cell lymphoma, nasal type, and CD4+/CD56+ hematodermic neoplasm are now fairly well-defined. However, considerable overlap is noted between cutaneous (and mucosal) GD-TCL and aggressive epidermotropic CD8+ CTCL. The similarities in clinical presentation and pattern of dissemination between these conditions may reflect similarities in homing profile and biologic function of normal gamma/delta-positive T-cells and activated CD8+ cytotoxic T cells, respectively. Apart from the group of SPTLs and the group of CD4+ small/medium-sized pleomorphic CTCLs, these rare types of CTCL have a very poor prognosis and are generally resistant to conventional chemotherapy. More aggressive regimens, including allogeneic bone marrow transplantation, for patients with aggressive types of CTCL including advanced stages of MF and SS are currently under investigation.179,180
Recently, studies have started to investigate gene and protein expression profiles in different types of cutaneous lymphoma. It is expected that these studies will not only contribute to a better understanding of the molecular pathways involved in the development and progression of these lymphomas, but will also provide new molecular targets for diagnosis and therapeutic intervention, and ultimately to more refined classification schemes.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2004-09-3502.
An Inside Blood analysis of this article appears at the front of the issue.

![Figure 1. Mycosis fungoides. (A) Typical patches and plaques on the trunk. (B) Infiltration of atypical T cells into the epidermis with formation of Pautrier microabscess (hematoxylin and eosin [H&E] staining; original magnification, × 200). The image in panel B was obtained through a Leica DM6000B microscope (Leica, Rijswijk, the Netherlands). Image acquisition was performed with a ProgResC10 camera and software (JenaOptik, Jena, Germany). An HC Plan APO 40×/0.85 objective was used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3502/6/m_zh80100578750001.jpeg?Expires=1765893776&Signature=ureP4aisSSxeqzOX8IUmXvRZWt-sjrELkCQmBYMuzXYCxGKF~0cbTD3byHP0kMNg5kafYrvfXGMdlLOAYbYoIi9OHPef2R-S3qv4kVSJq7aYKdB3ziwyXkJ4NsDmBa~Rbr19foyZY0hyL~dEBQNE1OkFUkhrus-EgjOhdUBDADetKUKxvYyQrHBUdxPXH45cIzg-HqMbiakI4mfgSIk-lbFNgjRIVWQG0qCmxw~zdKPipJqTmT2-5pkDYpK0ANQmtwiPykQCZ687a6HvPs5GameAWRYpkfZqWjLpVLalCwqhMDXEtdQ0Va0WJ8xYHl5SNV4UsXCxZ~uwxjrOdustog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
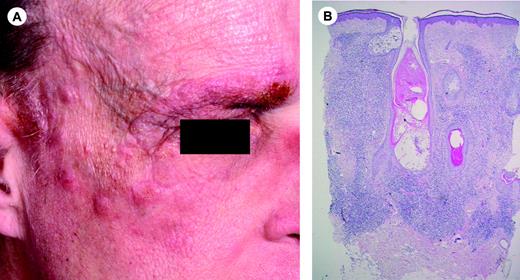
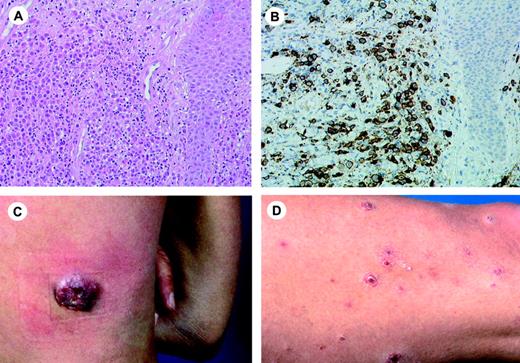

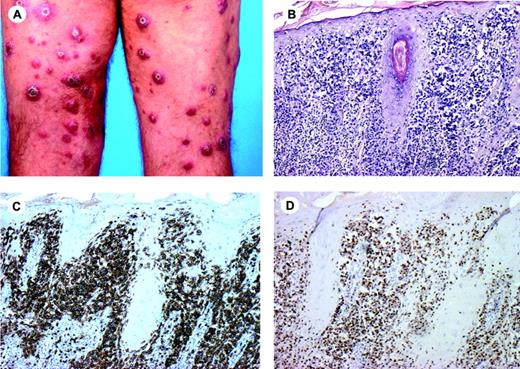
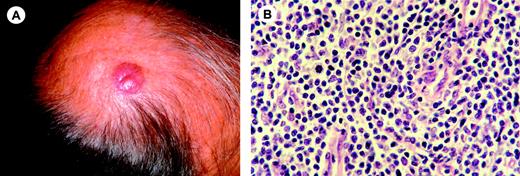
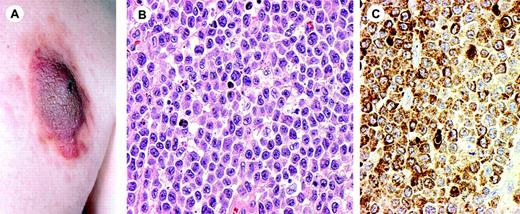
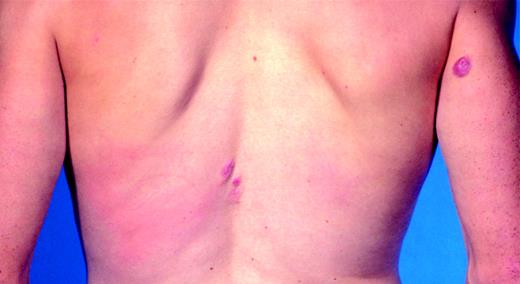
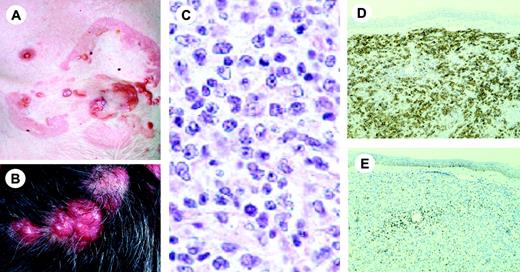
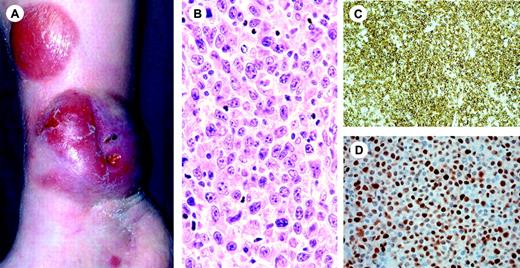
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal