Comment on Ingram et al, page 2752
Clonogenic assays define a new hierarchy among endothelial progenitor cells.
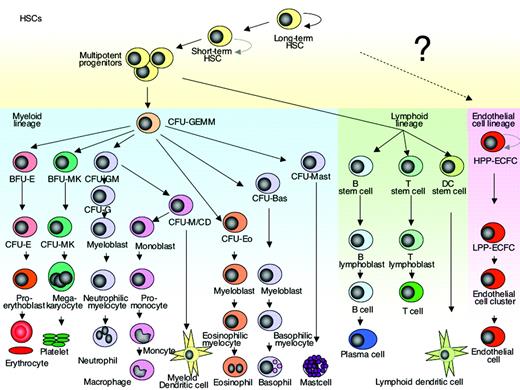
The classical view of stem cell differentiation is a hierarchical model that starts with a primitive stem cell at the top, giving rise to a proliferating progenitor pool from which differentiated blood cells develop. As the progeny of stem cells acquire more specific differentiated characteristics they lose their potential to proliferate. The ability for continuous self-renewal is characteristic of the most primitive stem cells. This ability is lost with advancing differentiation. Hematopoiesis is usually seen as a classical example of hierarchical stem cell differentiation and there are many experimental data that support such a hierarchical model.1-4 A more recent model of stem cell plasticity challenges the classical rigid archetype of hierarchical stem cell differentiation, suggesting that the stem cell, progenitor, and differentiated mature cell relationship is not a fixed hierarchy but rather a reversible continuum (reviewed in Balsam et al5 ).FIG1
Hierarchy of hematopoietic/endothelial stem cell differentiation. The model proposed by Ingram and colleagues implies that endothelial cell differentiation is hierarchical in nature and similar to the differentiation model of the myeloid and lymphoid lineage. The existence of a common progenitor cell that gives rise to both endothelial cells and hematopoietic cells in the adult human bone marrow is of great interest but still remains controversial. The curved arrow indicates self-renewing ability. HSC indicates hematopoietic stem cell; CFU-GEMM, colony-forming unit granulocyte erythrocyte monocyte macrophage; BFU-E, erythroid burst-forming unit; BFU-MK, megakaryocyte burst-forming unit; CFU-GM, granulocyte macrophage colony-forming unit; CFU-G; granulocyte colony-forming unit; CFU-E, erythroid cluster-forming unit; CFU-Eo, eosinophil colony-forming unit; CFU-Baso; basophil colony-forming unit; CFU-Mast; mast cell colony-forming unit; HPP-ECFC, high proliferative potential-endothelial colony-forming cells; LPP-ECFC, low proliferative potential-endothelial colony-forming cells; and EC-cluster, endothelial cell cluster.
Hierarchy of hematopoietic/endothelial stem cell differentiation. The model proposed by Ingram and colleagues implies that endothelial cell differentiation is hierarchical in nature and similar to the differentiation model of the myeloid and lymphoid lineage. The existence of a common progenitor cell that gives rise to both endothelial cells and hematopoietic cells in the adult human bone marrow is of great interest but still remains controversial. The curved arrow indicates self-renewing ability. HSC indicates hematopoietic stem cell; CFU-GEMM, colony-forming unit granulocyte erythrocyte monocyte macrophage; BFU-E, erythroid burst-forming unit; BFU-MK, megakaryocyte burst-forming unit; CFU-GM, granulocyte macrophage colony-forming unit; CFU-G; granulocyte colony-forming unit; CFU-E, erythroid cluster-forming unit; CFU-Eo, eosinophil colony-forming unit; CFU-Baso; basophil colony-forming unit; CFU-Mast; mast cell colony-forming unit; HPP-ECFC, high proliferative potential-endothelial colony-forming cells; LPP-ECFC, low proliferative potential-endothelial colony-forming cells; and EC-cluster, endothelial cell cluster.
In this issue of Blood, Ingram and colleagues use a single-cell colony assay to describe a novel hierarchy amongst human endothelial progenitor cells (EPCs) isolated from peripheral blood and umbilical cord. The identification of a distinct population of progenitor cells is based on their clonogenic and proliferative potential. High-proliferative potential-endothelial colony-forming cells (HPP-ECFCs) are large colonies that form secondary and tertiary colonies upon replating. HPP-ECFCs give rise to all subsequent stages of EPCs to secondary HPP-ECFCs. Low-proliferative potential-endothelial colony-forming cells (LPP-ECFCs) form colonies of more than 50 cells but no secondary LPP-ECFCs upon relating. Endothelial cell clusters (EC clusters) contain fewer than 50 cells and show recognizable features of differentiation; finally, the mature endothelial cells do not divide (Figures 1 and 7 in the paper by Ingram and colleagues in this issue of Blood). There has been a multitude of prior studies that describe the isolation of EPCs from human peripheral blood or umbilical cord. However, many of these so-called EPCs, although expressing some of the markers shared by both hematopoietic cells and EPCs, contained CD14+ cells that maintain the expression of the hematopoietic surface antigen CD45. Although these cells seem to support angiogenesis in vivo, this seemed to occur through the secretion of factors promoting angiogenesis rather than through proliferation and contribution to the lining of the neovasculature. Thus, these cells seemed more like circulating angiogenic cells than EPCs.6 The endothelial cells that outgrow from the progenitor cells isolated by the method described by Ingram and colleagues showed phenotypic and functional characteristics of endothelial cells and lack hematopoietic characteristics such as CD45 and CD14. Genetic studies in mice clearly demonstrate that during embryogenesis, and probably also in the adult bone marrow, mature blood cells and EPCs have a common progenitor cell, the hemangioblast (reviewed in Bailey and Flemming7 ). So far, compelling evidence that such a cell exists in humans is missing and awaits future rigorous genetic and/or functional studies. The existence of such a progenitor cell in adults would be a missing piece in the jigsaw of hematopoiesis and would have all kinds of implications for developing therapy. The ability to isolate and precisely assay EPCs is an important step and will stimulate future research to explore the use of endothelial cells as novel targets for diagnostic and therapeutic agents.
I thank Philip J. Mason and Dan C. Link for interesting discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal