Abstract
Common variable immunodeficiency (CVID) is characterized by hypogammaglobulinemia and defects in T-cell functions that could be primary or secondary. We addressed whether CVID is associated with impairment in the dendritic cell (DC) compartment, as DCs play a central role in the development of adaptive immunity. We demonstrate that DCs from CVID patients display severely perturbed differentiation, maturation, and function, and express markedly reduced levels of the costimulatory molecules that are critical for T-cell stimulation. Patients' DCs induced weak proliferation of allogeneic T cells and produced significantly low amounts of interleukin-12 (IL-12) upon CD40 signaling. Multiple defects in the immune system, including malfunctioning of DCs, appear to be prominent features of CVID patients. Impairment in both the innate and adaptive compartments of the immune system may thus cumulatively account for the inability of CVID patients to eradicate pathogens through conventional immune pathways, thus resulting in an increased risk for recurrent bacterial infections.
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous group of disorders characterized by hypogammaglobulinemia and a number of T- and B-cell defects.1-9 The fact that some patients' B cells can secrete immunoglobulin M (IgM) and IgG in vitro, while these patients are hypogammaglobulinemic in vivo, implies that B cells may not receive appropriate signaling for class switch and affinity maturation10-12 and that perturbed cellular interactions in germinal centers may be involved in the pathogenesis of the disease. Under physiologic conditions, immune responses are initiated in the T-cell areas of secondary lymphoid organs, where naive T cells encounter dendritic cells (DCs).13 DC-derived cytokines play a crucial role in the cascade of events leading to priming of naive T cells. The T-helper cells in turn induce B-cell growth and antibody production. However, direct interactions between DCs and B cells also occur.13 As DCs play a critical role in bridging innate and adaptive immunity, we addressed the issue of whether CVID is associated with an impaired DC compartment.
Study design
Heparinized blood samples were collected from 10 CVID patients at least 21 days following the last infusion of intravenous immunoglobulin (IVIg; patients 1 to 10), and from 2 newly diagnosed naive CVID patients prior to IVIg therapy (patients 11 and 12), upon approval by local ethics committees. CVID patients were heterogeneous in clinical presentation and were associated with recurrent pneumonia, granulomatous disease, lamblia, or autoimmune diseases (Supplementary Table 1). As control, blood samples were obtained from 3 patients (patients 1, 2, and 3) with selective antibody deficiencies (deficiency in IgG4; IgG2 and IgG4; and IgG4 and IgA, respectively), 1 patient with hyper IgM syndrome (patient 4) who received IVIg similar to CVID patients, and 6 healthy controls. Monocyte-derived DCs from patients' blood and from control groups were generated as described14 in the presence of 10% autologous plasma.
For allogeneic mixed lymphocyte reaction (MLR), DCs from CVID patients and healthy donors were exposed to CD4+ T-cells of third-party healthy donors.14 The CD4+ T cells were isolated by magnetic cell sorter (MACS) cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany).
Results and discussion
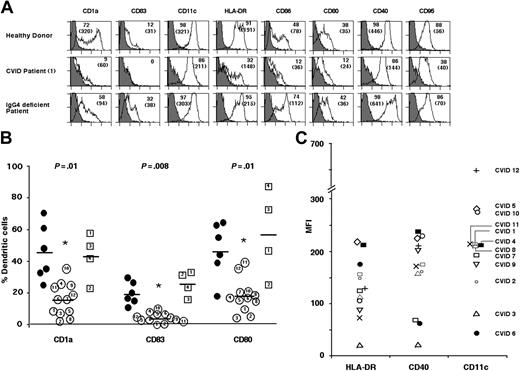
DCs of CVID patients expressed lower levels of CD1a (15.7 ± 10.6%) compared with DCs of healthy donors (46.1 ± 18.4%) and of control patients (42.3 ± 15.8%). The percentage of DCs from CVID patients expressing CD83 and CD80 was also significantly lower than in control groups (P ≤ .01, Mann-Whitney test) (Figure 1A-B). The expression of HLA-DR (mean fluorescence intensity [MFI], 131.6 ± 58.4), CD11c (224.4 ± 150.7), and CD40 (160.6 ± 72.4) (Figure 1C) on CVID patients' DCs was also significantly lower than that of DCs from healthy donors (n = 6) (201.5 ± 108.5, 397.7 ± 189.8, and 269.5 ± 112.9 for HLA-DR, CD11c, and CD40, respectively) and control patients (n = 4) (312.5 ± 182.7, 262.5 ± 27.4, and 323.8 ± 240.6 for HLA-DR, CD11c, and CD40, respectively) (P < .05; Mann-Whitney test) indicative of an impaired differentiation of DCs in patients with CVID. The defective differentiation of DCs from CVID patients was also observed in the presence of AB serum or serum-free medium, thus excluding the possible influence of patients' plasma components on differentiation. The defects in DCs were not due to IVIg replacement therapy since defects were also observed in newly diagnosed naive CVID patients, and in a patient having received IVIg 6 months prior to obtaining blood sample (patient 8). In addition, lymphoid DCs of CVID patients also presented with down-regulated CD86 and HLA-DR expression, while other markers were not altered (not shown).
Dendritic cells from CVID patients display impaired differentiation. (A) Differentiation of DCs is impaired in CVID patients. Flow cytometric analysis of the expression of differentiation markers on the surface of 6-day-old monocyte-derived DCs of healthy donors (upper panels), CVID patients (patient 1; middle panels), and selective antibody-deficient patients (patient 1; lower panels). Percentage of cells that are positive for the indicated markers are depicted, and mean fluorescence intensities are indicated in parentheses. (B) Comparison of percentage of cells expressing CD1a, CD83, and CD80 in 12 CVID patients (open circles; numbers 1-12 within each circle correspond to patients' numbers), 6 healthy donors (filled circles), 3 selective antibody-deficient patients (open squares; numbers 1-3 within each square correspond to patients' numbers), and 1 patient with hyper IgM syndrome (open squares with number 4) after differentiation of monocytes for 6 days. The mean values are indicated by a horizontal bar for each marker. Statistical significance as determined by the nonparametric Mann-Whitney test is indicated (*P ≤ .01). (C) Variations among CVID patients with respect to the expression of DC maturation markers in 6-day-old DCs. Each symbol in the figure denotes corresponding CVID patient. MFI indicates mean fluorescence intensity.
Dendritic cells from CVID patients display impaired differentiation. (A) Differentiation of DCs is impaired in CVID patients. Flow cytometric analysis of the expression of differentiation markers on the surface of 6-day-old monocyte-derived DCs of healthy donors (upper panels), CVID patients (patient 1; middle panels), and selective antibody-deficient patients (patient 1; lower panels). Percentage of cells that are positive for the indicated markers are depicted, and mean fluorescence intensities are indicated in parentheses. (B) Comparison of percentage of cells expressing CD1a, CD83, and CD80 in 12 CVID patients (open circles; numbers 1-12 within each circle correspond to patients' numbers), 6 healthy donors (filled circles), 3 selective antibody-deficient patients (open squares; numbers 1-3 within each square correspond to patients' numbers), and 1 patient with hyper IgM syndrome (open squares with number 4) after differentiation of monocytes for 6 days. The mean values are indicated by a horizontal bar for each marker. Statistical significance as determined by the nonparametric Mann-Whitney test is indicated (*P ≤ .01). (C) Variations among CVID patients with respect to the expression of DC maturation markers in 6-day-old DCs. Each symbol in the figure denotes corresponding CVID patient. MFI indicates mean fluorescence intensity.
A major function of DCs is their ability to trigger the activation and proliferation of T cells.13 DCs from CVID patients displayed a markedly weaker stimulatory effect on allogeneic T cells than DCs from healthy donors (Figure 2A). To further investigate the functional properties of CVID patients' DCs, we tested their ability to mature and produce interleukin-12 (IL-12) upon stimulation with CD40 ligand (CD40L)–transfected fibroblasts. There was a striking up-regulation of markers on DCs from control patients (Figure 2B), whereas DCs from CVID patients failed to up-regulate the maturation markers and costimulatory molecules (Figure 2B). We then measured the secretion of bioactive IL-12 (p70) from DCs of CVID patients upon stimulation with CD40L. A significantly low amount of IL-12 was produced by DCs of CVID patients compared with healthy donors and control patients (Figure 2C). Defective IL-12 production was observed in DCs of CVID patients whether naive or under IVIg therapy. Together, our results demonstrate that despite heterogeneous clinical presentation, CVID is generally associated with defective DC functions. Although our results are in contrast to a previous report indicating an enhanced proportion of intracellular IL-12–positive monocytes in CVID patients,15 the discrepancies could be due to differences in the methodologies (p70 versus p40; secretory versus intracellular IL-12; and isolated DCs versus whole peripheral blood mononuclear cells) or the stimuli used in the assays.
Dendritic cells from CVID patients display impaired functional properties. (A) Dendritic cells from CVID patients are impaired in their allogeneic CD4+ T-cell stimulatory capacity in an MLR, as measured by [3H]-thymidine uptake. Graded doses of DCs were seeded with 1 × 105 responder T cells in RPMI 1640 medium supplemented with 10% human AB serum. After 4 days, the cells were pulsed for 16 hours with 1 μCi (0.037 MBq) [3H]thymidine. Radioactive incorporation was measured by standard liquid scintillation counting, and results were expressed as counts per minute (cpm, mean ± SD of triplicate values) after subtraction of values from culturing stimulator cells alone. The level of [3H]-thymidine uptake by T cells in medium alone was in the range of 1320 ± 160 cpm and was less than 1000 cpm in stimulator cells alone when 10 000 cells were tested. (B) Dendritic cells from CVID patients display altered capacities for maturation. DCs (6 days old) from CVID patients (thick lines) and selective antibody-deficient patients (thin lines) were stimulated with CD40L-transfected fibroblasts (10:1) for 48 hours. The mean fluorescence intensity for 1 of the 4 CVID patients (patient 5, bold numbers) and 1 of the 3 selective antibody-deficient patients (patient 3, light numbers) analyzed are indicated. (C) Dendritic cells from CVID patients (n = 4, patients 3, 4, 5, and 12) produce significantly lower amounts of IL-12 compared with that of DCs from healthy donors (n = 3) (▪) and selective antibody-deficient patients (n = 3, patients 2, 3 and 4) (□). Cytokine secretion was measured in a cell-free culture supernatant by Quantikine kit (R&D Systems, Abingdon, Oxon, United Kingdom) following stimulation of cells with CD40L-transfected fibroblasts for 48 hours. The level of IL-12 production by 4 CVID patients (patients 3, 4, 5, and 12) was in the range of 5 to 86 pg/mL per 0.5 × 106 cells. DCs of patient 3 produced 29 pg/mL, those of patients 4 and 5 produced 5 pg/mL or less, and those of patient 12 secreted 86 pg/mL IL-12 upon CD40 stimulation. Statistical significance as determined by the nonparametric Mann-Whitney test is indicated (*P < .01).
Dendritic cells from CVID patients display impaired functional properties. (A) Dendritic cells from CVID patients are impaired in their allogeneic CD4+ T-cell stimulatory capacity in an MLR, as measured by [3H]-thymidine uptake. Graded doses of DCs were seeded with 1 × 105 responder T cells in RPMI 1640 medium supplemented with 10% human AB serum. After 4 days, the cells were pulsed for 16 hours with 1 μCi (0.037 MBq) [3H]thymidine. Radioactive incorporation was measured by standard liquid scintillation counting, and results were expressed as counts per minute (cpm, mean ± SD of triplicate values) after subtraction of values from culturing stimulator cells alone. The level of [3H]-thymidine uptake by T cells in medium alone was in the range of 1320 ± 160 cpm and was less than 1000 cpm in stimulator cells alone when 10 000 cells were tested. (B) Dendritic cells from CVID patients display altered capacities for maturation. DCs (6 days old) from CVID patients (thick lines) and selective antibody-deficient patients (thin lines) were stimulated with CD40L-transfected fibroblasts (10:1) for 48 hours. The mean fluorescence intensity for 1 of the 4 CVID patients (patient 5, bold numbers) and 1 of the 3 selective antibody-deficient patients (patient 3, light numbers) analyzed are indicated. (C) Dendritic cells from CVID patients (n = 4, patients 3, 4, 5, and 12) produce significantly lower amounts of IL-12 compared with that of DCs from healthy donors (n = 3) (▪) and selective antibody-deficient patients (n = 3, patients 2, 3 and 4) (□). Cytokine secretion was measured in a cell-free culture supernatant by Quantikine kit (R&D Systems, Abingdon, Oxon, United Kingdom) following stimulation of cells with CD40L-transfected fibroblasts for 48 hours. The level of IL-12 production by 4 CVID patients (patients 3, 4, 5, and 12) was in the range of 5 to 86 pg/mL per 0.5 × 106 cells. DCs of patient 3 produced 29 pg/mL, those of patients 4 and 5 produced 5 pg/mL or less, and those of patient 12 secreted 86 pg/mL IL-12 upon CD40 stimulation. Statistical significance as determined by the nonparametric Mann-Whitney test is indicated (*P < .01).
As the expression of markers on DCs can be modulated by several cytokines, we analyzed whether IL-10 was responsible for defective differentiation of DCs from CVID patients.16 Although CVID patients' DCs produced small amounts of IL-10 (69 ± 93 pg/mL, n = 6, Quantikine kit; Immunotech, Marseilles, France), the addition of neutralizing anti–IL-10 monoclonal antibodies (mAbs; 2 μg/mL) during differentiation only marginally restored the phenotypes on DCs. Furthermore, stimulation of cells with CD40 mAb (4 μg/106 cells) or with several-fold higher concentration of granulocyte-macrophage colony-stimulating factor and IL-4 during differentiation did not restore the normal phenotypes of DCs from CVID patients. These results indicate that IL-10 may not be the exclusive factor responsible for defective differentiation.
Costimulatory molecules on DCs play a critical role in the cascade of events leading to T-cell priming.13 IL-12 production by DCs polarizes CD4+ T cells into interferon-γ (IFN-γ)–producing T helper 1 cells. IFN-γ activates the antimicrobial activities of macrophages and together with IL-12 promotes T-cell differentiation into cytotoxic T lymphocytes. Various T-cell defects, including anergy, impaired proliferation, reduced expression of the CD40L, and impaired production of cytokines IL-2, IL-4, and IFN-γ, have been reported in CVID patients.2,17-20 A subgroup of CVID patients also presents with defective macrophage functions; and dysregulated T-cell function or macrophage activation has been implicated in the formation of granulomas in CVID patients.21,22 In the present study, although the patients displayed a normal or higher percent of T cells, they showed a defective proliferation (not shown). It is observed that the majority of CVID patients display normal T-cell numbers in their peripheral blood.9 Therefore, it is the impaired function of T cells that may be responsible for the pathogenesis in these patients. In addition, we report on the diminished production of IL-12 by DCs and reduced expression of CD40, CD80, and other costimulatory molecules involved in DC–T-cell cross-talk. We thus suggest that impairment in both the innate and adaptive compartments of the immune system cumulatively account for the inability of CVID patients to eradicate pathogens through conventional immune pathways.
CVID patients exhibit reduced serum levels of all immunoglobulin isotypes predisposing patients to frequent bacterial infections of the respiratory tract. In addition to T-cell stimulation, DCs regulate B-cell growth and immunoglobulin secretion.13 A direct interaction between CD40-activated DCs and B cells promotes the development of mucosal immunity. DCs together with IL-2 stimulate CD40-activated germinal center B-cell proliferation and drive their differentiation toward plasma cells.23 Thus, defective DC function along with impaired T-cell activity will have serious repercussion on the humoral immune response of CVID patients. Multiple defects in the immune system, including defective DC function, thus appear to be prominent features of CVID.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-04-1325.
Supported by INSERM and CNRS France, and by a grant from ZLB Bioplasma AG, Bern, Switzerland and Octapharma, Austria. J.B. is a recipient of a fellowship from the Fondation de la Recherche Médicale (France).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Dendritic cells from CVID patients display impaired functional properties. (A) Dendritic cells from CVID patients are impaired in their allogeneic CD4+ T-cell stimulatory capacity in an MLR, as measured by [3H]-thymidine uptake. Graded doses of DCs were seeded with 1 × 105 responder T cells in RPMI 1640 medium supplemented with 10% human AB serum. After 4 days, the cells were pulsed for 16 hours with 1 μCi (0.037 MBq) [3H]thymidine. Radioactive incorporation was measured by standard liquid scintillation counting, and results were expressed as counts per minute (cpm, mean ± SD of triplicate values) after subtraction of values from culturing stimulator cells alone. The level of [3H]-thymidine uptake by T cells in medium alone was in the range of 1320 ± 160 cpm and was less than 1000 cpm in stimulator cells alone when 10 000 cells were tested. (B) Dendritic cells from CVID patients display altered capacities for maturation. DCs (6 days old) from CVID patients (thick lines) and selective antibody-deficient patients (thin lines) were stimulated with CD40L-transfected fibroblasts (10:1) for 48 hours. The mean fluorescence intensity for 1 of the 4 CVID patients (patient 5, bold numbers) and 1 of the 3 selective antibody-deficient patients (patient 3, light numbers) analyzed are indicated. (C) Dendritic cells from CVID patients (n = 4, patients 3, 4, 5, and 12) produce significantly lower amounts of IL-12 compared with that of DCs from healthy donors (n = 3) (▪) and selective antibody-deficient patients (n = 3, patients 2, 3 and 4) (□). Cytokine secretion was measured in a cell-free culture supernatant by Quantikine kit (R&D Systems, Abingdon, Oxon, United Kingdom) following stimulation of cells with CD40L-transfected fibroblasts for 48 hours. The level of IL-12 production by 4 CVID patients (patients 3, 4, 5, and 12) was in the range of 5 to 86 pg/mL per 0.5 × 106 cells. DCs of patient 3 produced 29 pg/mL, those of patients 4 and 5 produced 5 pg/mL or less, and those of patient 12 secreted 86 pg/mL IL-12 upon CD40 stimulation. Statistical significance as determined by the nonparametric Mann-Whitney test is indicated (*P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2004-04-1325/6/m_zh80200468110002.jpeg?Expires=1765899522&Signature=IyAwjaP0S6gegxwZxzKhosTUa19o26ZAN8ciOuEHRVWoyvjs4oNEr4NvXoYZQXIP6JV1NMVWPfbffu6a3KXkZcHw-Y6zT27o9NMaWKs0VxbgM7f0blr43t-gFCLfHproIx2UUmfdfZASzFXnyaRa3DxGU~yXMFY1Cv9tL2fAz2ZSqNuvPElsLiUQ4z5g0qI-YxsmWkzcNRA87gsnOjmNJqN1Q5XnPTH8YVfkXSTGxHKhaRdnOxyrLLx251jl02xtDzSaQ-4PEEy9X98q0N-DJ2uwTjjPPbmXwr1fwo1f05we0XvFTjdaKip~HKLgjz5aLyLKcaurn77PW2bJZWNTGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal