Abstract
Homing of bone marrow stromal cells (MSCs) to bone and bone marrow after transplantation, important for the correction of conditions such as metabolic storage disorders, can occur but with poor efficiency. Substantial improvements in engraftment will be required in order to derive a clinical benefit from MSC transplantation. Chemokines are the most important factors controlling cellular migration. Stromal-derived factor-1 (SDF-1) has been shown to be critical in promoting the migration of cells to the bone marrow, via its specific receptor CXCR4. The aim of our study was to investigate CXCR4 expression on MSCs and its role in mediating migration to bone marrow. We show that CXCR4, although present at the surface of a small subset of MSCs, is important for mediating specific migration of these cells to bone marrow. (Blood. 2004; 104:2643-2645)
Introduction
Marrow stromal cells (MSCs) are nonhematopoietic progenitor cells capable of differentiating into tissues such as bone and cartilage.1 Several groups have shown that MSCs can be readily isolated by plastic adherence, expanded several fold, and genetically modified by viral vectors.2,3 Furthermore, they can support the survival and proliferation of human hematopoietic stem cells (HSCs).4 Clinically, MSCs may be used to enhance HSC engraftment after transplantation, to correct inherited disorders of bone and cartilage, or as vehicles for gene therapy.5,6
Crucial to the success of any of these strategies is efficient engraftment to bone and bone marrow. So far results have shown that although MSCs can engraft to those tissues, levels are at the limit of detection and clinically useful only in certain disorders such as osteogenesis imperfecta.7 Higher levels will be required to achieve a therapeutic benefit in the majority of applications.
The chemokine stromal-derived factor-1 (SDF-1) and its ligand CXCR4 play an important role in homing as shown by studies on engraftment of hematopoietic stem/progenitor cells8 and on colonization of bone and bone marrow by metastatic breast and prostate cancer cells.9
We have examined the expression of CXCR4 by MSCs and the migration of these cells to bone marrow stroma. Here we show for the first time that CXCR4 is important for MSC migration to bone marrow. However cell surface receptor levels are low, with large amounts found intracellularly.
Study design
Isolation of MSC cultures and CD34+ cells
Bone marrow aspirates were obtained, under local ethical approval, from the posterior iliac crest of donors aged 0 to 18 years after parental consent. MSCs were isolated by plastic adherence as previously described10 and cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen, Paisley, United Kingdom) + 10% fetal calf serum (FCS; Stem Cell Technologies, Vancouver, BC). CD34+ cells were isolated using the Minimacs system according to the manufacturer's instruction (Miltenyi Biotech, Bisley, United Kingdom)
Reverse-transcription-polymerase chain reaction (RT-PCR)
Total RNA from MSCs or peripheral blood mononuclear cells (PBMCs), the latter activated with 10 μg/mL phytohemagglutinin (PHA; Abbott Murex, Dartford, United Kingdom), was extracted using RNAzol (AMS Biotechnology, Abington, United Kingdom) according to the manufacturer's instructions, then reverse transcribed using a First Strand cDNA Synthesis kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). cDNA was analyzed by PCR for CXCR4 using 2 sets of primers: 668-bp amplicon forward, ATGGAGGGGATCAGTATATACAC and reverse, TGGAGTGTGCTATGTTGGCGTCT or a 206-bp amplicon forward, TTCTACCCCAATGACTTGTG and reverse, ATGTAGTAAGGCAGCCAACA. The following specific primers for β-actin were used to control for efficiency of reverse-transcription reaction: forward, GTGGGGCGCCCCAGGCACCA and reverse, CTCCTTAATGTCACGCACGATTTC. cDNA was subjected to PCR for 30 to 35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds, and with a final elongation step of 10 minutes at 72°C.
Flow cytometry
MSCs were stained with 10 μg/mL mouse monoclonal anti-human CXCR4 (clone 12G5; R&D Systems, Abington, United Kingdom), followed by anti-mouse IgG fluorescein isothiocyanate (FITC; Sigma, Poole, United Kingdom) according to the manufacturer's instructions. For intracellular staining, extracellular CXCR4 receptor was blocked with monoclonal anti-human CXCR4 (clone 12G5, 10 μg/mL, 1 hour, 4°C). Cells were then fixed with 4% paraformaldehyde (Sigma), permeabilized with 0.5% Triton X-100 (Sigma), and stained with anti-human CXCR4-phycoerythrin (PE; BD Biosciences, Oxford, United Kingdom). Cells were analyzed on a FACScalibur with CellQuest software (BD Biosciences).
Western blotting
Cells (105) were lysed in Laemmli buffer according to the manufacturer's instructions (Invitrogen) and run on a 12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) gel. Membranes were hybridized with rabbit anti-human CXCR4 at 1:1000 ± CXCR4 N-terminal peptide (Abcam, Cambridge, United Kingdom) or with anti-β actin (1:500; Sigma). Anti-rabbit horseradish peroxidase (HRP) secondary antibody was used at 1:1000 (Dako, Ely, United Kingdom). Deglycosylation was carried out using N-glycosidase F (PNGase) according to the manufacturer's instructions (New England Biolabs, Herts, United Kingdom).
Chemoinvasion assay
Migration was assayed using inserts with 8-μm pore membranes (BD Biosciences) as described.11 MSCs or PC3 cells were used at 4 × 105 cells/mL in the upper chamber. In the bottom chamber, bone marrow stroma (obtained from Dexter type culture12 after 5 weeks) or SDF-1 (R&D Systems) was used as chemoattractant. For neutralization studies, cells were incubated with anti-human CXCR4 monoclonal antibody (mAb, 10 μg/mL, clone 12G5; R&D Systems).
Statistical analysis
Data are presented as mean ± SEM with statistical significance tested using the 2-tailed paired t test.
Results and discussion
The expression of CXCR4 on human MSCs was studied in 8 MSC cultures (passage 1 to 7). All cultures were CD45- and CD34- and bound the Src homology 2 (SH2) monoclonal antibody raised against human MSCs. CXCR4 mRNA levels were assessed by RT-PCR with 2 sets of primers. The first set spans across both exons and detects isoform 1. The second set is targeted to exon 2 and detects both isoforms of CXCR4 described so far. In MSCs, CXCR4 mRNA levels were generally too low to be easily detected by routine RT-PCR regardless of the passage number of the cells (Figure 1A-B), although occasionally a faint signal was seen in some samples. Posttranscriptional regulation has been described to influence CXCR4 protein expression in Langerhans cells and prostate cancer cells.13-15 Analysis of 4 MSC cultures by staining with an antibody directed against the second extracellular loop of CXCR4 showed the receptor to be present at the cell membrane of less than 1% of the cells, with the exception of one line in which 3.9% of the cells stained positive (Figure 1C). However when we examined the intracellular expression of CXCR4, high levels of receptor expression were found in 83% to 98% of cells (n = 3).This was in contrast to CD34+ cells, in which only 12.2% of the cells were positive as previously published16 (Figure 1D), and to isotype controls, indicating that the signal seen was specific. CXCR4 intracellular storage has been previously described in response to cytokines.17 MSCs are known to secrete several cytokines and chemokines,18 which may be responsible for this phenomenon. Western blot analysis of 2 MSC cultures using an antibody directed against the N-terminal part of CXCR4 confirmed the expression of CXCR4 protein in 2 forms of about 66 and 130 kDa, a higher molecular weight than previously described and seen in our control cell line Jurkat (Figure 1E). This was due to N-glycosylation (Figure 1F). Specificity for CXCR4 was demonstrated by absence of any signal when the blocking CXCR4 N-terminal peptide was added to the assay. These data suggest that the receptor is present but in a highly glycosylated form.
CXCR4 expression in MSC cultures. (A) RT-PCR for CXCR4 expression using the sets of primers for the 668-bp amplicon (left panel) and β-actin expression (right panel). Lane 1 indicates PHA-activated PBMCs; lanes 2 to 4, MSC cultures passage 1 to 2; and lane 5, negative control. (B) RT-PCR for β-actin (top panel) and CXCR4 (bottom panel) expression. Lanes 1 to 2 indicate PHA-activated PBMCs; lanes 3 to 7, MSC cultures at passage 5 to 8; and lane 8, negative control. (C) A representative example of extracellular expression of CXCR4 in MSC cultures by antibody staining (right panel) and isotype-matched control (left panel). FSC indicates forward scatter. (D) A representative example of intracellular expression of CXCR4 in MSC cultures (i, isotype-matched control; ii, CXCR4 antibody staining) and in CD34+ bone marrow cells (iii, isotype-matched control; iv, CXCR4 antibody staining). (E) Western blot analysis using an anti-CXCR4 antibody alone (top left panel) or preadsorbed with a peptide blocking the antibody's binding domain (top right panel) and β-actin expression (bottom panels). Lane 1 indicates Jurkat cell line; and lanes 2 to 3, MSC cultures. (F) Western blot analysis of cellular extracts from MSC cultures treated with PNGase to remove N-glycosylation. Lane 1 indicates Jurkat cell line; lanes 2 and 4, MSC cultures following treatment with PNGase; and lanes 3 and 5, MSC cultures in absence of PNGase.
CXCR4 expression in MSC cultures. (A) RT-PCR for CXCR4 expression using the sets of primers for the 668-bp amplicon (left panel) and β-actin expression (right panel). Lane 1 indicates PHA-activated PBMCs; lanes 2 to 4, MSC cultures passage 1 to 2; and lane 5, negative control. (B) RT-PCR for β-actin (top panel) and CXCR4 (bottom panel) expression. Lanes 1 to 2 indicate PHA-activated PBMCs; lanes 3 to 7, MSC cultures at passage 5 to 8; and lane 8, negative control. (C) A representative example of extracellular expression of CXCR4 in MSC cultures by antibody staining (right panel) and isotype-matched control (left panel). FSC indicates forward scatter. (D) A representative example of intracellular expression of CXCR4 in MSC cultures (i, isotype-matched control; ii, CXCR4 antibody staining) and in CD34+ bone marrow cells (iii, isotype-matched control; iv, CXCR4 antibody staining). (E) Western blot analysis using an anti-CXCR4 antibody alone (top left panel) or preadsorbed with a peptide blocking the antibody's binding domain (top right panel) and β-actin expression (bottom panels). Lane 1 indicates Jurkat cell line; and lanes 2 to 3, MSC cultures. (F) Western blot analysis of cellular extracts from MSC cultures treated with PNGase to remove N-glycosylation. Lane 1 indicates Jurkat cell line; lanes 2 and 4, MSC cultures following treatment with PNGase; and lanes 3 and 5, MSC cultures in absence of PNGase.
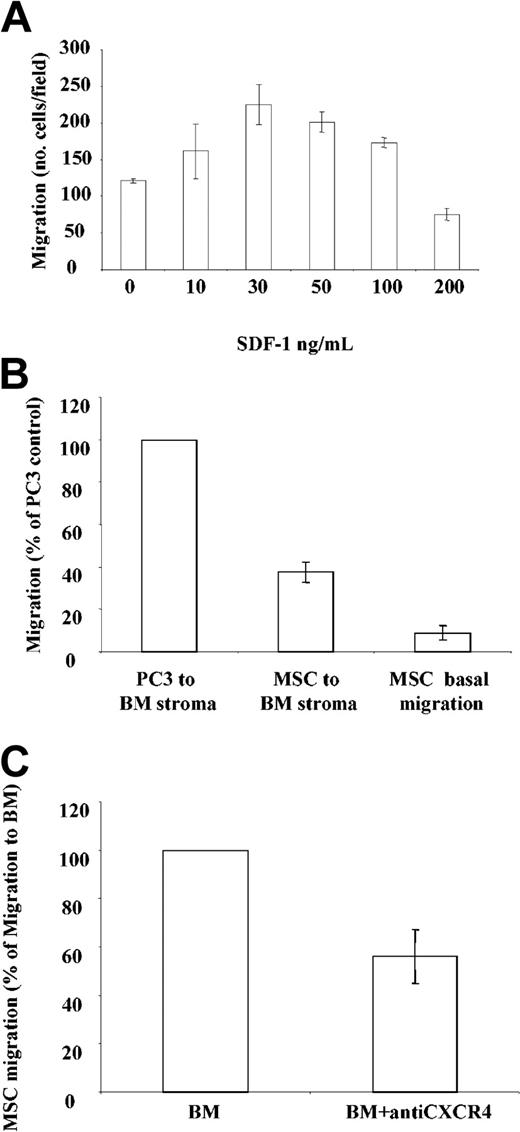
Next, the response of the CXCR4 receptor to the ligand SDF-1 was tested by studying the migration of MSCs in a transwell assay. Dose-dependent migration of MSCs (n = 3) occurred with maximal migration at 30 ng/mL SDF-1. Migration was most pronounced in cells expressing the highest amount of cell surface receptor (Figure 2A). The presence of bone marrow stroma induced significant migration in all MSC cultures compared with basal conditions (P < .002, n = 12; Figure 2B) at levels of 14% to 49% of that seen with the metastatic prostate cancer line PC3. The neutralizing anti-CXCR4 antibody inhibited MSC migration to bone marrow by 46% ± 31% (n = 8, P < .02; Figure 2C), indicating that MSCs express functionally active CXCR4 receptor, which contributes to much of MSC migration to bone marrow.
Chemotactic properties of MSC cultures. (A) Migration of MSCs in response to SDF-1. Results shown are from a representative experiment and are expressed as the average number of migrated cells in 5 fields examined. Each sample was run in duplicate. (B) Migration of MSCs and the prostate line PC3 to bone marrow, expressed as a percentage of PC3 migration. (C) Migration of MSCs to bone marrow in the presence of anti-CXCR4 (expressed as % of migration to bone marrow). All data shown are expressed as mean ± SEM.
Chemotactic properties of MSC cultures. (A) Migration of MSCs in response to SDF-1. Results shown are from a representative experiment and are expressed as the average number of migrated cells in 5 fields examined. Each sample was run in duplicate. (B) Migration of MSCs and the prostate line PC3 to bone marrow, expressed as a percentage of PC3 migration. (C) Migration of MSCs to bone marrow in the presence of anti-CXCR4 (expressed as % of migration to bone marrow). All data shown are expressed as mean ± SEM.
In conclusion, we have shown that the CXCR4 receptor, which is present at low levels at the cell surface, contributes to MSC migration. Strategies to mobilize the internalized receptor and increase its functional expression may be required to improve engraftment of MSCs to bone marrow and bone.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-02-0526.
I.B. is supported by the Jeans for Genes appeal (Mucopolysaccharidosis Society, United Kingdom); C.A.H. and L.J.F. are supported by Cancer Research UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Melissa Baxter for technical help. We thank Dr Donald Palmer and Nathelie Signoret for helpful discussion.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal