Abstract
Neutrophils are abundant, short-lived leukocytes, and their death by apoptosis is central to hemostasis and the resolution of inflammation, yet the trigger for their entry into apoptosis is unknown. We show here that death receptor signaling, including CD95 death-inducing signaling complex (DISC) formation and caspase 8 activation, occurred early in neutrophil apoptosis. However, death receptor ligation was not required for apoptosis, suggesting a novel mechanism for caspase 8 activation. We detected ceramide generation and clustering of CD95 in lipid rafts early in neutrophil apoptosis, and neutrophil apoptosis and ceramide generation were both significantly inhibited in acid sphingomyelinase knockout (ASM–/–) mice compared to wild-type littermates. Further studies revealed that ceramide generation, CD95 clustering, and neutrophil apoptosis were dependent on reactive oxygen species (ROSs) and were preceded by a fall in reduced glutathione levels. We propose that accumulation of ROSs, as a consequence of altered redox status, initiates ligand-independent death receptor signaling via activation of ASM and clustering of preformed DISC components in lipid rafts and is therefore a primary factor limiting neutrophil life span.
Introduction
Neutrophils are short-lived leukocytes that play a vital role in immune responses to rapidly dividing bacteria, yeast, and fungi. Their spontaneous entry in to apoptosis is a fundamental mechanism involved in maintaining a normal level of circulating neutrophils and ensuring the rapid resolution of inflammatory responses and prevention of chronic inflammatory diseases. However, our understanding of the mechanisms involved in triggering spontaneous neutrophil apoptosis is rudimentary. Apoptosis can be initiated by 2 principal pathways: the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway. In the former, ligation of a death receptor such as CD95 or TNFR1 induces formation of a death-inducing signaling complex (DISC) consisting of the death receptor, an adaptor protein (Fas-associated death domain [FADD] in the case of CD95), and an initiator caspase, predominantly pro-caspase 8.1 Clustering of death receptors following ligation promotes aggregation of pro-caspase 8 molecules within the DISC, inducing their autoproteolysis and generation of active caspase 8, which activates the downstream effector caspase 3.2 In the mitochondrial pathway the caspase cascade is initiated by mitochondrial membrane depolarization and release of proapoptotic factors such as cytochrome c. The latter associates with Apaf-1 and pro-caspase 9 to form the apoptosome, a complex analogous to the DISC. Caspase 9 is activated on the apoptosome and subsequently activates caspase 3.3 Death receptor signaling can be amplified through the mitochondria by caspase 8–mediated cleavage of a Bcl-2 family protein, Bid,4 which inserts in to the mitochondrial membrane and induces release of cytochrome c.
Although neutrophils express several death receptors, namely TNFR1, TRAIL R2,5 as well as CD95 and CD95 ligand,6 the current literature suggests that the mitochondria initiate the apoptotic signal.7,8 Neutrophils express few antiapoptotic proteins, with no detectable Bcl-2 or Bcl-xL, but do contain Mcl-1 and the proapoptotic protein Bax.9 Mcl-1 declines as neutrophils enter apoptosis, while Bax remains constant.9 Furthermore, neutrophil apoptosis is associated with Bax translocation and insertion in to the mitochondrial membrane, and factors delaying this process also delay neutrophil apoptosis.10 However, the literature also contains several reports that neutrophil apoptosis is caspase 8 dependent,11,12 and these observations suggested to us that an alternative signal for the initiation of neutrophil apoptosis must exist.
We therefore looked for evidence of signaling through the mitochondrial and death receptor pathways in neutrophils, focusing upon the kinetics of the events observed. We found significant activation of caspase 8 and cleavage of Bid prior to caspase 3 activation, indicative of a type II death receptor–initiated signal that was amplified through the mitochondria.2,4 However, neutrophil apoptosis was not blocked by neutralizing antibodies to the major death receptors expressed by neutrophils, a finding also reported by others,5 suggesting that death receptor signaling was initiated by a novel mechanism. Death receptor clustering in ceramide-rich lipid rafts is a crucial early event in their signaling,13 with CD95 ligation in the absence of ceramide generation and receptor clustering inducing less than 1% maximal caspase 8 activation.14 We found ceramide generation early in neutrophil apoptosis and showed that acid sphingomyelinase (ASM) was required for ceramide generation and apoptosis. In addition, CD95 became clustered in lipid rafts during culture, and disruption of lipid rafts delayed neutrophil apoptosis. In search of the factor causing activation of ASM, we found that levels of glutathione fell precipitously prior to ceramide generation, and CD95 clustering, ceramide generation, and apoptosis all were dependent upon reactive oxygen species (ROSs). Taken together, our data suggest that neutrophil life span is dictated by the redox status of the cell and that accumulation of ROSs triggers classic death receptor signaling pathways without the need for death receptor ligation.
Materials and methods
Isolation and culture of peripheral blood neutrophils
Human neutrophils were isolated from blood on Percoll density gradients as described previously15 and were greater than 97% neutrophils with the remainder mostly eosinophils. Neutrophils were resuspended in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (Sera International, United Kingdom), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma Aldrich, Poole, United Kingdom), and used immediately as healthy control cells, or were cultured in a humidified 5% CO2 atmosphere to provide apoptotic cells. To determine the role of death receptor ligation, neutrophils also were cultured in the presence of antagonistic IgG (ZB4) anti-CD95 antibodies (Upstate Biotechnology, Buckingham, United Kingdom) as well as antagonistic antibodies to tumor necrosis factorα (TNFα) (Biosource International, Belgium) and membrane-bound TNF-related apoptosis-inducing ligand (TRAIL) (Biosource International). Cytospin preparations (3 minutes, 10g) were made after 3 to 20 hours of incubation, differentially stained using a commercial May-Grunwald Giemsa stain (Diff-Quick, Gamidor, United Kingdom), and assessed for apoptotic morphology.12 Apoptosis also was routinely confirmed by at least one other method, namely caspase 3 activation assessed by fluorescence-activated cell-sorter scanner (FACS) using antibodies to activated caspase 3 (BD Pharmingen, Oxford, United Kingdom) and by binding of annexin V also assessed by FACS analysis using phycoerythrin-conjugated annexin V (BD Pharmingen). To determine the effect of antioxidants on apoptosis, neutrophils were incubated with N-acetylcysteine (100 μM) or desferrioxamine (10 μM) and apoptosis determined after 20 hours. Mouse neutrophils were isolated from blood using Percoll gradients exactly as for human neutrophils, with the exception that the Percoll layers contained 60% and 80% Percoll (Sigma Aldrich).
Measurement of caspase activation and Bid cleavage
Caspase 3, 8, and 9 activities were measured in lysates of neutrophils using commercial kits (R&D Systems, Abingdon, United Kingdom), which rely upon the cleavage of fluorochrome-conjugated caspase peptide substrates DEVD-AFC, IETD-AFC, and LEHD-AFC, respectively, and the release of the fluorochrome. Caspase activity was measured over a 60-minute period and expressed as mean fluorescence intensity per 106 cells. Caspase activation also was determined by Western blotting to detect cleavage of the pro-caspases to active caspases. In addition full-length Bid (22 kDa) is cleaved by caspase 8 to generate the 15-kDa fragment that promotes mitochondrial membrane permeability transition and release of cytochrome c. Cleavage of Bid also was detected by Western blotting. Neutrophils cultured for 0 to 20 hours were spun down, and the pellet was then precipitated with ice-cold 10% trichloroacetic acid and the precipitated proteins spun down at 14000g for 5 minutes at 4° C. The precipitate was washed 3 times in ice-cold acetone and taken up in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and proteins were separated on 12% SDS-PAGE gels. Antibodies to caspase 3 (BD Pharmingen), caspase 8 (Oncogene Research Products, Nottingham, United Kingdom), caspase 9 (BD Pharmingen), and Bid (Biosource International) were used in Western blotting, and blots were developed by enhanced chemiluminescence (ECL; Amersham Pharmacia, Amersham, United Kingdom).
Measurement of mitochondrial membrane permeability transition and GSH levels
Mitochondrial membrane potential was measured using a flow cytometric method based upon the ability of intact mitochondria to take up and retain lipophilic cationic fluorescent dyes. Neutrophils were loaded with 50 nM 3,3′-diheyloxacarbocyanine iodide (DiOC6; Molecular Probes, Cambridge, United Kingdom) or 10 μM 5,5′,6,6′-tetrachloro1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Sigma Aldrich) for 10 minutes and 30 minutes, respectively, at 37° C. Cells were then washed in phosphate buffered saline (PBS), resuspended in ice-cold PBS, and fluorescence analyzed using a Coulter Epics flow cytometer. Glutathione (GSH) concentration was determined in neutrophil lysates using a colorimetric assay using dithio-bis 2-nitrobenzoic acid (DTNB; Sigma Aldrich) as the substrate, and the color reaction was monitored at 405 nm.
Immunoprecipitation of the CD95 DISC
Formation of the CD95 DISC was assessed by immunoprecipitation of CD95 and detection of DISC proteins FADD and pro-caspase 8 in the immunoprecipitate as previously described.16 Briefly, healthy and cultured neutrophils were washed in cold PBS and incubated for 10 minutes at 4° C in PBS containing 0.02% di-(N-succinimidyl) 3,3′ dithiodipropionate (DTSP; Sigma Aldrich). The reaction was stopped by incubating cells for 5 minutes in PBS containing 10 mM ammonium acetate, followed by 2 further washes in cold PBS. Cells were then lysed on ice for 10 minutes in immunoprecipitation (IP) buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl pH 7.5 containing 150 mM NaCl, 1% Triton X-100, 10% glycerol, 50 μg/mL leupeptin, 10 μg/mL pepstatin A, 10 μg/mL aprotinin, and 4 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) and centrifuged at 1000g for 10 minutes at 4° C to remove nuclei and any remaining whole cells. The lysate was then incubated with a monoclonal anti-CD95 antibody (Apo-1, kindly provided by Dr P. H. Krammer, German Cancer Research Center, Heidelberg, Germany) and protein G linked to magnetic beads (μ MACS, Miltenyi Biotech, Surrey, United Kingdom) on ice for 30 minutes and the immunoprecipitated protein eluted in SDS-PAGE sample buffer per the manufacturer's instructions. The immunoprecipitate was analyzed by Western blotting, and blots were probed with a polyclonal rabbit antibody to CD95 ligand or a goat anti-FADD antibody (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit anti–caspase 8 antibody (Oncogene Research), or a rabbit anti-CD95 antibody (C20; Santa Cruz Biotechnology) to determine formation of the DISC. Horseradish peroxidase (HRP)–conjugated anti–goat IgG (Santa Cruz Biotechnology) and anti–rabbit IgG (Amersham Pharmacia) were used as secondary antibodies, and enhanced chemiluminescence was used to reveal immunoreactive bands.
Isolation of lipid raft proteins and chemical disruption of lipid rafts
Proteins located in lipid rafts were isolated exactly as described previously.17 Briefly, 5 × 107 neutrophils were lysed on ice in triton containing buffer (10 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM NaVO4, 10 μg/mL of pepstatin A, aprotinin and leupeptin, 4 mM AEBSF, 1% Triton X-100). Cell debris was removed, and the lysate was adjusted to 42% sucrose, transferred to a 15-mL polypropylene centrifuge tube, and overlaid with lysis buffer containing 30% and 5% sucrose and spun at 200 000g for 16 to 18 hours. Fractions of 1.25 mL were eluted from the centrifuge tube and the proteins collected by trichloroacetic acid (TCA) precipitation prior to analysis by Western blotting for the presence of CD95 and acid sphingomyelinase. Raft fractions were identified using Fyn (Chemicon International, Hampshire, United Kingdom) as positive marker and CD46 (Santa Cruz Biotechnology) as a negative marker of lipid rafts.
To disrupt lipid rafts neutrophils were incubated for 20 hours with a range of concentrations of nystatin or filipin (0.01-10 μg/mL; Sigma Aldrich) or appropriate solvent controls and neutrophil apoptosis determined as described above.
Measurement of cell surface ceramide and CD95 clustering in lipid rafts
Generation of ceramide on the surface of neutrophils was determined by immunostaining13 using an antibody that detects ceramide (15B4; Alexis, Nottingham, United Kingdom) and also by direct measurement of ceramide using a commercial DAG kinase assay kit (Amersham Pharmacia). Clustering of CD95 was detected by immunostaining using an anti-CD95 antibody (ZB4; Upstate Biotechnology) and a species-specific Texas red–linked secondary antibody (DAKO, Cambridge, United Kingdom). Colocalization in lipid rafts was assessed by dual staining of cells at 4° Cin 0.1% sodium azide with biotin-labeled cholera toxin B subunit (Sigma Aldrich) and streptavidin–fluorescein isothiocyanate (FITC) to identify GM1, a lipid raft localized glycolipid. Nonspecific staining was controlled for by the use of isotype-matched immunoglobulins (DAKO). Cells were examined using a Zeiss LSM 510 confocal scanning microscope equipped with a c-apochromat 63 ×/1.2 water objective (Carl Zeiss, Jena, Germany). Images were analyzed using LSM 510 software version 2.3 (Zeiss). Translocation of ASM was determined by differential centrifugation of neutrophil lysates followed by Western blotting with an anti-ASM antibody (Santa Cruz Biotechnology). The heavy membrane fraction containing lysosmes was obtained by centrifugation at 30000g for 20 minutes and the plasma membrane for 100000g for 30 minutes.
Studies with ASM–/– mice
To determine the requirement for ASM in neutrophil apoptosis and ceramide generation, neutrophils were isolated from ASM–/– knockout mice18 and their C57BL6 wild-type littermates. Apoptosis was determined after overnight culture by measurement of loss of DiOC6 retention by FACS analysis. Ceramide generation was measured after short-term culture (6 hours) using the DAG kinase ceramide assay as described above.
Statistics
Data presented here represent a minimum of 3 experiments, and, where appropriate, data are expressed as mean ± SD. Statistical significance was assessed by Student t test, and a P value less than .05 was taken as a significantly different value.
Results
Events characteristic of death receptor signaling occur during spontaneous neutrophil apoptosis
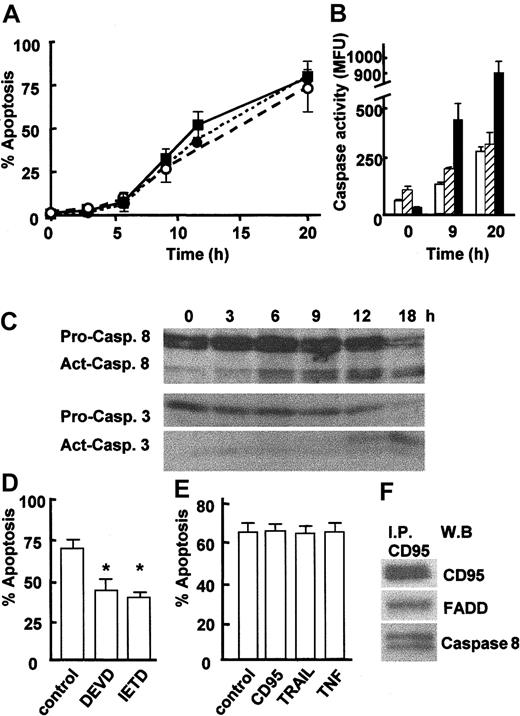
Neutrophils are short-lived cells that will enter apoptosis spontaneously within 24 to 36 hours when cultured in vitro. To investigate the timing of apoptotic events, neutrophils were cultured for up to 20 hours, and apoptosis was measured by assessment of cell morphology, detection of active caspase 3, and binding of annexin V (Figure 1A). Freshly isolated neutrophils contained very few apoptotic cells (2%-3%), but apoptosis gradually increased until approximately 70% of cells were apoptotic after 20 hours in culture (Figure 1A). Measurement of caspase activation using an enzymatic assay revealed that caspases 3, 8, and 9 all were activated during neutrophil apoptosis (Figure 1B). In order to identify the trigger for apoptosis, it was important to establish more precisely the kinetics of caspase 8 activation, as caspase 8 can be activated by caspase 3. Activation of caspases 3 and 8 was therefore monitored at 3-hour intervals by Western blotting to detect cleavage of pro-caspases. Figure 1C confirms the loss of full-length procaspase 8 (53/51 kDa) and pro-caspase 3 (32 kDa) and the appearance of their active cleavage products (41/43 kDa and 17 kDa, respectively) during neutrophil apoptosis, with active caspase 8 detected as early as 6 hours and prior to active caspase 3, which was not detected until 12 hours. Cleavage of pro-caspase 9 also was detected, but this did not occur until 12 hours of culture (data not shown). These data confirm previous reports showing that the activation of caspases 3 and 8 are involved in spontaneous neutrophil apoptosis11,12 and show for the first time that caspase 8 activation is an early event in neutrophil apoptosis occurring prior to caspase 3 activation. The requirement for caspase 8 activation in neutrophil apoptosis was demonstrated by incubating neutrophils with the caspase 8 inhibitor IETD-fmk, which significantly delayed neutrophil apoptosis (Figure 1D). The role of caspase 3 also was confirmed by incubating neutrophils with the caspase 3 inhibitor DEVD-fmk (Figure 1D). Activation of caspase 8 is achieved by its association with death receptor adaptor proteins (FADD) in a death receptor signaling complex (DISC) and subsequent clustering of the death receptor following its ligation. The close proximity achieved by DISC clustering allows autocleavage and activation of caspase 8.1,2 Neutrophils express TNFR1 and TRAIL R25 and CD95 and its ligand CD95 ligand.6 However, coculture of neutrophils with neutralizing antibodies to CD95, TNFα, or membranebound TRAIL had no effect on neutrophil apoptosis (Figure 1E), confirming the recent findings of Renshaw et al.5 These data suggested that a death receptor signaling pathway was activated during neutrophil apoptosis but that this occurred independently of receptor ligation. At the present time the only mechanism known for activation of caspase 8 is its recruitment to and clustering within a death receptor DISC. As the signaling pathway for CD95 is well defined, we next investigated whether the CD95 DISC was formed during neutrophil apoptosis. CD95 was immunoprecipitated from isolated neutrophils, and the immunoprecipitates were then assayed for the presence of FADD and pro-caspase 8, the other key elements of the DISC.1 FADD and pro-caspase 8 were found to coprecipitate with CD95 in freshly isolated neutrophils (Figure 1F), even though there was little active caspase 8 at this time point (Figure 1C). CD95 ligand was not found in the neutrophil DISC complex (data not shown).
Caspase activation during neutrophil apoptosis. (A) Neutrophils were isolated from human peripheral blood and then cultured at 37° C for up to 20 hours. Apoptosis was determined by examination of nuclear morphology in differentially stained cells (• with dotted line) or detection of active caspase 3 in cells by immunostaining and FACS analysis (▪ with solid line), or by measurement of annexin V–PE binding by FACS (○ with dashed line). (B) Caspase activity was determined in an enzymatic assay using lysates from freshly isolated neutrophils (0 hours) and neutrophils cultured for 9 hours or 20 hours. Caspase activity was measured by detecting release of fluorochrome from tetrapeptide substrates preferentially cleaved by caspase 3 (▪), caspase 8 (□), or caspase 9 (▨). Data for panels A and B are mean ± SD of 3 separate experiments. (C) Lysates were prepared from freshly isolated 0-, 3-, 6-, 9-, 12-, or 18-hour cultured apoptotic neutrophils and analyzed by Western blotting for the presence of caspase 8 and caspase 3. The full-length 32-kDa and 56-kDa (pro.) and cleaved 17-kDa and 41/42-kDa (act.) forms of caspase 3 and 8, respectively, are indicated. Representative of 3 experiments. (D) Neutrophils were cultured for 20 hours in the absence or presence of the caspase 8 inhibitor IETD-fmk (20 μM) or the caspase 3 inhibitor DEVD-fmk (50 μM), and apoptosis was measured by analysis of cell morphology. (E) Neutrophils were cultured in the absence or presence of antagonistic antibodies to CD95 (clone ZB4, 100 ng/mL), TNFα (100 ng/mL), or membrane-bound TRAIL (1 μg/mL) for 20 hours, and apoptosis was determined by analysis of cell morphology. Data for panels D and E are mean ± SD of 3 separate experiments. *P < .01. (F) Freshly isolated neutrophils were lysed and incubated with an anti-CD95 antibody (Apo-1). CD95 immunoprecipitates were then analyzed by Western blotting for the presence of FADD and pro-caspase 8. A representative of 3 experiments is shown.
Caspase activation during neutrophil apoptosis. (A) Neutrophils were isolated from human peripheral blood and then cultured at 37° C for up to 20 hours. Apoptosis was determined by examination of nuclear morphology in differentially stained cells (• with dotted line) or detection of active caspase 3 in cells by immunostaining and FACS analysis (▪ with solid line), or by measurement of annexin V–PE binding by FACS (○ with dashed line). (B) Caspase activity was determined in an enzymatic assay using lysates from freshly isolated neutrophils (0 hours) and neutrophils cultured for 9 hours or 20 hours. Caspase activity was measured by detecting release of fluorochrome from tetrapeptide substrates preferentially cleaved by caspase 3 (▪), caspase 8 (□), or caspase 9 (▨). Data for panels A and B are mean ± SD of 3 separate experiments. (C) Lysates were prepared from freshly isolated 0-, 3-, 6-, 9-, 12-, or 18-hour cultured apoptotic neutrophils and analyzed by Western blotting for the presence of caspase 8 and caspase 3. The full-length 32-kDa and 56-kDa (pro.) and cleaved 17-kDa and 41/42-kDa (act.) forms of caspase 3 and 8, respectively, are indicated. Representative of 3 experiments. (D) Neutrophils were cultured for 20 hours in the absence or presence of the caspase 8 inhibitor IETD-fmk (20 μM) or the caspase 3 inhibitor DEVD-fmk (50 μM), and apoptosis was measured by analysis of cell morphology. (E) Neutrophils were cultured in the absence or presence of antagonistic antibodies to CD95 (clone ZB4, 100 ng/mL), TNFα (100 ng/mL), or membrane-bound TRAIL (1 μg/mL) for 20 hours, and apoptosis was determined by analysis of cell morphology. Data for panels D and E are mean ± SD of 3 separate experiments. *P < .01. (F) Freshly isolated neutrophils were lysed and incubated with an anti-CD95 antibody (Apo-1). CD95 immunoprecipitates were then analyzed by Western blotting for the presence of FADD and pro-caspase 8. A representative of 3 experiments is shown.
The mitochondrial pathway is also involved in neutrophil apoptosis
As both caspase 8 and caspase 9 had been activated during neutrophil apoptosis, it was important to determine the kinetics of mitochondrial events during neutrophil apoptosis. Freshly isolated neutrophils contained mitochondria that took up and retained DiOC6 (Figure 2A) and JC-1 (Figure 2A right), markers of mitochondrial membrane integrity and function. However, in culture neutrophils began to lose DiOC6 and JC-1 retention after 9 hours (Figure 2A), confirming membrane permeability transition and loss of mitochondrial membrane potential in neutrophil apoptosis, but suggesting that this occurred after caspase 8 activation. Activation of caspase 8 can be linked to loss of mitochondrial membrane potential by the cleavage of the bcl-2 family protein Bid, as the cleaved form of Bid can insert in to the mitochondrial membrane and contribute to permeability transition.4 Figure 2B shows that full-length 22-kDa Bid was expressed in freshly isolated neutrophils and was cleaved to the 15-kDa form as neutrophils were cultured. Bid cleavage was readily detected after 9 hours of culture, supporting the DiOC6 and JC-1 data showing that the mitochondrial membrane was targeted during spontaneous apoptosis in neutrophils.
Mitochondrial permeability transition and Bid cleavage during neutrophil apoptosis. (A) Freshly isolated neutrophils and neutrophils cultured for 6, 9, or 20 hours were loaded with DiOC6 or JC-1 and analyzed for uptake and retention of the dyes by FACS analysis. A representative of 4 experiments is shown. (B) Lysates were prepared from freshly isolated neutrophils and neutrophils cultured for 0, 3, 6, 9, 12, and 18 hours and analyzed by Western blotting for the presence of full-length 22-kDa Bid and the cleaved 15-kDa form. A representative of 3 experiments is shown.
Mitochondrial permeability transition and Bid cleavage during neutrophil apoptosis. (A) Freshly isolated neutrophils and neutrophils cultured for 6, 9, or 20 hours were loaded with DiOC6 or JC-1 and analyzed for uptake and retention of the dyes by FACS analysis. A representative of 4 experiments is shown. (B) Lysates were prepared from freshly isolated neutrophils and neutrophils cultured for 0, 3, 6, 9, 12, and 18 hours and analyzed by Western blotting for the presence of full-length 22-kDa Bid and the cleaved 15-kDa form. A representative of 3 experiments is shown.
Taken together, these data suggest that a DISC forms spontaneously in human neutrophils and that a second event is then necessary to induce activation of caspase 8 and initiate neutrophil apoptosis. The caspase 8 signal is then amplified through the mitochondria via cleavage of Bid.
ASM and ceramide are required for neutrophil apoptosis
As the CD95 DISC was formed in freshly isolated neutrophils that did not have significant levels of active caspase 8 (Figure 1C), it was necessary to determine how DISC function was promoted to produce the caspase 8 activation seen by 6 to 9 hours of culture. Caspase 8 activation following CD95 ligation involves the translocation of acid sphingomyelinase (ASM) to the cell membrane, leading to the generation of ceramide-rich lipid rafts, which then induced clustering of the DISC and amplification of CD95 signaling and caspase 8 activation.10,11 To determine whether caspase 8 activation and apoptosis in neutrophils might also be driven by ASM activation and ceramide generation, we first looked for generation of ceramide during in vitro culture. Figure 3A shows that significant levels of ceramide were detected in neutrophils after 4 to 6 hours of culture and concomitant with the detection of caspase 8 activation (Figure 1C). To determine whether ASM was the enzyme involved in early ceramide generation during neutrophil apoptosis, we measured ceramide generation in neutrophils isolated from ASM knockout mice and looked for ASM mobilization from lysosomes (heavy membrane, HM fraction) to the cell membrane during neutrophil apoptosis. Ceramide generation was detected in neutrophils from wild-type mice after 6 hours in culture, but this increase was ablated in ASM–/– mice (Figure 3B), confirming that ASM was responsible for ceramide generation. Furthermore, fractionation of neutrophils revealed that although freshly isolated cells had significant levels of ASM in the cell membrane, this was further increased after 6 hours' culture (Figure 3C), confirming that ASM was mobilized to the cell membrane during the early stages of neutrophil apoptosis. Crucially, neutrophils from ASM–/– mice showed a significantly reduced level of apoptosis after overnight culture, evidenced by increased retention of DiOC6 in neutrophils from ASM–/– mice (Figure 3D). Only 10% to 15% of neutrophils retained DiOC6 in the wild-type mice, compared with approximately 60% in the ASM-deficient mice (Figure 3E). ASM is therefore required for both apoptosis and ceramide production during the early phases of neutrophil apoptosis. As well as confirming a requirement for ASM in neutrophil apoptosis, the data in Figure 3D also show that the ceramide generation is an early event in neutrophil apoptosis and could therefore drive caspase 8 activation.
ASM activation and ceramide generation in neutrophil apoptosis. (A) Freshly isolated human neutrophils and neutrophils cultured for 4 and 6 hours were lysed and assayed for ceramide content (picomoles per million cells) using a DAG kinase assay. (B) Neutrophils were isolated from C57BL6 wild-type (+/+) and ASM knockout (–/–) mice, and ceramide content was determined after 0 hours (□) and 6 hours (▪) of culture. (C) Human neutrophils were incubated for 0 hours or 6 hours, lysed, and the heavy membrane (HM), cytosol (Cyt), and plasma membrane (PM) fractions isolated by differential centrifugation and probed for ASM by Western blotting. A representative of 3 experiments is shown. Neutrophils were isolated from C57BL6 wild-type (+/+) and ASM knockout (–/–) mice and cultured for 20 hours. Apoptosis was measured by loss of DiOC6 staining assessed by FACS; open area indicates DiOC6 low cells and shaded area indicates DiOC6 high cells (D) and expressed as the percentage of apoptotic cells (E). For panels B and E data are mean ± SD of 4 mice.
ASM activation and ceramide generation in neutrophil apoptosis. (A) Freshly isolated human neutrophils and neutrophils cultured for 4 and 6 hours were lysed and assayed for ceramide content (picomoles per million cells) using a DAG kinase assay. (B) Neutrophils were isolated from C57BL6 wild-type (+/+) and ASM knockout (–/–) mice, and ceramide content was determined after 0 hours (□) and 6 hours (▪) of culture. (C) Human neutrophils were incubated for 0 hours or 6 hours, lysed, and the heavy membrane (HM), cytosol (Cyt), and plasma membrane (PM) fractions isolated by differential centrifugation and probed for ASM by Western blotting. A representative of 3 experiments is shown. Neutrophils were isolated from C57BL6 wild-type (+/+) and ASM knockout (–/–) mice and cultured for 20 hours. Apoptosis was measured by loss of DiOC6 staining assessed by FACS; open area indicates DiOC6 low cells and shaded area indicates DiOC6 high cells (D) and expressed as the percentage of apoptotic cells (E). For panels B and E data are mean ± SD of 4 mice.
Lipid raft disruption delayed neutrophil apoptosis
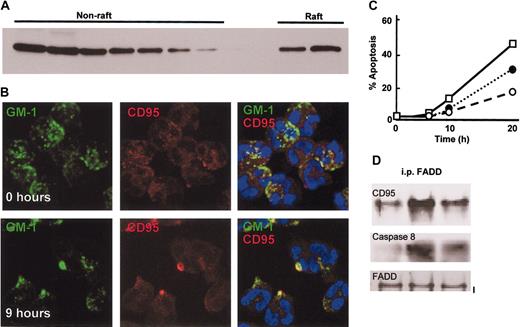
To determine whether neutrophil apoptosis might also involve ceramide-rich lipid raft structures, which are important for death receptor–induced apoptosis,13,19 lipid raft proteins were isolated from freshly isolated neutrophils and probed for CD95. Figure 4A shows that a proportion of CD95 was located within the raft fraction. The location of lipid raft containing fractions in the sucrose gradient was confirmed by assessing fractions for Fyn, a protein known to be present within rafts and using CD46 as a negative control (data not shown). CD95 signaling has been shown to require lipid rafts and the clustering and capping of CD95 at the cell surface.13 If spontaneous neutrophil apoptosis involves this pathway, then clustering of CD95 in lipid rafts should occur during culture of neutrophils, and entry in to apoptosis and apoptosis should be affected by the disruption of lipid rafts. Staining of neutrophils with biotin-labeled cholera toxin B, which binds the lipid raft localized glycolipid GM1, revealed that GM1 had a patchy appearance distributed over the entire surface of the cells in freshly isolated neutrophils. In contrast GM1 staining was clustered in larger patches as cells were cultured (Figure 4B). Increased clustering of GM1 was detected by 6 to 9 hours of culture with approximately 30% of cells showing clustered GM1. This process occurred subsequent to ceramide generation (Figure 3) and concomitant with caspase 8 activation (Figure 1C). Moreover, neutrophils were dual stained for CD95, and its distribution was also patchy and partially colocalized with GM1 in freshly isolated neutrophils (Figure 4B), in agreement with the lipid raft isolation data (Figure 5A). By 6 to 9 hours of culture CD95 staining showed clear clustering and colocalization with GM1 in lipid rafts (yellow pseudocolor; Figure 4B), resembling the capping seen following ligation of CD95 by CD95 ligand.13 All staining procedures were carried out at 4° C and in the presence of 0.1% sodium azide. Addition of the lipid raft disrupting agents nystatin and filipin inhibited neutrophil apoptosis (Figure 4C), and nystatin also reduced CD95 DISC formation during neutrophil in vitro culture and apoptosis (Figure 4D).
Neutrophil apoptosis involves clustering of CD95 in lipid rafts. (A) CD95 is partially localized in lipid rafts in freshly isolated neutrophils. Neutrophils were lysed in Triton X-100–containing buffer at 4° C, and the lysate was fractionated on a sucrose density gradient to separate raft and nonraft proteins. Gradient fractions were analyzed by Western blotting for the presence of CD95. (B) Lipid rafts containing CD95 cluster in aged neutrophils. Neutrophils were cultured for 0 hours or 9 hours and then stained with FITC-conjugated cholera toxin B subunit to locate GM1 in lipid rafts and a nonactivating anti-CD95 antibody (ZB4) and a Texas red–conjugated anti–mouse IgG1 antibody. The images shown are representative of 3 separate experiments performed. (C) Neutrophil spontaneous apoptosis depends upon intact lipid rafts; disruption of lipid rafts inhibited neutrophil apoptosis. Neutrophils were cultured for up to 20 hours in the absence or presence of 10 μg/mL nystatin (○), 10 μg/mL filipin (•), or 0.1% methanol as solvent control (□), and apoptosis determined by analysis of cell morphology. (D) Assembly of the DISC complex depends on intact lipid rafts. Neutrophils were cultured with and without nystatin, lysed, and incubated with an anti-FADD antibody. FADD immunoprecipitates were then analyzed by Western blotting for the presence of CD95 and pro-caspase 8. The blots shown are representative of 3 separate experiments performed.
Neutrophil apoptosis involves clustering of CD95 in lipid rafts. (A) CD95 is partially localized in lipid rafts in freshly isolated neutrophils. Neutrophils were lysed in Triton X-100–containing buffer at 4° C, and the lysate was fractionated on a sucrose density gradient to separate raft and nonraft proteins. Gradient fractions were analyzed by Western blotting for the presence of CD95. (B) Lipid rafts containing CD95 cluster in aged neutrophils. Neutrophils were cultured for 0 hours or 9 hours and then stained with FITC-conjugated cholera toxin B subunit to locate GM1 in lipid rafts and a nonactivating anti-CD95 antibody (ZB4) and a Texas red–conjugated anti–mouse IgG1 antibody. The images shown are representative of 3 separate experiments performed. (C) Neutrophil spontaneous apoptosis depends upon intact lipid rafts; disruption of lipid rafts inhibited neutrophil apoptosis. Neutrophils were cultured for up to 20 hours in the absence or presence of 10 μg/mL nystatin (○), 10 μg/mL filipin (•), or 0.1% methanol as solvent control (□), and apoptosis determined by analysis of cell morphology. (D) Assembly of the DISC complex depends on intact lipid rafts. Neutrophils were cultured with and without nystatin, lysed, and incubated with an anti-FADD antibody. FADD immunoprecipitates were then analyzed by Western blotting for the presence of CD95 and pro-caspase 8. The blots shown are representative of 3 separate experiments performed.
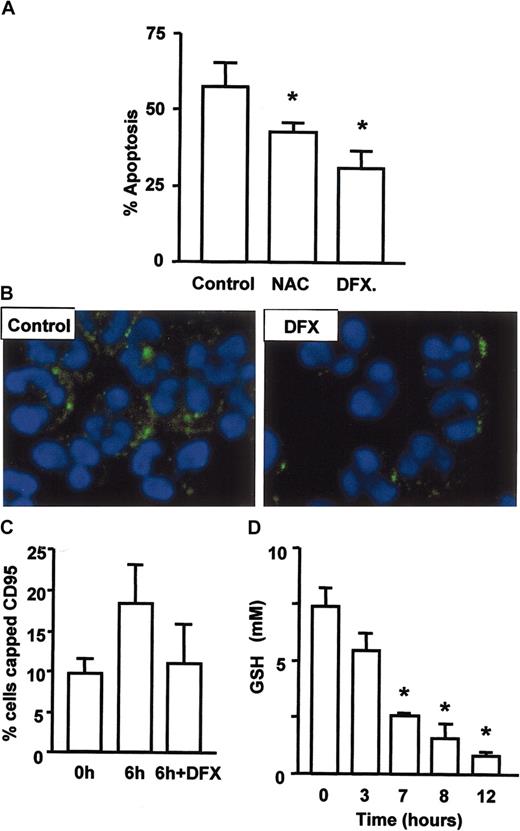
ROS regulate neutrophil apoptosis and CD95 clustering. (A) Apoptosis was delayed by inhibitors of oxygen radical formation. Neutrophils were cultured with 100 μM N-acetylcysteine or 10 μM desferrioxamine for 18 hours prior to assessment of apoptosis by cell morphology. Generation of ceramide and clustering of CD95 on aging neutrophils depends on the generation of hydroxyl radicals. Neutrophils were cultured with and without desferrioxamine, stained for ceramide (B) or CD95 and cells with clustered CD95 enumerated (C). (D) Intracellular levels of GSH were reduced within 3 to 7 hours of neutrophil culture to one third of the original level. Data for panels A, C, and D are mean ± SD of 3 separate experiments. *P < .05.
ROS regulate neutrophil apoptosis and CD95 clustering. (A) Apoptosis was delayed by inhibitors of oxygen radical formation. Neutrophils were cultured with 100 μM N-acetylcysteine or 10 μM desferrioxamine for 18 hours prior to assessment of apoptosis by cell morphology. Generation of ceramide and clustering of CD95 on aging neutrophils depends on the generation of hydroxyl radicals. Neutrophils were cultured with and without desferrioxamine, stained for ceramide (B) or CD95 and cells with clustered CD95 enumerated (C). (D) Intracellular levels of GSH were reduced within 3 to 7 hours of neutrophil culture to one third of the original level. Data for panels A, C, and D are mean ± SD of 3 separate experiments. *P < .05.
Taken together, these data show that many of the features of CD95 signaling are present in the early stages of neutrophil apoptosis, in the absence of death receptor ligation, and with ASM-generated ceramide playing a role in initiation of CD95 signaling and caspase 8 activation.
ROSs play a key role in neutrophil spontaneous apoptosis
As death receptor ligation was not the signal for ASM activation, CD95 clustering, and neutrophil apoptosis, we investigated possible alternatives. ROSs are known to activate neutral sphingomyelinase in cells undergoing apoptosis,20 and oxidation of a C-terminal cysteine residue has been proposed to induce acid sphingomyelinase activation.21 In addition, catalase has been shown to inhibit sphingomyelinase activation during daunorubicin-induced apoptosis.22 We therefore investigated the ability of antioxidants to modulate neutrophil apoptosis and the early events in the process such as CD95 clustering and ceramide generation. Co-incubation with N-acetylcysteine delayed neutrophil apoptosis, but the more effective agent was desferrioxamine (Figure 5A), an inhibitor of hydroxyl radical generation via the Fenton reaction. These data suggest a key role for hydroxyl radicals in neutrophil apoptosis, and interestingly, this ROS has a particularly high oxidation potential (2.31V compared with 0.33V for superoxide). That ROSs were mediating their effect through activation of ASM and death receptor signaling was confirmed by the inhibition of both ceramide generation (Figure 5B) and CD95 clustering (Figure 5C) by desferrioxamine. The ability of ROSs to induce cell death is well documented,23 though the toxic potential of ROSs is limited by several mechanisms, one of the most important being the presence of reduced glutathione (GSH), a powerful antioxidant and the major determinant of redox status in mammalian cells. We therefore measured GSH levels in neutrophils and found that they fell dramatically in the first 3 to 7 hours of culture (Figure 5D), prior to detection of any of the physiologic changes indicative of commitment to apoptosis, such as mitochondrial membrane depolarization or caspase activation. These data show that a change in redox status is the earliest event in neutrophil apoptosis and could be the trigger for cell death.
Discussion
Neutrophils are very short-lived cells with an average survival time in vivo of fewer than 2 days, but a number of cytokines found at inflammatory sites such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon-β (IFN-β)24 can extend their life for several days. However, the homeostatic regulation of neutrophil numbers is largely at the level of production, with 1 to 2 × 1011 cells produced every day. The mechanisms that lead to cell death so rapidly after the release of neutrophils in to the circulation have been unclear. The dominant paradigm has suggested that neutrophil entry in to spontaneous apoptosis is regulated at the level of the mitochondria.7,8 However, several lines of evidence now question this model. Crucially, the activation of caspase 8 occurs before caspase 3 in spontaneous neutrophil apoptosis, and neutrophil apoptosis is caspase 8 dependent,11,12 which suggests a death receptor–mediated mechanism. Neutrophils express a variety of specific death receptors, namely TNFR1 and CD95 and receptors for TRAIL, and we have shown here that CD95 DISC formation and signaling occurs during the induction of neutrophil death. Intriguingly, disruption of lipid rafts significantly inhibits CD95 DISC formation, and neutrophil apoptosis and lipid rafts are also required for CD95 signaling and apoptosis.13
These data suggest a key role for death receptor signaling in the induction of spontaneous neutrophil apoptosis, but these signals do not appear to require ligation of the death receptors. However, neutrophils begin to express CD95 ligand as they are cultured in vitro,6 so it was important to rule out DISC formation and signaling as a result of autocrine CD95 ligation. Several lines of evidence would appear to eliminate CD95 ligation as the initiator of neutrophil apoptosis, for example, antibodies that block CD95 ligand binding do not inhibit neutrophil apoptosis.5,25 Also, neutrophil apoptosis is not affected in gld mice, which completely lack the CD95 ligand, or in lpr mice, which express a form of the CD95 molecule incapable of binding the ligand.26 Finally, the DISC complex present in neutrophils did not contain CD95 ligand (data not shown). These data strongly suggest that the DISC formed spontaneously in neutrophils, possibly upon their release from the bone marrow. There is a precedent for this proposal, as spontaneous DISC formation also has been reported to occur in anoikis,27 which is cell death resulting from the detachment of adherent cells from neighboring cells or stromal support matrix. Survival signals in this situation may therefore act to prevent formation of the DISC, which will occur spontaneously in their absence. Neutrophils are produced from precursors in the bone marrow, and upon their release in to the circulation they lose contact with marrow survival factors such as G-CSF and GM-CSF and die within 48 hours. Neutrophil apoptosis therefore represents a classic example of death occurring as a result of the removal of a survival signal, and the presence of a preformed DISC in neutrophils may determine their short life span. There is another example of DISC formation occurring in the absence of death receptor ligation: Hennino et al28 reported that the CD95 DISC was formed, independently of CD95 ligation, in germinal center B cells. In these cells the DISC also contained the caspase 8 inhibitor c-FLIPL, and apoptosis was prevented only if the B cells received a survival signal from CD40L. We could not detect c-FLIPL in the DISC immunoprecipitated from neutrophils (data not shown), though it is possible that this could be induced at inflammatory sites by cytokines that inhibit neutrophil apoptosis.
We and others have shown that CD95 and other members of the TNF receptor family19,29 localize to lipid rafts. During ligand-induced activation of death receptors, a crucial step is the clustering of receptors30 and DISCs, driven by coalescence of lipid rafts. This process is dependent on the activation of ASM and consequent generation of ceramide within the cell membrane.13,14 This suggested to us a possible mechanism for the generation of ligand-independent death receptor signals through DISC aggregation. We observed both generation of ceramide and clustering of CD95 during spontaneous apoptosis in human neutrophils and neutrophils from mice deficient in the acid sphingomyelinase gene (ASM–/–) did not show an early increase in ceramide during cell culture, and their entry in to apoptosis was profoundly retarded. These results clearly suggest that DISC aggregation mediated by ceramide-induced coalescence of lipid rafts leads to death receptor signals through CD95, even in the absence of ligand. As several TNF family receptors also reside in lipid rafts,19,29 it is possible that the activation of ASM also will lead to clustering of other death receptors, including TNFR1, providing a further explanation for the normal rate of neutrophil apoptosis in lpr and gld mice.26 Indeed, preliminary data from Edwin Chilvers laboratory have shown the presence of a preassembled TNFR1 DISC in neutrophils (Edwin R. Chilvers, Cambridge University, oral communication, November 19, 2003).
The mechanisms that lead to the activation of ASM in spontaneous neutrophil apoptosis effectively constitute a biologic time switch, and our data reveal a key role for ROSs in this process. Recent reports indicate that activated neutrophils also die via caspase 8–mediated apoptosis, and the trigger appears to be dependent upon reactive oxygen species.31 Furthermore, neutrophils deficient in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase undergo apoptosis at a reduced rate,32 and incubation of neutrophils under hypoxic conditions significantly inhibits apoptosis.33 Our observation that spontaneous neutrophil death, ceramide generation, and CD95 clustering are inhibited by desferrioxamine clearly suggests that ROS accumulation, and possibly hydroxyl radicals in particular, is the initial trigger in this process. In support of this proposal, a recent report has shown that the induction of apoptosis by DNA-damaging agents was mediated by ROS-dependent clustering of CD95.34
What, then, is the role of the mitochondria in neutrophil apoptosis, and how can our data be reconciled with the extensive literature suggesting a central role for this pathway? Others have proposed that alterations in the ratio of pro- to anti-apoptotic bcl-2 family proteins occur as neutrophils age and enter apoptosis9 and that the insertion of proteins such as Bax in to the mitochondrial membrane is the trigger for apoptosis.10 The data reported here also support a role for loss of mitochondrial membrane integrity in spontaneous neutrophil apoptosis, but secondary to DISC formation, caspase 8 activation, and Bid cleavage. In fact the events of death receptor signaling (caspase 8 activation) and Bax translocation to the mitochondria may be linked, as ceramide has been shown here to play a role in caspase 8 activation and by others to induce Bax translocation to the mitochondria in myeloid cells.35 The generation of ceramide may therefore trigger and coordinate signals for neutrophil apoptosis occurring at the cell membrane and within the mitochondria.
In summary, we propose that neutrophil spontaneous apoptosis occurs through the effects of ROSs, leading directly to the generation of ceramide and consequent clustering of death receptors. A clear implication of this pathway is that any death receptor–expressing cell exposed to a threshold level of ROSs is likely to activate ligand-independent clustering of death receptors leading to apoptosis.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-01-0191.
Supported by a program grant from the Arthritis Research Campaign (D.S.T.), a PhD studentship from the United Birmingham Hospitals Endowment Fund (K.W.), The Biotechnology and Biological Sciences Research Council and Rhone-Poulenc Rohrer (R.C.), and the European Union (P.R.W.). L.E.C. was the recipient of a Wellcome Trust Vacation Scholarship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal