Abstract
Bone marrow (BM)-derived cells are thought to participate in the growth of blood vessels during postnatal vascular regeneration and tumor growth, a process previously attributed to stem and precursor cells differentiating to endothelial cells. We used multichannel laser scanning confocal microscopy of whole-mounted tissues to study angiogenesis in chimeric mice created by reconstituting C57BL mice with genetically marked syngeneic BM. We show that BM-derived endothelial cells do not significantly contribute to tumor- or cytokine-induced neoangiogenesis. Instead, BM-derived periendothelial vascular mural cells were persistently detected at sites of tumor- or vascular endothelial growth factor-induced angiogenesis. Subpopulations of these cells expressed the pericyte-specific NG2 proteoglycan, or the hematopoietic markers CD11b and CD45, but did not detectably express the smooth muscle markers smooth muscle α-actin or desmin. Thus, the major contribution of the BM to angiogenic processes is not endothelial, but may come from progenitors for periendothelial vascular mural and hematopoietic effector cells. (Blood. 2004;104: 2084-2086)
Introduction
Several recent reports have suggested that adult bone marrow (BM)-derived stem and progenitor cells for vascular endothelial cells (ECs) may contribute to vascular healing following trauma, as well as to pathologic postnatal neoangiogenesis.1-10 The mechanism of the mobilization and recruitment of the putative adult EC precursors has been argued to be vascular endothelial growth factor (VEGF) driven.2,5,6,11 Intriguingly, recruitment of BM-derived endothelial precursors has been shown to be both necessary and sufficient for tumor angiogenesis and growth in mice.5 It has therefore been suggested that inhibition of the function of these EC precursors might provide a novel approach to blocking tumor angiogenesis (reviewed in Rafii et al12 ). Correspondingly, therapeutic endothelial stem cell transplantation could be a promising approach to restore tissue vascularization after ischemic events (reviewed in Rafii et al13 ). However, the true in vivo differentiation capacity of adult BM stem and progenitor cells and their possible contribution to nonhematopoietic cells and tissues including ECs remains controversial.14-18 Therefore, we set out to test the angiogenic cell fate potential of adult BM-derived stem cells and their progeny in vivo.
Study design
Animals and bone marrow transplantations
Chimeric mice reconstituted with enhanced green fluorescent protein positive (GFP+) syngeneic bone marrow (BM) cells were created to study the behavior of BM cells in vivo. Briefly, BM was collected by flushing femurs of C57BL/6-TgN(ACTbEGFP)1Osb mice (Jackson Laboratory, Bar Harbor, ME). Unselected BM cells (2 × 106) were transplanted into C57BL/6JO1aHsd wild-type mice via tail vein injection. The recipients were irradiated 1 day before transplantation by a sublethal dosage of 4.0 Gy. Fluorescence activated cell sorter (FACS) analysis showed that peripheral blood cells of the recipients were almost completely (80%-95%) reconstituted with GFP+ cells 5 to 8 weeks after transplantation. FACS analysis of the BM using antibodies against the stem and progenitor cell markers Sca-1 and CD117 (BD Pharmingen, Palo Alto, CA) was carried out 7 to 11 weeks after the transplantation when the mice were killed. Typically, 36% to 43% of the total BM cells were GFP+, while 10% to 14% and 15% to 28% of the GFP+ cells were positive for Sca-1 or CD117, respectively, confirming the successful engraftment of the donor-derived stem cells. The Provincial State Office of Southern Finland approved all experiments.
Tumor-induced model of angiogenesis
The B16-F1 melanoma cell line (American Type Culture Collection, Manassas, VA) was maintained in Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% fetal bovine serum (PromoCell, Heidelberg, Germany). In order to induce tumor angiogenesis, the B16 cells (2 × 106 cells in 20 μL) were injected subcutaneously into the ears of mice 5 to 8 weeks after the BM transplantation. The tumors were excised and processed for tissue analyses 14 to 21 days later.
VEGF polypeptide-induced model of angiogenesis
The mice that underwent BM transplantation were injected subcutaneously at the same injection site in the ear with murine VEGF164 protein (60 ng/injection per mouse; R&D Systems, Minneapolis, MN) every other day. The experiment started 5 to 8 weeks after the BM transplantation. The mice were killed after 15 days and the ears were processed for tissue analyses.
Immunohistochemistry
For the whole-mount staining, the ears were collected and the cartilage was removed. Tissues were fixed in 4% paraformaldehyde (PFA), blocked with 3% serum/0.3% Triton-X in phosphate-buffered saline (PBS), and incubated with primary antibodies overnight at 4°C. The ears were then washed and incubated with fluorescence-conjugated secondary antibodies (Alexa 594 antirat, Alexa 594 antirabbit, Alexa 647 antirat, Alexa 680 antirabbit; Molecular Probes, Eugene, OR) overnight at 4°C. Finally, the samples were flattened and mounted (DABCO; Sigma-Aldrich, St Louis, MO). For cryosectioning, tissues were fixed in 2% PFA for one hour, incubated in 20% sucrose/PBS overnight, and embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek Europe, Zoeterwoude, the Netherlands). Sections (10 μm) were immunostained with the primary antibodies overnight at 4°C, followed by incubation with secondary antibodies for 30 minutes at room temperature. The primary antibodies used were rat antimouse CD31/Pecam-1, rat antimouse CD105/endoglin, rat antimouse CD45, rat antimouse CD11b/Mac-1 (BD Pharmingen), rat antimouse alpha-smooth muscle α-actin (cyanin-3 [Cy3]-conjugated; Sigma-Aldrich), rabbit antimouse/human von Willebrand factor, rabbit antimouse Desmin (DAKO, Glostrup, Denmark), and rabbit anti-2.19-21
The samples were analyzed with a Zeiss Axioplan 2 immunofluorescence microscope using a 20 × (0.5 numerical aperture) Plan-Neofluar oil immersion objective, AxioCam Hrc camera, and Axiovision 3.1 software (Carl Zeiss, Göttingen, Germany). Additionally, the samples were analyzed with a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss) using multichannel (sequential) scanning in frame mode. A 40 × (1.3 numerical aperture) Plan-Neofluar oil immersion objective and LSM 5 Software version 3.2 were used (Carl Zeiss). Single XY scans had an optical slice thickness of 0.9 μm or less. Three-dimensional projections were digitally reconstituted from stacks of confocal optical slices.
Results and discussion
To study the possible contribution of BM-derived cells to angiogenesis, we studied the tissues of chimeric mice reconstituted with GFP+ BM cells 5 to 8 weeks after transplantation. Angiogenesis was induced in the ears of the mice that underwent BM transplantation by repeated subcutaneous injections with recombinant murine VEGF164 protein. Alternatively, angiogenesis was induced by subcutaneous inoculation of the syngeneic B16 melanoma tumor. In addition to inducing angiogenesis, tumor growth or VEGF delivery should also lead to the mobilization and vascular incorporation of BM-derived EC precursors.2,5,11 After allowing the blood vessels to grow for 14 to 21 days, the mice were killed and blood vessels and BM-derived cells were analyzed in whole mounts or tissue sections by fluorescence microscopy or by using multichannel laser scanning confocal microscopy.
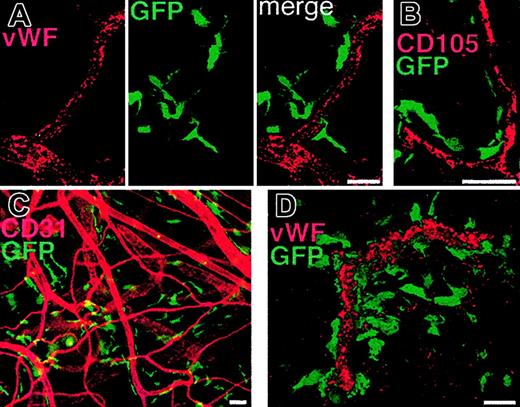
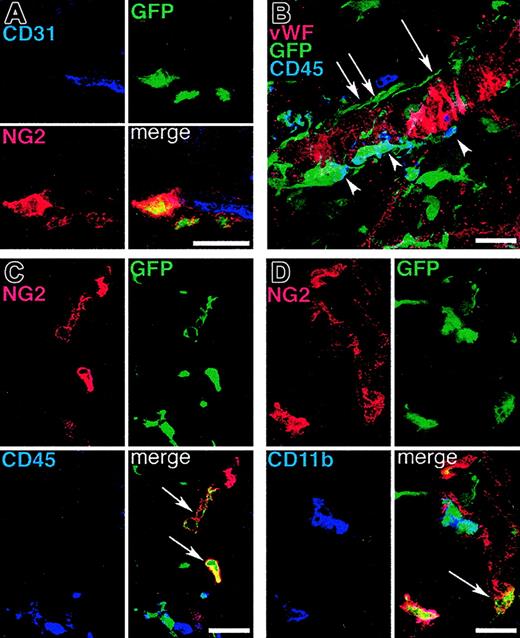
ECs were detected by using antibodies against CD31, von Willebrand factor (VWF), or CD105. In extensive analyses of the tissues from more than 50 mice that underwent BM transplantation, we were unable to find evidence of donor-derived vascular ECs (Figure 1). Instead, we constantly observed very high numbers of BM-derived GFP+ periendothelial cells in both VEGF- and tumor-induced vessels (Figure 1). These cells were often in very close contact with the underlying ECs. The majority of the GFP+ periendothelial cells were immunoreactive for the hematopoietic markers CD11b and CD45 (Figure 2). Many of the GFP+ cells had the distinctive shape and location described for pericytes on capillaries and on tumor blood vessels.22 In addition, many of the periendothelial BM-derived GFP+ cells expressed the NG2 proteoglycan (Figure 2), a marker for developing pericytes that is also expressed in the pericytes of angiogenic blood vessels in tumors.19,20,23 In contrast, we found no periendothelial GFP+ cells expressing detectable levels of desmin or smooth muscle α-actin, which are typically found in differentiated vascular smooth muscle cells (not shown). However, smooth muscle markers appear to be specific for differentiated periendothelial cells in rodents, and therefore may be poorly expressed in developing angiogenic microvasculature.21,24
Periendothelial location of bone marrow-derived cells in angiogenic vessels. Angiogenesis was induced in the ears of mice that underwent BM transplantation by repeated subcutaneous VEGF protein injections (A) or by subcutaneous implantation of B16 melanoma (B-D). The tissues were studied by immunofluorescence microscopy (C) or by laser scanning confocal microscopy (A-B, D). (A-B) Vascular endothelial cells stained for von Willebrand factor (VWF, A) or CD105 (B) demonstrate the periendothelial location of the GFP+ BM-derived cells. Note that no GFP+ endothelial cells can be detected. (C) The abundant number and close association of BM-derived cells to endothelial cells (CD31 staining, red) can also be seen in the peritumoral area where larger arterioles with no apparent angiogenic activity are seen. (D) A 3-dimensional projection digitally reconstituted from stacks of confocal optical slices demonstrates the periendothelial location of the BM-derived GFP+ cells bordering the vascular endothelial cells expressing VWF (red). Bars represent 40 μm.
Periendothelial location of bone marrow-derived cells in angiogenic vessels. Angiogenesis was induced in the ears of mice that underwent BM transplantation by repeated subcutaneous VEGF protein injections (A) or by subcutaneous implantation of B16 melanoma (B-D). The tissues were studied by immunofluorescence microscopy (C) or by laser scanning confocal microscopy (A-B, D). (A-B) Vascular endothelial cells stained for von Willebrand factor (VWF, A) or CD105 (B) demonstrate the periendothelial location of the GFP+ BM-derived cells. Note that no GFP+ endothelial cells can be detected. (C) The abundant number and close association of BM-derived cells to endothelial cells (CD31 staining, red) can also be seen in the peritumoral area where larger arterioles with no apparent angiogenic activity are seen. (D) A 3-dimensional projection digitally reconstituted from stacks of confocal optical slices demonstrates the periendothelial location of the BM-derived GFP+ cells bordering the vascular endothelial cells expressing VWF (red). Bars represent 40 μm.
Subpopulations of periendothelial BM-derived vascular mural cells express the pericyte marker NG2 proteolygan and/or the hematopoietic markers CD45 or CD11b. Angiogenesis was induced in mice that underwent BM transplantation by subcutaneous implantation of B16 melanoma (A, C-D) or by repeated subcutaneous VEGF protein injections (B), and the tissues were analyzed by confocal microscopy. (A) Some of the BM-derived mural cells covering angiogenic blood vessel endothelium (CD31 staining, blue) express the pericyte marker NG2 (red). (B) A 3-dimensional projection demonstrates that BM-derived GFP+ periendothelial cells encompass both cells expressing CD45 (arrowheads) and cells with no CD45 immunoreactivity (arrows). (C-D) Similarly, BM-derived NG2+ cells also include cells that do not coexpress CD45 or CD11b (arrows). Bars represent 30 μm.
Subpopulations of periendothelial BM-derived vascular mural cells express the pericyte marker NG2 proteolygan and/or the hematopoietic markers CD45 or CD11b. Angiogenesis was induced in mice that underwent BM transplantation by subcutaneous implantation of B16 melanoma (A, C-D) or by repeated subcutaneous VEGF protein injections (B), and the tissues were analyzed by confocal microscopy. (A) Some of the BM-derived mural cells covering angiogenic blood vessel endothelium (CD31 staining, blue) express the pericyte marker NG2 (red). (B) A 3-dimensional projection demonstrates that BM-derived GFP+ periendothelial cells encompass both cells expressing CD45 (arrowheads) and cells with no CD45 immunoreactivity (arrows). (C-D) Similarly, BM-derived NG2+ cells also include cells that do not coexpress CD45 or CD11b (arrows). Bars represent 30 μm.
Our data provide an alternative explanation for the reports in which BM-derived EC precursors have been described, although it is not possible to unconditionally conclude that these cells would not altogether exist. Pericytes are often in a very intimate contact with the underlying ECs, with occasionally only an extremely thin (even< 1 μm) process of an EC separating a pericyte from the blood vessel lumen.22 As we show in our report, this close spatial association to blood vessel lumen can be seen to apply also to BM-derived vascular mural NG2+ cells or CD11b+/CD45+ cells. Only by using confocal scanning of very thin slices could we separate marker colocalization from superimposition of endothelial and periendothelial cells. Use of multichannel (sequential) scanning also eliminated crosstalk between the GFP signal and the fluorescent dyes. With mural cells in such a close contact to each other and to the vascular lumen, it is impossible to reliably tell endothelial and periendothelial mural cells apart by histologic analysis of sections using ordinary microscopy. Therefore, our results raise the possibility that most of the putative BM-derived ECs that have been described earlier in many publications may in fact be BM-derived pericyte-like cells and/or periendothelial hematopoietic cells that have, because of their extremely close proximity to vascular lumen, been misinterpreted as ECs.
We conclude that adult BM-derived cells participate in angiogenesis, and a small subpopulation differentiates to vascular mural periendothelial cells that are morphologically indistinguishable from pericytes. Pericytes are thought to have a critical role for vascular morphogenesis, maturation, and function, possibly by regulating EC proliferation and differentiation.25 Also known as mural cells or Rouget cells, they constitute a heterogenous population of cells that has been difficult to define, and their ontogeny is not well understood. Pericytes are known to be plastic, having the capacity to differentiate into other mesenchymal cell types, such as smooth muscle cells and fibroblasts.23,25 Our results suggest that during postnatal neoangiogenesis, progenitors for these mural cells are mobilized from the BM and integrated in the vascular structures.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2004-01-0336.
Supported by grants from the Finnish Academy of Sciences, Biocentrum Helsinki, Sigrid Juselius Foundation, the Novo Nordisk Foundation, the European Union (Angionet QLC1-CT-2001-01172), and the National Institutes of Health (HD37243).
K.A. and P.S. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal