Abstract
We have shown that human high molecular weight kininogen is proangiogenic due to release of bradykinin. We now determined the ability of a murine monoclonal antibody to the light chain of high molecular weight kininogen, C11C1, to inhibit tumor growth compared to isotype-matched murine IgG. Monoclonal antibody C11C1 efficiently blocks binding of high molecular weight kininogen to endothelial cells in a concentration-dependent manner. The antibody significantly inhibited growth of human colon carcinoma cells in a nude mouse xenograft assay and was accompanied by a significant reduction in the mean microvascular density compared to the IgG control group. We also showed that a hybridoma producing monoclonal antibody C11C1 injected intramuscularly exhibited markedly smaller tumor mass in a syngeneic host compared to a hybridoma producing a monoclonal antibody to the high molecular weight kininogen heavy chain or to an unrelated plasma protein. In addition, tumor inhibition by purified monoclonal antibody C11C1 was not due to direct antitumor effect because there was no decrease of tumor cell growth in vitro in contrast to the in vivo inhibition. Our results indicate that monoclonal antibody C11C1 inhibits angiogenesis and human tumor cell growth in vivo and has therapeutic potential for treatment of human cancer. (Blood. 2004;104:2065-2072)
Introduction
Growth and metastasis of solid tumors depend on the formation of new blood vessels, which originate from the existing vasculature by angiogenesis. Modulation of angiogenesis is now a well-accepted experimental strategy for control of tumor growth. Proteolytic fragments of proangiogenic molecules, including plasma proteins, which are potent inhibitors of this complex process,1 include angiostatin, derived from plasminogen and endostatin,2 derived from collagen XVIII. We have discovered a new antiangiogenic polypeptide, kininostatin,3 which is domain 5 (D5) of high molecular weight kininogen (HK) produced by proteolysis by plasma kallikrein and factor XIa.4 In this study, we use a monoclonal antibody (mAb) to HK to inhibit angiogenesis. The profibrinolytic, antiadhesive, and antiangiogenic activities of the plasma kallikrein-kinin system have been reviewed.5 A single gene codes for plasma HK. Following the cleavage of bradykinin (BK) from HK domain 4 (D4) by kallikrein, the resulting active cofactor, cleaved HK (HKa), consists of a heavy chain (65 kDa) containing domains 1, 2, and 3 (D1, D2, and D3) and a light chain (55 kDa) containing domains 5 and 6 (D5 and D6), both of which contain endothelial cell-binding sites. HKa undergoes major conformational changes and acquires the ability to bind to anionic surfaces and endothelial cell receptors including urokinase plasminogen activator receptor (uPAR),6 cytokeratin, and gC1q receptor. These new properties render it antiangiogenic7 because it induces endothelial cell apoptosis and cell cycle interference.8 The C-terminal domain of the light chain of HK (D6) contains the binding site for prekallikrein (PK). When HK binds to endothelial cells, either plasma-activated factor XII or membrane-bound prolylcarboxypeptidase cleaves PK to the active enzyme plasma kallikrein.9 Plasma kallikrein then cleaves BK from HK. The tight binding of BK to bradykinin receptors B1 or B2 (or both) allows stimulation of endothelial cells before BK is metabolized by kininases and aminopeptidases.5
Over the past 2 years, BK has been proposed as a proangiogenic agent. Parenti et al10 have shown that BK binding to B1 promotes angiogenesis in the rabbit cornea by up-regulation of endogenous fibroblast growth factor 2 (FGF-2). B1 antagonists or B1 knockouts reduce angiogenesis,11 further supporting the notion that BK is proangiogenic. BK promotes the early phases of angiogenesis by increasing vascular permeability through the B2 receptor.12 The finding of suppressed in vivo angiogenesis in kininogen-deficient rats suggests that endogenous BK produced by proteolytic cleavage of HK by plasma kallikrein on the endothelial cell surface is strongly proangiogenic.13 In a recent study,14 we demonstrated that HK as well as BK stimulate neovascularization more than 2-fold and thus are proangiogenic. We also demonstrated that soybean trypsin inhibitor, which inactivates plasma kallikrein, inhibits the proangiogenic effect of HK, indicating that release of BK is important. Using mAb C11C1 to HK, we showed that angiogenesis in the chicken chorioallantoic membrane (CAM) assay induced by FGF-2, vascular endothelial growth factor (VEGF), or HK is inhibited by the mAb in a dose-dependent manner14 as is a human tumor grown on CAM. In the present study, we tested the hypothesis that mAb C11C1 could inhibit human and murine tumor growth in vivo in mammals (mice). We first explored whether mAb C11C1 could inhibit the binding of HK to human endothelial cells and thus prevent or decrease the angiogenic effect of BK. We tested whether mAb C11C1 had a direct antitumor effect. We then examined the effects of mAb C11C1 on athymic nude mice bearing human colon carcinoma (HCT-116) as well as a syngeneic murine model to determine if mAb C11C1 might have a potential therapeutic effect on human tumors. Our findings suggest that mAb C11C1 has therapeutic potential for human malignant tumors.
Materials and methods
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid antibodies
The mAbs C11C1 and 2B5 to HK and B38 to factor V were produced and characterized in our laboratory as previously described.15-17 All are IgG1 isotypes. The mAbs 2B5 and B38 were produced from ascites in pristane-primed mice after intraperitoneal injection of the hybridomas. C11C1 does not produce ascites and therefore was produced in tissue culture. C11C1 is a murine mAb directed to an epitope on the unique 46-kDa light chain of HK.15 The epitope was mapped to a portion of the D5 domain, H441-K502, by proteolytic digestion, immunoaffinity chromatography, and N-terminal sequencing.4 C11C1 inhibits the in vitro coagulation activity of HK (HKa). 2B5 reacted with HK D2 and D3 and inhibited thiol protease activity.15 The control mAb B38 is directed to the light chain of factor V.16 IgG fractions were isolated by protein-A affinity chromatography (Pro-Chem, Acton, MA) according to the manufacturer's recommendations. The IgG antibodies were single bands of 160 kDa on nonreduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and 2 bands of 55 and 25 kDa in the presence of reducing conditions. The IgG fractions were dialyzed against 0.01 M HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.15 M NaCl, pH 7.4, for 20 hours at 4°C. Mouse IgG1κ (MOPC-21; mIgG), purchased from Sigma-Aldrich (St Louis, MO), was used in all experiments but the binding studies. Those studies (Figure 1) used mouse IgG (Sigma catalog no. T-5381).
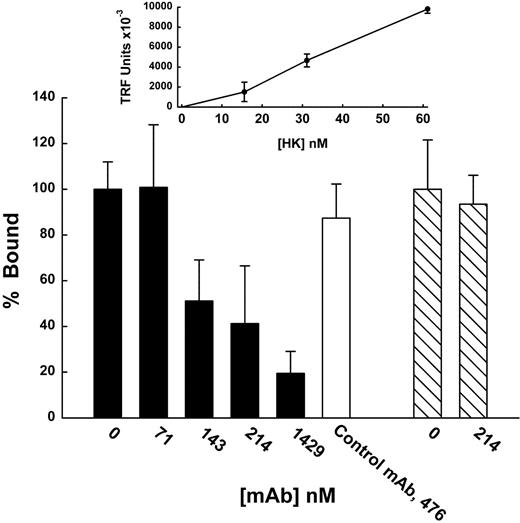
Concentration-dependent binding and inhibition of HK binding to cultured endothelial cells by mAb C11C1. HUVECs cultured to confluence on a fibronectin-coated 96-well Immulon 2HB plate. Mixtures were incubated with HUVECs in binding buffer for 30 minutes at 4°C. Binding was measured as described in “Materials and methods” in the presence of 50 μM ZnCl2. Nonspecific binding of HK or factor XII was determined using Eu-labeled HK or factor XII (Eu-HK, Eu-XII) in the absence of ZnCl2 and was subtracted from total binding to determine specific binding. The inset linear graph shows the concentration-dependent binding of Eu-HK to HUVECs as assessed using 200 μL of each Eu-HK concentration/well. The bar graph shows 100 μL of mixtures of Eu-HK (31 nM, ▪, □) or EU-XII (43 nM, ▧) and various indicated concentrations of mAb C11C1 were added to each well. Control antibody used with Eu-HK is normal purified mouse IgG (Sigma I-52381). The data are plotted as percent bound and comparisons were made to the TRF value obtained in the absence of antibody (0 nM). Mean ± SEM, n = 3. For the concentrations of mAb C11C1, 214 nM (P = .013), and for 1429 nM (P = .004).
Concentration-dependent binding and inhibition of HK binding to cultured endothelial cells by mAb C11C1. HUVECs cultured to confluence on a fibronectin-coated 96-well Immulon 2HB plate. Mixtures were incubated with HUVECs in binding buffer for 30 minutes at 4°C. Binding was measured as described in “Materials and methods” in the presence of 50 μM ZnCl2. Nonspecific binding of HK or factor XII was determined using Eu-labeled HK or factor XII (Eu-HK, Eu-XII) in the absence of ZnCl2 and was subtracted from total binding to determine specific binding. The inset linear graph shows the concentration-dependent binding of Eu-HK to HUVECs as assessed using 200 μL of each Eu-HK concentration/well. The bar graph shows 100 μL of mixtures of Eu-HK (31 nM, ▪, □) or EU-XII (43 nM, ▧) and various indicated concentrations of mAb C11C1 were added to each well. Control antibody used with Eu-HK is normal purified mouse IgG (Sigma I-52381). The data are plotted as percent bound and comparisons were made to the TRF value obtained in the absence of antibody (0 nM). Mean ± SEM, n = 3. For the concentrations of mAb C11C1, 214 nM (P = .013), and for 1429 nM (P = .004).
To produce anti-HK (anti-D5 440) antibody, peptide HK440 (sequence G440-H455, Mr 1657 Da) from the D5 region of HK was synthesized and purified by high-performance liquid chromatography (HPLC) by the Protein Chemistry Laboratory of the University of Pennsylvania (Philadelphia). Conjugation and antibody production was performed by Sigma Genosys Biotechnologies (The Woodlands, TX). Briefly, 4 mg HK440 was conjugated to keyhole limpet hemocyanin (KLH) for immunization into New Zealand white rabbits. Following preimmune sera collection, immunization was initiated with a 200-μg injection in complete Freund adjuvant. Three more immunizations were each made with an additional 100 μg incomplete Freund adjuvant (IFA) at 14-day intervals. The first bleed was taken 7 days later. Two additional doses of 100 μg IFA immunizations with subsequent bleeds were performed at 7-day intervals. The sera resulting from the second bleed were raised to 40% saturation with ammonium sulfate (AS), stirred for 30 minutes at 4°C, then pelleted at 10 000g for 20 minutes. The resulting pellet was washed and recentrifuged twice in 50% AS, then solublized in 0.05 M Tris (tris(hydroxymethyl)aminomethane), pH 8.0, and dialyzed overnight in the same buffer with 2 exchanges. The final immunoglobulin preparation was judged more than 80% by SDS-PAGE (Mr = 220 kDa) and yielded a protein concentration of 70 mg/mL. Anti-D5 440 antibody (1:8000 dilution) recognizes HK, HKa, and purified D5, but not low molecular weight kininogen (LK) by Western blotting under both reduced or nonreduced conditions.
Proteins
Purified HK and factor XII polyclonal antibody to anti-human PK were obtained from Enzyme Research Laboratories (South Bend, IN).
Europium labeling of HK and factor XII
Time-resolved fluorometry (TRF) was used with europium chelating labeling of HK (Eu-HK) and factor XII (Eu-XII) as the tracer ligand (DELPHIA; Perkin Elmer Life Sciences, Shelton, CT). HK and factor XII were labeled according to the manufacturer's instructions for protein labeling using their Eu-chelated N1-(p-isothiocyanatobenzyl)-diethylenetriamine-N1,N2,N3,N3-tetraacetic acid (DTTA) reagent. Both retained their functional coagulant activity. TRF was measured in the 96-well culture Immulon HB plate using 200 μL/well DELPHIA enhancement solution (which contains Triton X-100 adequate to dissolve cells and release the label) and read in a Victor2 1420 multilabel counter (Perkin Elmer Life Sciences). Under these conditions, 1 nM Eu results in about 1 × 106 TRF units of fluorescence.
HUVECs
Human umbilical vein endothelial cells (HUVECs) were obtained and cultured according to the procedures of Clonetics (Warrenale, PA). They were maintained in endothelial cell growth medium (EGM) containing growth factors and 10% fetal calf serum at 37°C in a humidified incubator (5% CO2/95% air). HUVECs from 3 to 6 passages were subcultured to confluence onto fibronectin-treated (10 μg/mL overnight, 37°C; Sigma Chemical, St Louis, MO) Immulon 2HB 96-well flat-bottom plates (Thermo Labsystems, Franklin, MA) in EGM. Immulon 2HB plates were used because it was determined that nonspecific HK binding was high on standard culture plates but was negligible on these plates when blocked with binding buffer. Binding buffer with or without 50 μM ZnCl2 was composed of 0.01 M HEPES, 0.137 M NaCl, 0.004 M KCl, 0.011 M glucose, 0.001 M CaCl2, and 500 μg/mL bovine serum albumin (BSA; fatty acid-free), pH 7.35.
Xenograft model
The HCT-116 human colon carcinoma cell line was obtained from the American Type Culture Collection (Manassas, VA; CCL-247) and cultured in 100-mm dishes in Dulbecco modified Eagle medium (DMEM; Gibco Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Summit Biotechnology, Summit, NJ), 3.5 g glucose/L, and 2.5 mL penicillin-streptomycin/L at 37°C and 5% CO2 until its growth was exponential. Tumor formation was induced by injecting 1.0 × 107 HCT-116 cells subcutaneously into the right hind flank of female athymic nude mice (Taconic Farms, Germantown, NY) using a similar protocol to that of Sheng et al.18 The nude mice were randomized into groups with 10 mice in each group. mAb C11C1 or control mouse IgG was injected intraperitoneally on the same day of tumor cell inoculation. The antibody was administered every 2 days at a dose of 320 μg/mouse per injection. Tumor volume was determined by measuring length and width every 2 or 3 days using a caliper (Fowler Ultra-Cal III, Fisher Scientific, Pittsburgh, PA). Mice were maintained in a pathogen-free environment and handled according to Institutional Animal Care and Use Committee (IACUC) guidelines. Tumor volume was determined by the equation V = (L × W2) × 0.5, where V = volume, L = length, and W = width. The mice were humanely killed on day 16 and the tumors were removed and weighed. All animal experimentation was performed in accordance with the guidelines of the IACUC of Temple University.
Syngeneic tumor model
Balb/c murine hybridoma cell lines were grown in plastic tissue culture flasks (Falcon, Franklin Lakes, NJ) in DMEM, supplemented with 100 μM sodium hypoxanthine, 16 μM thymidine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FBS, at 37°C in a humidified 5% CO2 atmosphere. The recipients were syngeneic Balb/c mice, which allowed the assessment of tumor growth in an immunocompetent environment. The animals were randomly placed in cages in groups of 5 and housed in the modern animal facilities at Temple University. They had free access to food and water. The research protocols were carried out under the guidelines of Temple University's IACUC. The intramuscular tumor growth was limited to 2 cm (by 1 or more mice in the group) to avoid excess discomfort. In both protocols, the animals were killed by an overdose of CO2 inhalation (consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association). Twenty-nine female Balb/c mice were divided in 2 groups: 2B5 group (n = 9) and C11C1 group (n = 20). We injected 5 × 105 cells (suspended in 1 × Hanks solution) intramuscularly in the shaved mouse back. At day 11, the 2B5 group was humanely killed because their tumors reached the humane limit. Five mice of the C11C1 group were killed on day 11 and the remaining 15 animals were followed for 6 months. The tumors were dissected from the surrounding tissues and weighed.
Histology and immunohistochemistry processing
The tumor tissue was preserved in zinc fixative (BD Biosciences Technical Protocol, Media, PA) and processed for paraffin embedding. Paraffin sections (4 μM) were cut and air-dried overnight, deparaffinated with xylene, and hydrated in decreasing concentrations of ethanol. Endogenous peroxidase activity was eliminated with a solution of 3% hydrogen peroxide and methanol. We blocked endogenous biotin binding (Vector, Burlingame, CA) and induced antigen retrieval by proteolytic digestion (Protease XXIV; Biogenex, San Ramon, CA). The slides were sequentially incubated with CD31 antibody to highlight endothelial cells (mAb ER-MP12; BMA Peninsula Laboratories, San Carlos CA), with anti-D5 440 antibody to detect HK (see “Antibodies”) or with anti-human PK antibody to detect PK. This incubation was followed by the appropriate species secondary (biotinylated) antibody, and streptavidin-peroxidase complex (Vectasin Elite; Vector Laboratory, Burlingame, CA). CD31 is directed to the platelet-endothelial cell adhesion molecule (PECAM), which is widely used to highlight endothelial cells and measure microvascular density. Antihuman PK antibody cross-reacts with mouse PK/kallikrein. Diaminobenzidine was used as a chromagen in the presence of hydrogen peroxide (Dako, Carpinteria, CA). Slides were then counterstained with Mayer hematoxylin (Fisher Scientific, Fair Lawn, NJ), dehydrated in alcohol, cleared in xylene, and mounted.
iMVD, HK, and PK analysis and testing for apoptosis
For intratumoral microvascular density (iMVD), we have followed the recommendations of Vermeulen et al.19 Briefly, digitalized images from the 10 most vascularized high-power fields (× 400) per tumor (hot spots) were analyzed using Image-Pro-Plus 4.1 software (Media Cybernetics, Silver Spring, MD). The evaluation was limited to those vessels with a cross-sectional area ranging from 10 to 50 μm. The total tumor surface area was examined. The total number of vessels ranging in size from 10 to 50 μm/tumor and their surface area were recorded and the following parameters calculated: percentage of area occupied by vessels (ratio of vessel area to total area) and mean number of vessels/mm2/group. To assess the in vivo binding of HK and PK, the total number of vessels (> 50 μm) present in 3 high-power fields (× 200) per tumor were counted and the percentage of immunopositive vessels tabulated. Testing for apoptosis was also done in each tumor with annexin V-biotin and converter-POD (antifluorescent antibody, Fab fragment from sheep, conjugated with peroxidase) as described by Roche Laboratories (Mannheim, Germany). A pathologist who did not know the identity of each group analyzed the data. The microscope used was a Leica DMSL (Leica, Heerbrugg, Switzerland). The digital camera was a Fuji MX-700 (Fuji, Tokyo, Japan). The numerical aperture of the lenses was F3.2/F8 (automatic).
In vitro measurements of cell proliferation
The glutathione-S-transferase (GST)-D5 was prepared as previously described.20 The coding sequence for GST was linked to the cDNA coding for the K420-S513 sequence of HK and the construct ligated into the pGEX2T vector. The resulting phage was expressed in Escherichia coli and purified on a glutathione column to more than 90% purity.20 The recombinant protein GST-D5 (270 nM) was previously shown to inhibit endothelial cell proliferation by 100%.3 For these experiments, HCT-116 cells were maintained in McCoy 5A medium supplemented with 10% FBS and 10 mL penicillin/streptomycin in 500 mL medium. All cell lines were grown at 37°C in a humidified incubator with 5% CO2. Before each specific experiment, cells were grown to 70% to 80% confluence, harvested with trypsin, and then resuspended to the required density for each particular assay. Before cell proliferation assays, cells were resuspended in 10% FBS to inactivate the trypsin and then spun for 5 minutes at 300g and the supernatant discarded. The cell pellet was resuspended in 30 mL culture medium and the cell concentration determined at 0 time using a cell-counting method in a hemocytometer. Trypan blue exclusion was used to enumerate the number of viable cells in each group. The cell proliferation assay was initiated by resuspending 25 × 103 cells/mL in 2% FBS medium and these cells were then plated onto 6-well plates and grown for 2 days. To synchronize these cells, they were grown in serum-free culture medium for 2 hours before they were treated with 500 nM of the recombinant proteins GST (control) and GST-D5 or mAb C11C1 (1 μM), also in serum-free media. These cells were then allowed to grow for 24, 48, and 72 hours in the incubator. All assays involving GST, GST-D5, and mAb C11C1 were performed with culture media containing 15 μM final concentration of ZnCl2. At the end of each incubation period, cells were counted by the hemocytometer after detaching cells from each group with trypsin-EDTA (ethylenediaminetetraacetic acid) solution. Only cells excluding trypan blue were counted. Each count was performed in triplicate and 3 independent experiments were used. The results were expressed as the percent of the control-treated cells (GST treated) and then expressed as mean ± SD.
Surface plasmon resonance
Surface plasmon resonance was performed and analyzed using BiaCore 2000 methodology (BiaCore, Uppsala, Sweden). Pure antibodies were linked using standard amine coupling to a B1 Pioneer Sensor chip to achieve a saturated chip surface with a 5000 to 6000 RU difference (representing an antibody base of 5-6 ng). MOPC-21 was linked to Fc1 as a control mAb. mAb C11C1 was linked to Fc4. Because the epitope recognition site in mAb C11C1 is sensitive to denaturization at low pH, Fc1 and Fc4 were regenerated using 3 × 35 μL 0.1 M sodium carbonate, pH 10.0. The dilution and system buffer for the plasma runs was Hanks balanced solution (HBS)-EDTA (0.01 M HEPES, 0.15 M NaCl, 1 mM EDTA, pH 7.4). Human citrated pooled normal plasma (PNP) (obtained from George King Bio-Medical, Overland Park, KS) or citrated athymic nude mouse plasma was diluted 1:4 in HBS-EDTA. The plasma runs were performed at 60 μL/min with 150-μL injections. The data were analyzed by subtraction of the control run sensor signal (Fc1) from the sensor signal generated by each linked antibody.
Statistical analysis
All results were analyzed using Student t test or analysis of variance (ANOVA). All data are expressed as mean ± SEM except where indicated.
Results
mAb C11C1 blocks HK binding to endothelial cells
mAb C11C1 inhibits the coagulant activity of HK, a property of the light chain. In contrast, mAb 2B5 blocks the cysteine protease inhibitory activities of the heavy chain. The epitope C11C1 was mapped to H441-K502 of the light chain (kininostatin) by proteolytic digestion, immunoaffinity chromatography, and N-terminal sequencing.4 Both D3 and D5 are involved in cell binding.5 To further explore the action that mAb C11C1 has on HK in vivo, we examined whether the effect in vivo could hinge on its ability to prevent binding of HK to cultured endothelial cells. Using DELPHIA technology with a Europium-tagged HK (Eu-HK), we studied the binding of HK to HUVECs and found that it was inhibited by mAb C11C1 (Figure 1). Eu-HK was able to bind to HUVECs in a concentration-dependent manner (Figure 1 inset). At higher concentrations of HK, we have demonstrated saturation of binding to endothelial cells.6 This experiment is designed to reveal inhibition if present and thus binding was observed on the lower portion of the binding isotherm. Incubating Eu-HK (31 nM) in the presence of increasing concentrations of mAb C11C1 resulted in significant blocking of Eu-HK binding to the HUVECs (Figure 1 bar graph). These experiments suggest that one mode of action of mAb C11C1 effect on HK on angiogenesis14 is to block its ability to bind to its various receptors on the endothelial cell surface. To test for the specificity of the antibody, we studied the effect of mAb C11C1 on the binding of factor XII to endothelial cells, which bind in a zinc-dependent manner to the same receptor, gC1qR, as HK.21 The mAb C11C1 concentration (214 nM) that blocked Eu-HK binding failed to inhibit the binding of Eu-FXII (43 nM).
mAb C11C1 recognizes HK in mouse plasma
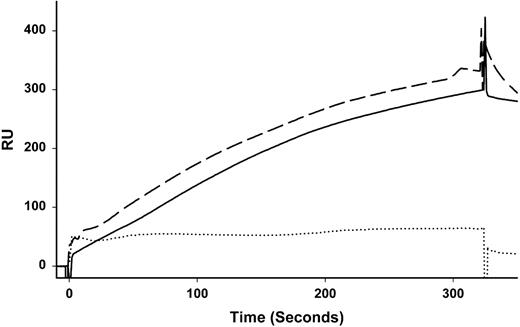
The mAb C11C1 recognizes D5 in both human HK and HKa4 but reacts poorly with HK or HKa in a Western blot. mAb C11C1 also recognizes chicken HK (ornithokininogen).14 To demonstrate that mAb C11C1 recognizes kininogen in mouse plasma, we used surface plasmon resonance (BiaCore). The mAb C11C1 was bound to a sensor chip and human or mouse plasma was injected and pumped over the chip. The results show typical binding sensor-grams for human and mouse plasma to mAb C11C1 (Figure 2) that are virtually identical. In contrast, human plasma from a patient deficient in HK (and LK) and studied in our laboratory22 did not bind to mAb C11C1.
Surface plasmon resonance of mAb C11C1 binding to mouse kininogen present in mouse plasma. Diluted plasma prepared as outlined in “Materials and methods” was injected over a sensor chip to determine the ability of mAb C11C1 to bind to HK in mouse plasma. Control mAb (MOPC-21) was used to subtract nonspecific binding. Mouse plasma (solid line) and human plasma (dashed line) bound with similar kinetics. HK-deficient plasma (dotted line) did not bind. Each line represents averaged signals of 3 separate experiments.
Surface plasmon resonance of mAb C11C1 binding to mouse kininogen present in mouse plasma. Diluted plasma prepared as outlined in “Materials and methods” was injected over a sensor chip to determine the ability of mAb C11C1 to bind to HK in mouse plasma. Control mAb (MOPC-21) was used to subtract nonspecific binding. Mouse plasma (solid line) and human plasma (dashed line) bound with similar kinetics. HK-deficient plasma (dotted line) did not bind. Each line represents averaged signals of 3 separate experiments.
mAb C11C1 decreases human colon carcinoma growth rate in a murine xenograft model
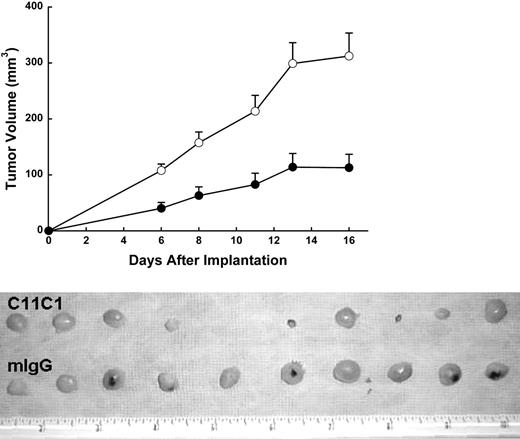
To test the ability of the mAb C11C1 to prevent colon tumor growth in the xenograft model, athymic nude mice bearing colon carcinoma (HCT-116) were treated systemically with mAb C11C1 (320 μg/dose) or control antibody at the same dose level every 2 days. Ten (100%) of 10 mice in the IgG group developed tumors compared to 9 (90%) of 10 mice from group C11C1. On day 16, when the experiment terminated, the IgG group had a mean volume of 312 ± 41 mm3, whereas group C11C1 showed a significant reduction (125 ± 23 mm3; P = .002). As shown in the top of Figure 3, on day 16, mAb C11C1 significantly inhibited the growth of subcutaneous HCT-116 tumor compared to the control group, mIgG (P < .002). Moreover, 2 additional experiments showed that the treatment with mAb 2B5 (n = 10, n = 12), which is an antibody targeted to an epitope in the heavy chain of HK,15 failed to significantly (P > .05) inhibit the growth of the colon tumor on day 16 (data not shown). The bottom of Figure 3 shows the gross appearance of the excised tumor nodules from each group. The tumors derived from mAb C11C1-treated animals appeared pale or translucent with few and very small hemorrhagic foci. In contrast, large tumors with more prominent and frequent hemorrhagic foci were observed in the group receiving mIgG. The excised tumors (on day 16) showed the control mIgG group had a weight of 275 ± 97 mg (mean ± SEM), whereas the tumors from the C11C1 group excised on day 16 showed a highly significant reduction in weight to 131 ± 52 mg (P = .001). Regression analysis showed a strong correlation between the tumor volume and weight in each group (P < .001).
Human colon carcinoma (HCT-116) growth response to different mAb treatments in nude mice. (Top) Each mouse was treated systemically with 320 μg mIgG (○) and 320 μg mAb C11C1 (•). Data are mean ± SEM. The treatment was administered intraperitoneally every 2 days for 16 days. The C11C1 mice showed a tumor growth plateau from days 13 to 16, whereas the group receiving mIgG continued to grow. The difference in volume was statistically significant at all points mIgG versus C11C1 (P < .003). (Bottom) Note that the C11C1-treated group had smaller, paler tumors, whereas the mIgG group had larger tumors and hemorrhagic foci. Nine of 10 mice had tumors at the time of autopsy (day 16) versus 10 of 10 in the mIgG group. The decrease in weight in the C11C1 group was statistically significant (P = .0012). The ruler in the picture measures inches.
Human colon carcinoma (HCT-116) growth response to different mAb treatments in nude mice. (Top) Each mouse was treated systemically with 320 μg mIgG (○) and 320 μg mAb C11C1 (•). Data are mean ± SEM. The treatment was administered intraperitoneally every 2 days for 16 days. The C11C1 mice showed a tumor growth plateau from days 13 to 16, whereas the group receiving mIgG continued to grow. The difference in volume was statistically significant at all points mIgG versus C11C1 (P < .003). (Bottom) Note that the C11C1-treated group had smaller, paler tumors, whereas the mIgG group had larger tumors and hemorrhagic foci. Nine of 10 mice had tumors at the time of autopsy (day 16) versus 10 of 10 in the mIgG group. The decrease in weight in the C11C1 group was statistically significant (P = .0012). The ruler in the picture measures inches.
mAb C11C1 decreases tumor angiogenesis in a murine xenograft model
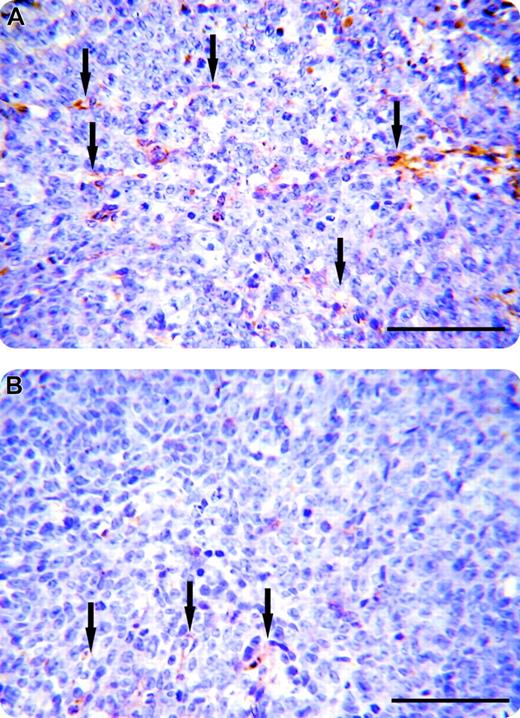
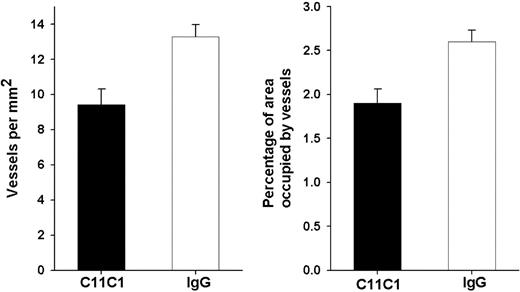
To examine whether the inhibition of tumor growth by mAb C11C1 treatment was due to suppression of proliferation of tumor blood vessels or to a different mechanism, the iMVD of the excised tumors was assessed. Figure 4 shows a representative microscopic field of each group, demonstrating that the number of blood vessels is decreased (arrows) in the mAb C11C1-treated group as compared to control group mIgG. Figure 5 displays the iMVD reported as vessels/mm2 and the percentage of total tumor area occupied by the vessels (percent vessel area/total area). The iMVD is significantly decreased in the mAb C11C1-treated group (P < .003) in comparison to the control group (mIgG). These data support the hypothesis that there is a significant relationship between tumor development and iMVD in this setting. Testing for apoptosis revealed that there was no endothelial cell apoptosis in the specimens treated with mAb C11C1 (data not shown).
Visualization of vessels in human colon carcinoma. Immunochemistry sections. The tumor sections were incubated with monoclonal CD31 antibody (PECAM) using the immunoperoxidase technique. The vessel walls (arrows) stained golden-brown. (A) Human colon carcinoma treated with murine IgG. (B) Human colon carcinoma treated with mAb C11C1. There were fewer vessels apparent in the C11C1-treated group. Bars represent 100 μm.
Visualization of vessels in human colon carcinoma. Immunochemistry sections. The tumor sections were incubated with monoclonal CD31 antibody (PECAM) using the immunoperoxidase technique. The vessel walls (arrows) stained golden-brown. (A) Human colon carcinoma treated with murine IgG. (B) Human colon carcinoma treated with mAb C11C1. There were fewer vessels apparent in the C11C1-treated group. Bars represent 100 μm.
Graphical representation of iMVD. (A) The mean ± SEM number of vessels/mm2 is shown and is significantly less in the C11C1 group (P < .003). (B) The mean ± SEM percentage of tumor area occupied by vessels is displayed and is significantly less in the C11C1 group (P < .004).
Graphical representation of iMVD. (A) The mean ± SEM number of vessels/mm2 is shown and is significantly less in the C11C1 group (P < .003). (B) The mean ± SEM percentage of tumor area occupied by vessels is displayed and is significantly less in the C11C1 group (P < .004).
Hybridomas secreting mAb C11C1 decrease tumor weight in a murine syngeneic model
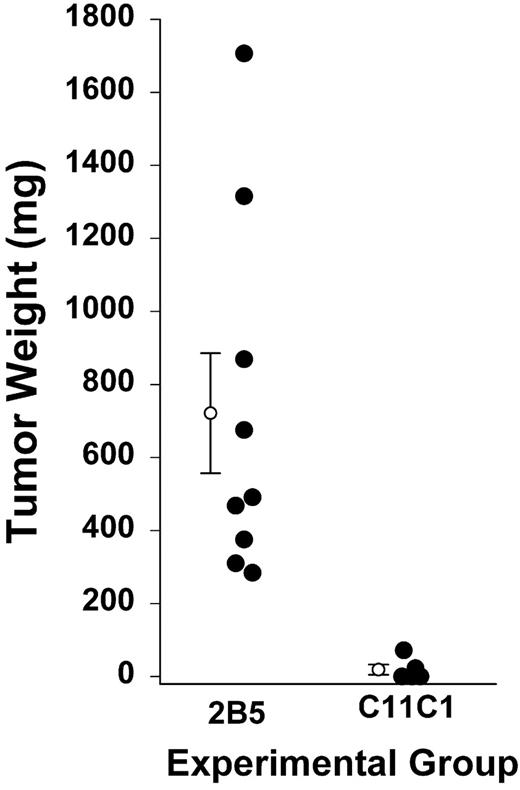
To show that the results are not confined to the xenograft model, we studied a syngeneic model (Figure 6). At day 11, after intramuscular inoculation of hybridomas secreting mAbs 2B5 and C11C1, all animals from group 2B5 had developed tumors. Group 2B5 neoplasms had a mean weight of 721 ± 164 mg. A cage with 5 animals from group C11C1 was selected at random to compare tumor growth. Two of the 5 mice had detectable tumors but 3 did not. The mean weight was 19 ± 13.9 mg. The 2B5 tumors were heavier than C11C1 tumors (P = .008).23 The iMVD of the C11C1 hybridomas was 6.8 ± 1.8 vessels/mm2, whereas the iMVD of the 2B5 tumors was 12.4 ± 0.9 vessels/mm2 (P = .02).
Tumor growth of intramuscular hybridomas. Hybridomas were excised on day 11 of treatment and weighed. Solid circles represent the hybridoma weight from each animal. The open circles with error bars are mean ± SEM; n = 9 and n = 5, respectively, for mAb 2B5 and mAb C11C1 (P = .015). An additional 15 animals treated with mAb C11C1 had no apparent tumor growth and were not killed until day 22, at which time no tumors were found.
Tumor growth of intramuscular hybridomas. Hybridomas were excised on day 11 of treatment and weighed. Solid circles represent the hybridoma weight from each animal. The open circles with error bars are mean ± SEM; n = 9 and n = 5, respectively, for mAb 2B5 and mAb C11C1 (P = .015). An additional 15 animals treated with mAb C11C1 had no apparent tumor growth and were not killed until day 22, at which time no tumors were found.
mAb C11C1 decreases endothelial HK and PK binding in a colon carcinoma murine xenograft model
Endothelial cells lining the tumor blood vessels showed immunopositivity to HK and PK. The entire endothelial cell layer of each vessel examined was either immunopositive or nonreactive.
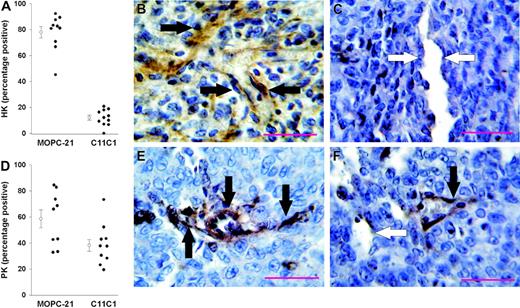
HK. The tumors were tested for HK immunoreactivity with rabbit polyclonal anti-D5 440. Animals treated with control isotype-specific immunoglobulin MOPC-21 showed 77.9% ± 4.4% of the vessels immunopositive for HK (Figure 7A-B). Animals treated with mAb C11C1 showed markedly reduced immunopositivity for HK, 12.1% ± 1.9% (Figure 7A,C).
Percentage of vessel immunopositivity to HK and PK. HK results are on the top, PK results on the bottom. (A) Percentage of tumor vessels (entire endothelial cell layer) immunopositive to anti-HK in animals treated with MOPC-21 and mAb C11C1. There was a dramatic and significant decrease in the percentage of vessels positive for HK in the C11C1-treated group. (B) Representative microphotograph (cell at × 730) from animals treated with MOPC-21 showing endothelial cells staining brown (black arrows). (C) Representative microphotograph from animals treated with mAb C11C1; most vessels were immunonegative for HK. White arrows point to immunonegative endothelial cells. (D) Percentage of tumor vessels immunopositive to anti-PK in animals treated with MOPC-21 and mAb C11C1. There was a significant decrease in PK immunopositivity in the animals treated with mAb C11C1. (E) Representative microphotograph from animals treated with MOPC-21 showing endothelial cells with brown staining (black arrows). (F) Representative microphotograph from animals treated with mAb C11C1. Thirty-eight percent of the vessels examined were immunopositive to PK. The black arrow points to a positive vessel and the white arrow points to a negative vessel. The red bars in panels B, C, E, and F indicate 100 μM. The open circles in panels A and D are the mean ± SEM.
Percentage of vessel immunopositivity to HK and PK. HK results are on the top, PK results on the bottom. (A) Percentage of tumor vessels (entire endothelial cell layer) immunopositive to anti-HK in animals treated with MOPC-21 and mAb C11C1. There was a dramatic and significant decrease in the percentage of vessels positive for HK in the C11C1-treated group. (B) Representative microphotograph (cell at × 730) from animals treated with MOPC-21 showing endothelial cells staining brown (black arrows). (C) Representative microphotograph from animals treated with mAb C11C1; most vessels were immunonegative for HK. White arrows point to immunonegative endothelial cells. (D) Percentage of tumor vessels immunopositive to anti-PK in animals treated with MOPC-21 and mAb C11C1. There was a significant decrease in PK immunopositivity in the animals treated with mAb C11C1. (E) Representative microphotograph from animals treated with MOPC-21 showing endothelial cells with brown staining (black arrows). (F) Representative microphotograph from animals treated with mAb C11C1. Thirty-eight percent of the vessels examined were immunopositive to PK. The black arrow points to a positive vessel and the white arrow points to a negative vessel. The red bars in panels B, C, E, and F indicate 100 μM. The open circles in panels A and D are the mean ± SEM.
PK. The tumors were tested for PK immunoreactivity with anti-human PK antibody. Animals treated with control immunoglobulin MOPC-21 had 58% ± 6.9% tumor blood vessels immunopositive for PK (Figure 7D-E). Animals treated with mAb C11C1 showed decreased tumor blood vessel immunopositivity for PK, 38.1% ± 4.5% (Figure 7D,F). In summary, there was a significant decrease in HK (P < .001) and PK (P < .002) detected in tumors from animals treated with mAb C11C1 (n = 11) when compared to animals treated with MOPC-21 (n = 10).
mAb C11C1 fails to inhibit in vitro tumor proliferation
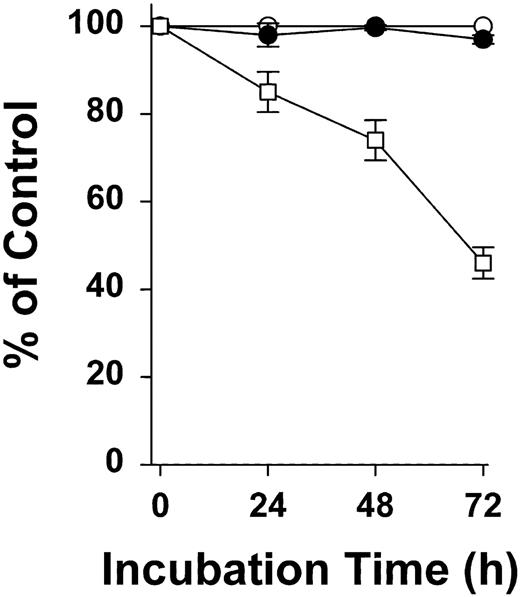
We have shown that mAb C11C1 inhibits endothelial cell proliferation.14 The decrease in tumor volume and weight was postulated to be associated with a decrease in the microvessels supplying the tumor. An additional explanation would be if mAb C11C1 had a direct inhibitory effect on tumor growth. HCT-116 human colon carcinoma was cultured for 72 hours as described (see “Xenograft model”). All experiments were performed using cells from passage 20 or less. The total nucleic acid in control cultures doubled within 24 to 36 hours. Using the cell-counting method (Figure 8), proliferation in the presence of mAb C11C1 was indistinguishable from the group with addition of GST alone; thus, mAb C11C1 did not inhibit tumor cell proliferation. In another experiment, isotype-matched normal murine IgG (MOPC-21) also failed to inhibit cell proliferation (data not shown). In contrast, GST-D5 used as a positive control (see “Materials and methods”) progressively inhibited the proliferation as a function of time, reaching a value less than 50% at 72 hours (P < .001). Preliminary results indicate that the mechanism of action of GST-D5 on tumor cells differs from that on endothelial cells because GST-D5 causes apoptosis in endothelial cells24 but not in tumor cells.
The effect of GST-D5 and mAb C11C1 on the proliferation of HCT-116 cells. HCT-116 cells were grown to 50% confluence and counted as outlined in “Materials and methods.” This count at 0 time was designated as 100%. Equal numbers of cells were added to media and treated at intervals as indicated with 500 nM recombinant proteins, GST (○) as control, GST-D5 (□), or 1 μM mAb C11C1 (•) as test proteins. Aliquots were counted at 24, 48, and 72 hours and expressed as mean ± SD (n = 3).
The effect of GST-D5 and mAb C11C1 on the proliferation of HCT-116 cells. HCT-116 cells were grown to 50% confluence and counted as outlined in “Materials and methods.” This count at 0 time was designated as 100%. Equal numbers of cells were added to media and treated at intervals as indicated with 500 nM recombinant proteins, GST (○) as control, GST-D5 (□), or 1 μM mAb C11C1 (•) as test proteins. Aliquots were counted at 24, 48, and 72 hours and expressed as mean ± SD (n = 3).
Discussion
Folkman25 was the first to postulate that if new blood vessels were indeed essential for tumor growth, then inhibiting angiogenesis should inhibit tumor expansion. Each step including detachment, migration, proliferation, proteolysis, and tube formation involved in tumor-associated angiogenesis provide opportunities for specific therapeutic interventions. Of the many growth factors derived from malignant cells that have been shown to stimulate neovascularization in neoplastic diseases, VEGF-A seems to play a crucial role in both the proliferation and migration of endothelial cells and an association between VEGF-A expression and tumor aggressiveness in colon cancer has been demonstrated.25 We have explored the effect of mAb C11C1 on the VEGF-A165-stimulated CAM.14 Vessel formation is markedly increased by VEGF-A165 and this increase is inhibited as a function of the amount of mAb C11C1 applied to the CAM from 0.2 to 10 μg.14
Our present studies in athymic mice indicate that mAb C11C1 can inhibit human tumor growth in a mammalian xenograft model. The inhibition by mAb C11C1 is consistent with HK serving as a proangiogenic protein. In considering the significance and interpretation of these results, we postulate that the major effect of the antibody was antiangiogenic with a decrease in the vessels supplying the human tumor accounting for the slow growth observed. One objection that might be raised is that the human tumor of necessity is growing in an immunologically incompetent host, which does not represent the situation in patients with cancer. Accordingly, we used a unique syngenetic model in mice. We followed the growth of these hybridomas derived from murine plasma cell tumors fused to splenic lymphocytes immunologically instructed to produce murine antibodies against 2 different epitopes of HK as well as an unrelated hybridoma to coagulation factor V (B38). These hybridomas were injected subcutaneously in the back of the mice. The complete analysis of this model is the subject of another study.23 Here, we present the weights of the tumors at the time of autopsy of the mice. The marked decrease of the hybridoma weights in the mice treated with mAb C11C1 compared with 2B5 suggests that the antibody produced by the tumor was responsible for the marked decline in weight.
A second consideration is that mAb C11C1 has a direct antitumor effect in addition to the effect on angiogenesis. To study this issue, we measured tumor cell proliferation in vitro where no vascular supply is required. We used as a comparison a recombinant peptide, GST-D5, representing the epitope of the antibody, which had been found to have direct antiangiogenic activity.3 GST-D5 inhibited the proliferation of the HCT-116 human colon carcinoma cells, but mAb C11C1 had no effect. This finding indicates that the mechanism of mAb C11C1 differs from that of HKa or GST-D5.
In this report, we demonstrate that the significant decrease of iMVD was synchronously accompanied by a significant decrease of human colon tumor growth. The fact that mAb C11C1 has no effect on HCT-116 cell proliferation in vitro argues against a direct antitumor effect and is consistent with the hypothesis that antiangiogenic activity is most important in vivo. Our results support the paradigm that tumors, which lack the ability to induce adequate neovascularization due to antiangiogenic therapy, show limited growth and invasive potential.
The current study provides new information that broadens our knowledge of the role of kininogen in regulating angiogenesis. HK stimulates angiogenesis by binding to endothelial cells14 and proteolytically cleaving to BK. The inhibition of tumor growth by mAb C11C1 suggested that the antibody might prevent the binding of HK, a proangiogenic molecule, to endothelial cells. In this report, we provide direct evidence that in vitro mAb C11C1 at a 4-fold excess over HK inhibited binding to endothelial cells about 50% with a maximum inhibition of 85% at a 50-fold excess. HK is present in human plasma in moderately high concentration, 80 μg/mL or 670 nM. Based on the dose of the mAb given, the blood volume of the mouse, and the half-life of mAbs, we estimate that the concentration of mAb C11C1 in the murine plasma was 3 μM or 4.5-fold that of HK.26 Therefore, mAb C11C1 could block the binding and subsequent proteolysis of HK on the endothelial surface, thus inhibiting the release of angiogenic BK. Evidence that soybean trypsin inhibitor, which inhibits plasma kallikrein, can block the proangiogenic effect of HK in a CAM model argues that a proteolytic cleavage product of HK, BK, is responsible for the proangiogenic effect.14 One cannot rule out that the source of BK is LK; however, this is unlikely because mAb C11C1 would not block the binding of LK because LK does not contain its epitope. BK is angiogenic because it directly stimulates neovascularization in the CAM. The other product of cleavage is HKa, which is antiangiogenic. Thus, mAb C11C1 may block the binding of HK to endothelial cells and its subsequent cleavage to BK and thus inhibit angiogenesis and tumor growth.
To demonstrate the involvement of HK and PK in vivo, we examined the tumor vessels with specific antibodies to these human plasma proteins (Figure 7). The tumor vessels were 77.9% immunopositive for HK in the group treated with a control antibody. This finding indicates that HK binds to endothelial cells that have been exposed to tumor angiogenic proteins. In the group treated with the mAb to HK, C11C1, the percentage of vessels immunopositive for HK was markedly reduced to 12.1%, representing an 85% inhibition. This finding reproduces in vivo the observation that mAb C11C1 prevents the binding of HK to endothelial cells in vitro (Figure 1). PK circulates in plasma in a 1:1 complex with HK.27 However, because the concentration of HK in human plasma is 670 nM and PK is 490 nM,5 73% of PK is present as the complex with HK. Therefore, it is not surprising that the tumor vessels were only 58.1% immunopositive for PK (Figure 7D), which is 74% of HK immunopositivity. The mAb C11C1 reduced the immunopositivity for PK to 38.1% of tumor vessels (Figure 7F), which represents a less complete inhibition compared to HK, as predicted. Because PK is present on over half of the endothelial cells, it is positioned to be activated by factor XIIa or prolylcarboxypeptidase and release BK as angiogenic stimulus. This process would also be down-regulated by mAb C11C1.
We suggest that the use of anti-HK therapy as an antiangiogenic treatment in combination with conventional cytotoxic therapy, radiation, or immunotherapy may improve the efficacy of these anticancer therapies. Because patients with kininogen deficiency are asymptomatic from the deficiency and long-lived,28 inhibition of kininogen functions by a mAb should have a high probability of being safe.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-02-0449.
Supported in part by grants R01 CA83121 and T32 HL07777 from the National Institutes of Health.
J.S.S. and I.M.S. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Virginia Sheaffer for administrative assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal