Comment on Baron et al, page 2254
The level of donor chimerism in T and NK cells early after allogeneic nonmyeloablative transplantation identifies patients at risk for rejection or GVHD, thus allowing possible preemptive strategies for their prevention.
Hematopoietic stem cell transplantation for HLA-matched related or unrelated donors using myeloablative conditioning regimens is increasingly used in the treatment of patients with hematologic malignancies. The reduced antileukemic effect of the nonmyeloablative conditioning is compensated by a presumed graft-versus-leukemia effect exerted by donor-derived effector cells. These effector cells might be T cells, natural killer (NK) cells, and/or other as yet undefined lymphocyte subpopulations. While posttransplantation immunosuppression is necessary to avoid graft-versus-host disease (GVHD), it can also reduce the graft-versus-leukemia effect. In addition, stable donor engraftment is the major requirement for the graft-mediated antileukemic effect, and patients who either do not engraft or who reject after initial engraftment will not benefit from this form of immunotherapy. It would therefore be very helpful for the clinical management of patients to know early after transplantation which patients will be at risk for rejection and which will develop acute graft-versus-host disease. These complications have been identified only through their clinical manifestation, which makes their treatment more complicated.FIG1
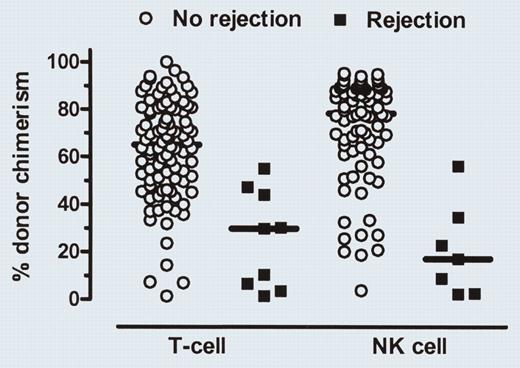
Day-14 donor T-cell and NK-cell chimerism levels in patients with or without subsequent graft rejection. See the complete figure in the article beginning on page 2254.
Day-14 donor T-cell and NK-cell chimerism levels in patients with or without subsequent graft rejection. See the complete figure in the article beginning on page 2254.
In this issue, Baron and colleagues present data that allow the early identification of patients at risk for rejection or acute GVHD before clinical manifestation. They have analyzed the kinetics of donor engraftment among various peripheral blood cell populations in 120 patients with hematologic malignancies who received hematopoietic stem cell grafts from HLA-matched related or unrelated donors after nonmyeloablative conditioning. By measuring the donor T-cell and donor NK cell chimerism levels on day 14, they found that patients with low donor T-cell and low donor NK cell chimerism (below 50%) had an increased probability of graft rejection. In contrast, high donor T-cell chimerism on day 28 after transplantation was associated with an increased probability of acute graft-versushost disease. Forty-one of 93 patients with measurable malignancies at transplantation achieved complete remission at a median of 199 days after hematopoietic cell transplantation (HCT), despite the fact that 19 of the 41 patients had a mixed T-cell chimerism when they achieved remission. Interestingly, an earlier establishment of donor NK cell chimerism was associated with an improved progression-free survival. The findings by Baron and colleagues might help to identify patients at risk for graft rejection and acute graft-versus-host disease before these events occur. Future studies should investigate whether these complications can be avoided using preemptive strategies such as early donor lymphocyte infusions (DLIs) or donor NK cell infusions in patients with a low day-14 T-cell and NK cell chimerism or by increasing immunosuppression in patients with high T-cell chimerism at day 28. Likewise, immunosuppression could be reduced in patients not found to be at risk on day 28, thus allowing more graft-versus-leukemia effect without increasing the risk of GVHD. Such an individualized approach might further enhance the graft-versus-leukemia effect of allogeneic hematopoietic cell transplantation using nonmyeloablative conditioning regimens and thus improve the overall survival rate. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal