Abstract
Mutations within the BCR-ABL kinase domain in imatinib-treated chronic myeloid leukemia (CML) are the main mechanism of acquired resistance. The early detection of mutations should provide clinical benefit by allowing early intervention. Quantitative polymerase chain reaction (RQ-PCR) results of BCR-ABL mRNA were correlated with mutation analysis in 214 patients treated with imatinib. We determined whether there was a difference in the incidence of mutations between the patients with a more than 2-fold rise in BCR-ABL and patients with stable or decreasing levels. Of the 56 patients with a more than 2-fold rise, 34 (61%) had detectable mutations (median rise, 3.0-fold; 25th-75th percentiles, 2.3-5.2). In 31 (91%) of these 34 patients, the mutation was present at the time of the rise and became detectable within 3 months in the remaining patients. Only 1 (0.6%) of 158 patients with stable or decreasing BCR-ABL levels had a detectable mutation, P less than .0001. Thus, a more than 2-fold rise identified 34 (97%) of 35 patients with a mutation. We conclude that a rise in BCR-ABL of more than 2-fold can be used as a primary indicator to test patients for BCR-ABL kinase domain mutations.
Introduction
The selective inhibitor of the BCR-ABL tyrosine kinase, imatinib mesylate (Glivec; Novartis Pharmaceuticals, Basel, Switzerland), has demonstrated excellent safety and important therapeutic benefit for patients with chronic myeloid leukemia (CML).1-5 However, resistance to imatinib can occur in all phases of the disease. Most patients who acquire resistance after an initial response have reactivation of the BCR-ABL tyrosine kinase.6 Mutation within the kinase domain is now recognized as the principal mechanism of reactivation7-10 with 31 different point mutations described to date.7,9-15 Mutations have been reported to be detectable in 35% to 90% of resistant patients.
Biochemical and cellular assays have demonstrated that the different BCR-ABL mutations result in varying levels of resistance,7,10,11,16,17 and clinical studies have shown that the location of the mutation within the kinase domain may affect the disease outcome.9,13,18 The different mutations may, therefore, require differing strategies to overcome resistance such as dose escalation for those that confer moderate resistance, combination therapy or transplantation for highly resistant mutations. Mutations have been detected prior to relapse,7-9 and in some cases very low levels of mutant clones have been detected prior to commencing imatinib.7,12,19,20 Most mutations are not detectable at the start of imatinib therapy, although this may reflect the relative insensitivity of the methodology for detecting the mutations at very low levels. The evidence suggests that monitoring patients treated with imatinib for mutations provides an important guide for clinical management and has prognostic relevance. However, frequent sequencing of BCR-ABL for the early detection of mutations in all patients with CML is unlikely to be a cost-effective strategy.
We sought to address the question of whether molecular monitoring of BCR-ABL transcript levels can guide the frequency and timing of sequencing analysis for the early recognition of mutations. Monitoring of patients treated with imatinib by quantitative reverse transcriptase-polymerase chain reaction (RQ-PCR) of BCR-ABL mRNA has proved effective for defining patient response. Early reduction of BCR-ABL can predict a subsequent cytogenetic response.21-24 The level of BCR-ABL predicts for disease-free survival25,26 and clinical outcome,23,27 whereas the proportional reduction in BCR-ABL is closely correlated with the level of cytogenetic response.27 Patients who achieve at least a 3-log reduction in BCR-ABL, which is termed a major molecular response, have a low incidence of disease progression.25 Rising levels of BCR-ABL are strongly predictive of cytogenetic and hematologic relapse after an allogeneic transplantation.28-30
In the current study we performed a retrospective examination of serial BCR-ABL measurements, mutation analysis, and clinical outcome in 214 patients treated with imatinib to evaluate the hypothesis that the emergence of a detectable mutant clone will lead to a rise in the BCR-ABL level and that this rise can be used as a sensitive trigger to screen for mutations.
Patients and methods
Patients
A total of 214 patients with CML treated with imatinib were included in the study, and informed consent was provided according to the Declaration of Helsinki. The criteria for inclusion of patients and data points were the following: (1) at least 2 peripheral blood samples were collected within 6 months and tested for BCR-ABL levels; (2) mutation analysis was performed at least every 6 months, and the last sample received for each patient was analyzed for mutations; and (3) the imatinib dose was not interrupted or reduced (the data points for 9 of the 214 patients were excluded after dose interruption or cessation). Patients had received between 3 and 39 months of imatinib therapy (median, 15 months). At the start of imatinib therapy 5 patients were in blast crisis, 27 in accelerated phase as defined previously and included clonal evolution as a sole criterion for classification as accelerated phase,9 48 in late chronic phase (patients who commenced imatinib 12 months or more since diagnosis), and 134 in early chronic phase (patients who commenced imatinib < 12 months since diagnosis).
The definition of acquired imatinib resistance was described previously9 and incorporates the criteria for defining a loss of response proposed by Goldman.31 Briefly, acquired resistance was defined as a sustained complete hematologic remission (CHR) which was followed by transformation to accelerated or blastic phase, loss of a sustained CHR without transformation, the development of new cytogenetic abnormalities in the Philadelphia chromosome-positive (Ph+) clone, an increase of 30 or more points in the percentage of Ph+ bone marrow metaphases examined at an interval of 3 months or longer, loss of a major cytogenetic response (MCR; 0%-35% Ph+), or loss of a complete cytogenetic response (CCR, Ph-).
Quantitation of BCR-ABL mRNA
Patients were monitored by real-time quantitative RT-PCR (RQ-PCR) for BCR-ABL transcript levels in peripheral blood at 1- to 6-month intervals for up to 39 months of imatinib therapy. The RQ-PCR method used TaqMan probes and the 7700 Sequence Detection System (Applied Biosystems, Foster City, CA).32 The performance of the RQ-PCR assay was monitored by analysis of a high- and low-quality control RNA that differ in the BCR-ABL level by approximately 3 logs. The controls were prepared in house from BCR-ABL-positive and -negative cells that were stored in Trizol stabilization solution (Invitrogen Life Technologies, Carlsbad, CA) and processed in every RT and quantitative PCR assay.22 A total of 1536 patient samples was analyzed by RQ-PCR (median, 5 samples per patient; range, 2-26 samples).
Mutation analysis
The patients were screened for mutations approximately every 6 months by isolation of the BCR-ABL allele in a seminested PCR followed by direct sequencing.8,9 This technique permitted the detection of single or multiple mutations in patients with disease status ranging from a CCR to overt relapse at a sensitivity of approximately 20%. The frequency of mutation screening and RQ-PCR analysis was increased in patients with evidence of acquired resistance. Overall, 15% of patients had an increased frequency of analysis. Mutation screening every 6 months was continued in patients with an ongoing response to imatinib unless a mutation was detected, in which case the frequency of mutation analysis and RQ-PCR was increased. When a mutation was detected in a patient for the first time, the unscreened cDNA samples from prior collection time points that were stored after RQ-PCR analysis were tested to determine when the mutation first became detectable by the direct sequencing technique. Mutation analysis was performed on a total of 804 samples.
Statistical and result analysis
Contingency tables using the chi-square statistic or the Fisher exact test as appropriate were used to determine the significance of the difference in frequency of mutations and acquired resistance in patients with a more than 2-fold rise in BCR-ABL level in consecutive samples. Groups were compared by the Mann-Whitney test. To determine whether a 2-fold rise in BCR-ABL transcript level is statistically significantly different, a mixed model analysis of variance was performed in which the samples with an increase in gene expression were fixed effects and duplicates within the low level control sample were random effects. SAS statistical analysis software (SAS Institute, Cary, NC) was used.
The BCR-ABL doubling times were calculated by the application of the formula t2 = ln 2/k, where t2 is the doubling time and k is the slope of the line between the 2 points that correspond to the BCR-ABL values before and after the rise. Slope k = ln (b) - ln (a)/d, where (a) is the BCR-ABL value before the rise, (b) is the BCR-ABL value after the rise, and (d) is the number of days over which the rise occurred. For this formula to be valid it was necessary to confirm that the BCR-ABL transcript level increased exponentially. This was confirmed by graphing the BCR-ABL values of relapsing patients with 3 or more consecutive results. The number of days over which the rise in BCR-ABL occurred was plotted against the log10BCR-ABL values for the period in which the rise occurred. The equation of the correlation coefficient was calculated by using least squares analysis.
Results
Measurement reliability of the RQ-PCR assay
A number of procedures were implemented to maximize the reliability and reproducibility of the RQ-PCR assay. To compensate for variations in the RQ-PCR assay we quantitated the normal BCR gene as a control gene and reported the results as a percentage ratio of BCR-ABL/BCR%. For each sample the BCR control gene and the BCR-ABL target gene were reverse transcribed in the same RT reaction by using random hexamers that compensated for differences in the efficiency between RT reactions. The BCR control degraded at a similar rate to that of BCR-ABL and, therefore, controlled for variations in the quality of the RNA.22 The RNA of each sample was reverse transcribed on 2 separate occasions and tested in separate quantitative PCR analyses. The quantitative value was recorded as the average of the duplicate RQ-PCR results.
The estimate of measurement reliability for the RQ-PCR assay was assessed by using 2 levels of quality control RNA. The standard deviation of the control samples was determined by repeated analysis over a 6-month period (64 assays). The low control value in our assay is approximately equivalent to a 3-log reduction from a standardized baseline value.25 The RQ-PCR assay measured BCR-ABL levels over a more than 4-log dynamic range and not unexpectedly, the low control level had a larger random error than the high level. The 2 SD range as shown in Table 1 provides an estimate of measurement reliability at approximately the 95% level of confidence for the controls.
Estimate of measurement reliability at approximately the 95% level of confidence was determined by using 2 levels of quality control
. | Mean BCR-ABL/BCR, % . | 2 SD range . | CV, % . |
|---|---|---|---|
| Low control | 0.072 | 0.036-0.108 | 25.0 |
| High control | 77 | 57-97 | 13.0 |
. | Mean BCR-ABL/BCR, % . | 2 SD range . | CV, % . |
|---|---|---|---|
| Low control | 0.072 | 0.036-0.108 | 25.0 |
| High control | 77 | 57-97 | 13.0 |
We did not have a control sample at a 4-log reduction from the baseline level. However, there was a larger variability of the BCR-ABL RQ-PCR assay at that level as evidenced by the variability of the lowest BCR-ABL standards. A 10-fold dilution series of plasmid standards, ranging from approximately 106 to 10 BCR-ABL copies, were used to determine the quantitative values.32 The variability of very low BCR-ABL values was calculated from the variation in the Ct value (fractional PCR cycle number at which a plot of the increase in fluorescence crosses a defined threshold) of the low standard. The Ct value varied from 35.36 to 37.54 cycles (2 SD range) which was calculated from the Ct values of the b3a2 BCR-ABL low standard recorded over a 2-year period with a threshold of 0.04. One cycle is approximately equivalent to a 2-fold change in expression when the PCR amplifies with 100% efficiency. We can conclude from these data that the quantitative values at very low levels of BCR-ABL fluctuated by approximately 22.18, which is 4.5-fold (2.18 is the number of cycles within the 2 SD range, refer to User Bulletin no. 2, ABI Prism Sequence Detection System, Applied Biosystems). In contrast, a similar calculation for the standard with 102BCR-ABL copies had a measurement reliability ranging more than approximately 2.5-fold at the 95% level of confidence. The highest standard at 106 copies had a measurement reliability ranging more than approximately 1.8-fold at the 95% level of confidence. Therefore, there was a stratification of assay variability over the dynamic range with up to a 4.5-fold variability at -4 logs, 2.5-fold at -3 logs, and less than 2-fold at baseline levels.
Correlation of RQ-PCR results, mutation analysis, and patient outcome
The aim of this study was to test the hypothesis that the emergence of a detectable mutant clone will reliably lead to a rise in the BCR-ABL level and that this rise can be used as an indicator to test for mutations. We considered it necessary to select the amount of increase that had the optimal positive predictive power, while limiting false-negative results.
The measurement reliability of the RQ-PCR assay indicated a stratification of variability over the dynamic range with values below -3 logs having the largest variability. We demonstrated that the assay could reliably detect at least a 2-fold change in BCR-ABL at the level of a 3-log reduction and above on the basis of the quality control data and on our observation that the difference in the patient duplicate RQ-PCR results was more than 2-fold on only 5.5% of occasions (calculated from a subset of 1114 duplicate analyses). We confirmed that this was the case by using a mixed model analysis of variance in which the samples with an increase in gene expression were fixed effects, and duplicates within the low level control sample were random effects. The test confirmed that a 2-fold change in expression level was statistically different at the level of a 3-log reduction, P = .003 (F value, 9.31). At lower BCR-ABL values the measurement reliability could fluctuate by more than 2-fold. It is also possible that samples with poorer quality RNA may have a higher coefficient of variation compared with good quality RNA samples. However, for the purpose of this analysis these samples were included in the investigation.
Samples were not included when the BCR-ABL value fluctuated between an undetectable level and very low levels (positive BCR-ABL/BCR% values ranging from 0.005% to 0.02%). This level of BCR-ABL is too low for mutation analysis and occurred in 10 of 24 patients who had negative BCR-ABL values at some time during imatinib therapy. None of these patients had further increases in BCR-ABL, and none acquired resistance. The detection of BCR-ABL in these patients at some time points and not at others is largely dependent on the quality of the RNA as demonstrated by the control gene BCR transcripts, rather than having clinical significance.
Patients with a rise in BCR-ABL of more than 2-fold had a high incidence of mutations
We retrospectively grouped the 214 patients into 2 groups according to whether a rise of more than 2-fold in BCR-ABL occurred in consecutive samples at any time during the course of analysis. Fifty-six patients had such a rise, and 34 (61%) of these had detectable mutations (median rise of 3.0-fold, 25th-75th percentiles, 2.3-5.2). Only 1 (0.6%) of the 158 patients with stable or decreasing levels had a detectable mutation, P less than .0001 (Table 2).
Results of mutation analysis in patients with a greater than 2-fold rise in BCR-ABL and those with stable or decreasing levels
Consecutive BCR-ABL values in 214 patients . | No. patients (%) . | Mutation analysis (%) . | Acquired resistance (%) . |
|---|---|---|---|
| Rise of more than 2-fold | 56 of 214 (26) | ||
| Mutations | 34 of 56 (61) | 31 of 34 (91) | |
| No mutations | 22 of 56 (39) | 13 of 22 (59) | |
| Stable or decreasing levels | 158 of 214 (74) | ||
| Mutations | 1 of 158 (0.6) | 1 of 1 (100) | |
| No mutations | 157 of 158 (99) | 1 of 158 (0.6) |
Consecutive BCR-ABL values in 214 patients . | No. patients (%) . | Mutation analysis (%) . | Acquired resistance (%) . |
|---|---|---|---|
| Rise of more than 2-fold | 56 of 214 (26) | ||
| Mutations | 34 of 56 (61) | 31 of 34 (91) | |
| No mutations | 22 of 56 (39) | 13 of 22 (59) | |
| Stable or decreasing levels | 158 of 214 (74) | ||
| Mutations | 1 of 158 (0.6) | 1 of 1 (100) | |
| No mutations | 157 of 158 (99) | 1 of 158 (0.6) |
A mutation event was associated with a more than 2-fold rise in 34 (97%) of 35 patients. Of the 179 patients without a mutation, 157 (88%) had a stable or decreasing BCR-ABL level. Thus, by using a more than 2-fold rise in BCR-ABL as a predictor of a mutation we find that the estimated false-positive rate is 12% and the estimated false-negative rate is 3%. A more than 2-fold rise provided the optimal positive predictive power for mutations, while limiting the false-negative results.
Mutations in patients with a rise in BCR-ABL of more than 2-fold. In the group of 34 patients with mutations and a more than 2-fold rise in BCR-ABL, 31 (91%) had detectable mutations at the time of the rise. The mutations in the remaining 3 patients became detectable at 2, 3, and 3 months after the rise (Figure 1). In 16 (47%) of the 34 patients the mutations were also detectable from 1.5 to 8 months before the rise (median, 4.5 months) (Figure 2). Eight of the 34 patients had a CCR at the time the mutation was detected.
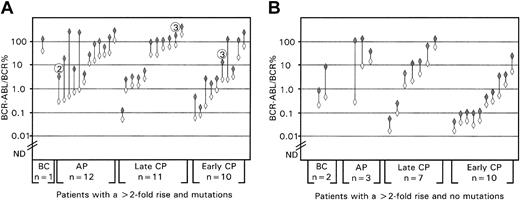
High incidence of mutations in patients with a more than 2-fold rise in BCR-ABL in consecutive analyses. The graphs represent the BCR-ABL values before (open diamonds) and after the rise (shaded diamonds). ND = the level below which BCR-ABL was not detectable. (A) Patients with a mutation. In 31 of the 34 patients, the mutation was detectable at the time of the rise and within 3 months in the remaining 3 patients. The circled number above the 3 patients indicates the month after the rise that the mutation became detectable. (B) Patients without a mutation.
High incidence of mutations in patients with a more than 2-fold rise in BCR-ABL in consecutive analyses. The graphs represent the BCR-ABL values before (open diamonds) and after the rise (shaded diamonds). ND = the level below which BCR-ABL was not detectable. (A) Patients with a mutation. In 31 of the 34 patients, the mutation was detectable at the time of the rise and within 3 months in the remaining 3 patients. The circled number above the 3 patients indicates the month after the rise that the mutation became detectable. (B) Patients without a mutation.
Serial measurement of BCR-ABL transcript levels in patients with a rise in BCR-ABL of more than 2-fold. Patient A (A) commenced imatinib in late chronic phase (≥ 12 months since diagnosis). The T315I mutation was detected prior to a steady rise in BCR-ABL, which highlights that mutation analysis is warranted when the rise in multiple samples exceeds 2-fold from the nadir level. Escalated doses of imatinib were ineffective, and the patient proceeded to an autologous transplantation. Patient B (B) was 1 of only 3 patients whereby the mutation was detected after the rise in BCR-ABL. The patient proceeded to an autologous transplantation after progression to accelerated phase (AP). In both patients A and B, the mutations remained detectable at the last measurement before transplantation. In patient C (C) a steady rise in the BCR-ABL level preceded a return to myeloid blast crisis (MBC); however, no mutations were detected. Patient D (D) commenced imatinib in early chronic phase (< 12 months since diagnosis), and the Y253H mutation was first detected at 6 months of imatinib. A rise in the BCR-ABL level on consecutive occasions followed; however, the patient maintained a CCR from 3 to 12 months.
Serial measurement of BCR-ABL transcript levels in patients with a rise in BCR-ABL of more than 2-fold. Patient A (A) commenced imatinib in late chronic phase (≥ 12 months since diagnosis). The T315I mutation was detected prior to a steady rise in BCR-ABL, which highlights that mutation analysis is warranted when the rise in multiple samples exceeds 2-fold from the nadir level. Escalated doses of imatinib were ineffective, and the patient proceeded to an autologous transplantation. Patient B (B) was 1 of only 3 patients whereby the mutation was detected after the rise in BCR-ABL. The patient proceeded to an autologous transplantation after progression to accelerated phase (AP). In both patients A and B, the mutations remained detectable at the last measurement before transplantation. In patient C (C) a steady rise in the BCR-ABL level preceded a return to myeloid blast crisis (MBC); however, no mutations were detected. Patient D (D) commenced imatinib in early chronic phase (< 12 months since diagnosis), and the Y253H mutation was first detected at 6 months of imatinib. A rise in the BCR-ABL level on consecutive occasions followed; however, the patient maintained a CCR from 3 to 12 months.
We identified mutations at 3 amino acids not previously described: E279K, 835G>A, M388L, 1162A>T, and E450G, 1349G>A. In addition, a new nucleotide substitution was identified at amino acid F317, 951C>A. This substitution resulted in the same mutation that has been described previously, F317L, which arises from the nucleotide substitution 951C>G.7,8
Patients with a rise in BCR-ABL of more than 2-fold had a high incidence of acquired resistance. The number of patients with acquired imatinib resistance was determined in those patients with a rise in BCR-ABL of more than 2-fold and those patients with stable or decreasing levels. Acquired resistance occurred in 44 (79%) of the 56 patients with a rise compared with 2 (1.3%) of the 158 patients with stable or decreasing BCR-ABL levels, P less than .0001. Resistance was evident at the time of the rise in 24 (43%) of the 56 patients. Of the remaining 32 patients with a rise, 20 (63%) subsequently acquired resistance at a median of 4 months after the rise (25th-75th percentiles, 3-5 months). Table 3 shows the nature of the resistance in each of the disease phases for the patients with a rise in BCR-ABL. Nine patients progressed to accelerated phase and 8 of these patients developed new cytogenetic abnormalities in addition to the Ph+ clone. The interpretation of these data suggests that a more than 2-fold rise in BCR-ABL can also be used as a screen for patients with a high risk of clonal evolution.
Mutation analysis and clinical outcome in patients with a greater than 2-fold rise in BCR-ABL according to the disease phase at the start of imatinib
. | . | . | Acquired resistance . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Disease phase at the start of imatinib . | More than 2-fold rise in BCR-ABL (%) . | No. patients . | AP or BC . | Loss of CHR . | Cytogenetic relapse . | No acquired resistance . | ||
| Blast crisis, n = 5 | 3 (60) | |||||||
| Mutation | 1 | 1 | — | — | — | |||
| No mutation | 2 | 2 | — | — | — | |||
| Accelerated phase, n = 27 | 15 (56) | |||||||
| Mutation | 12 | 6 | 2 | 4 | — | |||
| No mutation | 3 | 1 | — | 1 | 1 | |||
| Late chronic phase, n = 48 | 18 (38) | |||||||
| Mutation | 11 | 6 | 2 | 1 | 2 | |||
| No mutation | 7 | 2 | — | 2 | 3 | |||
| Early chronic phase, n = 134 | 20 (15) | |||||||
| Mutation | 10 | 3 | 3 | 3 | 1 | |||
| No mutation | 10 | 1 | — | 4 | 5 | |||
. | . | . | Acquired resistance . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Disease phase at the start of imatinib . | More than 2-fold rise in BCR-ABL (%) . | No. patients . | AP or BC . | Loss of CHR . | Cytogenetic relapse . | No acquired resistance . | ||
| Blast crisis, n = 5 | 3 (60) | |||||||
| Mutation | 1 | 1 | — | — | — | |||
| No mutation | 2 | 2 | — | — | — | |||
| Accelerated phase, n = 27 | 15 (56) | |||||||
| Mutation | 12 | 6 | 2 | 4 | — | |||
| No mutation | 3 | 1 | — | 1 | 1 | |||
| Late chronic phase, n = 48 | 18 (38) | |||||||
| Mutation | 11 | 6 | 2 | 1 | 2 | |||
| No mutation | 7 | 2 | — | 2 | 3 | |||
| Early chronic phase, n = 134 | 20 (15) | |||||||
| Mutation | 10 | 3 | 3 | 3 | 1 | |||
| No mutation | 10 | 1 | — | 4 | 5 | |||
Late chronic phase indicates patients who commenced imatinib 12 months or more after diagnosis. Early chronic phase indicates patients who commenced imatinib less than 12 months after diagnosis. Dashes indicate that there were no patients in the category.
Most patients with a rise in BCR-ABL had a CHR at the time of the rise (36 [64%] of 56 patients). Twenty-five (69%) of these 36 patients acquired resistance. Nine (25%) of the 36 patients had lost their cytogenetic response at the time of the rise and 2 of the 9 subsequently lost a CHR. Sixteen (44%) of the 36 patients subsequently acquired resistance at a median of 4 months after the rise (25th-75th percentiles, 3-5 months). Of these 16 patients, 2 progressed to blast crisis, 4 to accelerated phase, 2 lost a CHR, and 8 lost their cytogenetic response. The interpretation of these data indicates that a substantial number of patients with a rise in BCR-ABL while in a CHR subsequently acquire resistance, and a rise in BCR-ABL is of positive prognostic value in this situation.
Of the 2 patients with acquired resistance who did not have a rise in BCR-ABL of more than 2-fold, one had a 1.5-fold rise in consecutive samples at which time a mutation was detected. The patient died in blast crisis within 3 months, and no further samples were received. The other patient developed new cytogenetic abnormalities in the Ph+ clone and proceeded to an allogeneic transplantation. This patient was the only patient in the series who had a significant finding by bone marrow cytogenetic analysis that was not accompanied by a rise in BCR-ABL. Both of these patients were in early chronic phase.
In total, acquired resistance was evident in 32 (91%) of the 35 patients with mutations. In 20 (63%) of the 32 patients, the mutation was detectable before the resistance was evident (median, 3.5 months; 25th-75th percentiles, 2.0-7.0 months). Of the 3 patients with mutations who have not acquired resistance, one had a mutation that was first detected at last follow-up, 1 is still in a CCR at 6 months after detection of the mutation and the BCR-ABL value continues to increase, and the third patient received an increased dose of imatinib and achieved a CCR at 23 months after the detection of the mutation (E355G).
Long-term follow-up data on treatment response are available for 6 patients in chronic phase who acquired resistance after the detection of the mutation and received an increased imatinib dose for at least the next 12 months. These patients were in a CHR at the time of mutation detection. Two of these patients had the highly resistant T315I mutation. Both were unresponsive to increased doses and lost a CHR but remained in chronic phase. In contrast, 4 patients with mutations that confer moderate resistance (M244V, F317L, M351T, and E355G) either regained CCR or achieved a MCR after the dose increase.
Patients who progressed to blast crisis or accelerated phase had a significantly shorter BCR-ABL transcript doubling time. BCR-ABL quantification is valuable in predicting the disease phase of relapse after allogeneic transplantation in which a shorter doubling time indicates relapse into a more aggressive disease.28 To determine whether the rate of increase is also predictive for patients treated with imatinib, we calculated the BCR-ABL doubling times of patients with a more than 2-fold rise. Patients were included in the analysis if they had achieved at least an MCR before the rise in BCR-ABL (n = 38).
We established that the rise in BCR-ABL transcripts represented a constant logarithmic increase, which is equivalent to a constant BCR-ABL doubling time. Twenty patients had 3 or more BCR-ABL values measured during the time of the increase. The data for each patient were plotted on semilogarithmic graphs, and the correlation coefficient (r) for each patient was calculated. In each case the correlation coefficient was close to 1 (mean, 0.976; range, 0.940-1.0), which indicates a constant logarithmic increase. Because these data were consistent with an exponential growth model, the BCR-ABL transcript doubling times for each patient were calculated. The doubling time for 14 patients who progressed to blast crisis or accelerated phase was a median of 17 days (25th-75th percentile, 13-35 days), which was significantly shorter than for 24 patients who remained in chronic phase without disease progression at 53 days (25th-75th percentile, 39-87 days), P < .001. The interpretation of these data suggests that a more rapid BCR-ABL transcript doubling time in patients treated with imatinib who achieve at least a MCR may indicate relapse into a more aggressive disease.
Clinical significance of a more than 2-fold rise in patients with very low levels of BCR-ABL. Patients in chronic phase who achieve significant reductions of BCR-ABL of at least 3 logs have a low risk of subsequent disease progression.25 The assay variability at very low levels of BCR-ABL is also greater at these levels. We, therefore, examined the patients who achieved significant reductions in BCR-ABL, which in our assay is approximately equivalent to a BCR-ABL/BCR% value of less than 0.1%, to determine whether a 2-fold rise in this situation was still predictive of mutations. The patients were divided into those who had achieved a BCR-ABL/BCR% value of less than 0.1% at any sampling time point (n = 79) and those who had not (n = 135). The highest incidence of mutations was in the patients who did not achieve a BCR-ABL/BCR% value of less than 0.1% (32 [24%] of 135 patients). However, among these patients there was a significantly higher incidence of mutations in those who had a more than 2-fold rise compared with those who had stable or decreasing BCR-ABL levels (66% versus 1.1%), P < .0001. Therefore, using a rise of more than 2-fold as a signal for mutation screening is more sensitive than using the sole criterion of failure to achieve a significant BCR-ABL reduction. Similarly, in the patients who had achieved a BCR-ABL/BCR% value of less than 0.1%, there was a significantly higher incidence of mutations in those who subsequently had a more than 2-fold rise, compared with those with stable or decreasing BCR-ABL levels (33% versus 0%), P = .0001 (Table 4).
The incidence of mutations in patients who had achieved very low BCR-ABL levels at any time during imatinib therapy
Consecutive BCR-ABL values in 214 patients . | No. patients (%) . | No. patients (%) . | Mutation analysis (%) . |
|---|---|---|---|
| Rise of more than 2-fold | 56 of 214 (26) | ||
| Lowest BCR-ABL/BCR% value achieved less than 0.1 | 9 of 56 (16) | Mutations, 3 of 9 (33) | |
| Lowest BCR-ABL/BCR% value achieved 0.1 or greater | 47 of 56 (84) | Mutations, 31 of 47 (66) | |
| Stable or decreasing levels | 158 of 214 (74) | ||
| Lowest BCR-ABL/BCR% value achieved less than 0.1 | 70 of 158 (44) | Mutations, 0 of 70 (0) | |
| Lowest BCR-ABL/BCR% value achieved 0.1 or greater | 88 of 158 (56) | Mutations, 1 of 88 (1.1) |
Consecutive BCR-ABL values in 214 patients . | No. patients (%) . | No. patients (%) . | Mutation analysis (%) . |
|---|---|---|---|
| Rise of more than 2-fold | 56 of 214 (26) | ||
| Lowest BCR-ABL/BCR% value achieved less than 0.1 | 9 of 56 (16) | Mutations, 3 of 9 (33) | |
| Lowest BCR-ABL/BCR% value achieved 0.1 or greater | 47 of 56 (84) | Mutations, 31 of 47 (66) | |
| Stable or decreasing levels | 158 of 214 (74) | ||
| Lowest BCR-ABL/BCR% value achieved less than 0.1 | 70 of 158 (44) | Mutations, 0 of 70 (0) | |
| Lowest BCR-ABL/BCR% value achieved 0.1 or greater | 88 of 158 (56) | Mutations, 1 of 88 (1.1) |
The interpretation of these data indicates that even in the setting of very low BCR-ABL values a rise of more than 2-fold may have clinical significance in terms of detecting mutations, although it is less likely to yield significant findings than in the setting of higher BCR-ABL levels.
More than 2-fold rise in the patients not having mutations or acquired resistance. Nine of the 56 patients with a more than 2-fold rise in BCR-ABL did not have detectable mutations, acquired resistance, or both. None of these 9 patients have had subsequent increases in the BCR-ABL level. Eight of the 9 patients had very low levels of BCR-ABL at the time of the increase. The rise of more than 2-fold in these patients may be primarily associated with the greater assay variability at low BCR-ABL levels rather than having clinical significance (Figure 3).
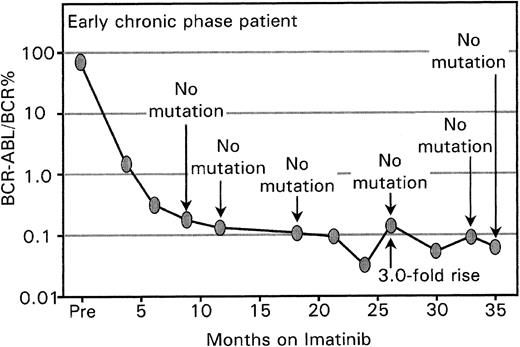
A rise in BCR-ABL in patients with very low levels may not always have biologic significance. Mutation screening in the patient in early chronic phase commenced at 9 months of imatinib. The BCR-ABL level increased by 3-fold from months 24 to 26, but no mutations were detected and the patient maintained a CCR. The BCR-ABL values remained at a very low level, and the rise and fall may reflect the greater variability of the assay at low BCR-ABL levels. A rise at this level may not necessarily have biologic significance.
A rise in BCR-ABL in patients with very low levels may not always have biologic significance. Mutation screening in the patient in early chronic phase commenced at 9 months of imatinib. The BCR-ABL level increased by 3-fold from months 24 to 26, but no mutations were detected and the patient maintained a CCR. The BCR-ABL values remained at a very low level, and the rise and fall may reflect the greater variability of the assay at low BCR-ABL levels. A rise at this level may not necessarily have biologic significance.
In summary, of the 214 patients analyzed, clinical significance as defined by the detection of mutations, acquired resistance, or both, was associated with a more than 2-fold rise in BCR-ABL in 47 (96%) of 49 patients. Of the 165 patients without a mutation or acquired resistance, 156 (95%) had a stable or decreasing BCR-ABL level. Thus, by using a more than 2-fold rise in BCR-ABL as a predictor of clinical significance we find that the estimated false-positive rate is 5% and the estimated false-negative rate is 4%.
Discussion
Identification of the molecular basis of imatinib resistance in patients with CML is valuable because it could provide prognostic information and contribute to determining appropriate therapy to prevent or overcome resistance. An important issue that needs to be resolved is whether screening for mutations at a defined interval, such as every 6 months is sufficient or indeed necessary for every patient. The cost effectiveness of frequent mutation screening in standard clinical practice is not established. Our study establishes a very strong association between a rise in the BCR-ABL transcript level and the detection of kinase domain mutations in patients with CML treated with imatinib. The findings indicate that screening patients for mutations when the BCR-ABL level remains stable or decreases has a low yield and may not be warranted outside the setting of clinical trials.
The measurement reliability of our RQ-PCR assay is an improvement over the original quantitative PCR assays that used competitive PCR,33-36 whereby differences in BCR-ABL levels of half an order of magnitude could be detected.34 By using competitive PCR techniques to monitor patients after transplantation or while on interferon-α therapy, the terms “PCR relapse” or “PCR progression” were proposed to identify patients with pending hematologic relapse. The definition was a more than 10-fold rise in BCR-ABL level in consecutive analyses, which accounted in part for the inaccuracy inherent in the technique.37 In our study we have demonstrated that a rise in BCR-ABL of more than 2-fold reflects a true rise in most cases and can identify most patients with mutations.
Our analysis was retrospective, and various physicians treated the patients with mutations on an individual basis. No controlled trials of therapeutic options to overcome or prevent resistance for the patients with various disease phases were undertaken. The biochemical and biologic sensitivity to imatinib has not been calculated for all of the mutations. It is, therefore, premature to offer specific therapeutic guidelines for every one of the 22 individual mutations that we have detected. However, we have previously shown a strong association of mutations located in the P-loop with a poor prognosis in patients in both accelerated phase and chronic phase.9 Such patients may, therefore, require prompt treatment intervention. Although dose increases may be appropriate for many of the non-P-loop mutations, including patients who are still in hematologic remission, a better understanding of the biologic and clinical effect of each mutation will be needed before specific recommendations can be made. If the decision were made to increase the imatinib dose, close molecular monitoring would be appropriate. Other therapeutic options such as a transplantation or a change of drug therapy should be considered if the BCR-ABL level continues to increase. A more rapid BCR-ABL transcript doubling time may indicate that the patient is relapsing into an aggressive disease.
It is possible that many of the patients with mutations could also have been identified by cytogenetic assessment of the percentage of Ph+ cells in the marrow. We do not have simultaneous marrow cytogenetics and RQ-PCR available in enough cases to make this comparison. However, even if an increase in the percentage of Ph+ cells could identify patients with mutations, it has a limited role as a primary screening strategy. Frequent bone marrow aspirates are difficult and unpleasant for the patient, and marrow cytogenetics often yields insufficient metaphases for accurate quantitation. In addition, 24% of the patients with mutations in our study were still in a CCR at the time the mutation was detected.
With the inclusion of the 3 new mutations reported in this study, a total of 34 different point mutations have been described within the BCR-ABL kinase domain.7,9-15 There is the possibility that further mutations will be detected. Indeed, in a comprehensive survey of amino acid substitutions that confer resistance by using an in vitro screen of randomly mutated BCR-ABL, numerous substitutions were identified in addition to those reported in patients.38 We have now detected some of these in vitro mutations in patients, including the E279K mutation reported in our current study and the L248V and F486S mutations recently identified.9 This suggests that the range of mutations will increase and reinforces the requirement for mutation monitoring. It particularly strengthens the case for screening the entire kinase domain rather than focusing on a strategy that recognizes known mutations.
We conclude that mutation analysis should be initiated if the BCR-ABL level rises by more than 2-fold by means of a reproducible RQ-PCR technique. Furthermore, this rule is applicable even when the BCR-ABL values are very low. It should be noted, however, that we have used our data to both develop a decision rule to initiate mutation analysis and then checked the performance of the rule against the same data set. Therefore, this decision rule needs to be validated by further studies at ours and other centers.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2004-03-1134.
Supported in part by grants from Novartis Pharmaceuticals Australia
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rebecca Lawrence and Chani Field for the excellent technical support provided; Graeme Casey for assistance with the mathematical calculations; John Reynolds for assistance with the statistical calculations; the data managers and laboratory staff of the participating centers, in particular Alison Harper, Rosie Hoyt, Melinda Higgins, Robyn Western, Jenny Larsen, Jenny Muirhead, Laima Muceniekas, Claire Inglis, Sonja Downey, and Karen Wallis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal