Abstract
Hemophilia is a genetic disease caused by a deficiency of blood coagulation factor VIII or IX. Bleeding into joints is the most frequent manifestation of hemophilia. Hemarthrosis results in an inflammatory and proliferative disorder termed hemophilic synovitis (HS). In time, a debilitating, crippling arthritis, hemophilic arthropathy, develops. Although the clinical sequence of events from joint bleeding to synovitis to arthropathy is well documented, the component or components in blood and the molecular changes responsible for hemophilic synovitis are not known. Iron has long been suspected to be the culprit but direct evidence has been lacking. Previously, we showed that iron increased human synovial cell proliferation and induced c-myc expression. Here we show that bleeding into a joint in vivo and iron in vitro result in increased expression of the p53-binding protein, mdm2. Iron induced the expression of mdm2 by normal human synovial cells approximately 8-fold. In a murine model of human hemophilia A, hemarthrosis resulted in pathologic changes observed in human hemophilic synovitis and a marked increase in synovial cell proliferation. Iron, in vitro, induced the expression of mdm2. The molecular changes induced by iron in the blood may be the basis of the increase in cell proliferation and the development of hemophilic synovitis. (Blood. 2004;104:2060-2064)
Introduction
Hemophilia is a genetic disease caused by a deficiency of blood coagulation factor VIII or IX.1 Bleeding into joints is the most frequent manifestation of hemophilia.2 Hemarthrosis results in an inflammatory and proliferative disorder termed hemophilic synovitis (HS).3 In time, a debilitating, crippling arthritis, hemophilic arthropathy, develops.3 The clinical events from joint bleeding to synovitis to arthropathy are well documented.4 After only a few episodes of joint bleeding, there is deposition of hemosiderin in the superficial and deeper layers of the synovial membrane as well as a proliferation of synovial fibroblasts and vascular cells.5 Over the ensuing years, there is destruction of the cartilage and underlying bone.6 Fibrosis and ankylosis develops.7 The pathogenesis of and molecular changes leading to blood-induced HS are poorly understood.8 The gross,9 radiologic,10 microscopic,11 and ultrastructural12 changes that occur in the synovial membrane of human and experimental hemarthrosis are reminiscent of changes described in malignant tissues. Villous hypertrophy may be the result of promotion of cell growth and/or abrogation of cell death. Repeated episodes of bleeding induce synoviocyte hypertrophy and hyperplasia, an intense neovascular response, and inflammation of the synovial membrane. The component(s) in blood that initiate these changes are not known. Iron is often proposed as one possibility.13-15 Iron plays a role in malignant cell growth,16,17 local invasion, and tumor progression, possibly due to changes in the expression of oncogenes.13 We hypothesized that iron plays a similar role in HS. In support of this hypothesis, Wen et al showed that in vitro iron increases human synovial fibroblast cell (HSFC) proliferation and induces expression of the c-myc oncogene.13 Here we examined blood-induced synovitis and mdm2 expression in vivo in a murine model of human hemophilia and iron-induced mdm2 gene expression in vitro by normal human and murine synovial cells.
Materials and methods
This project was approved by Rush University's Institutional Animal Care and Use Committee for the protection of animals in experimental research.
Tissue explants and cell culture
Primary cultures of normal human and murine synovial fibroblast cells were a gift from Dr Katalin Mikecz (Department of Biochemistry, Rush University). Synovial fibroblast cells were cultured in Dulbecco modified Eagle medium (DMEM; Biowhittaker, Walkersville, MD) with 10% fetal bovine serum, penicillin G (100 IU/mL), and streptomycin (100 μg/mL) in a humidified, 5% CO2 atmosphere at 37°C. All experiments were performed with cells between the fifth and eighteenth passage. Testing for bacterial, fungal, and mycoplasma contamination was done routinely.
Mouse model and breeding
Haig Kazazian at the University of Pennsylvania generously provided breeder pairs of mice. The E16/E17 factor VIII knockout B6;129S4-F8tm1kaz mouse line was generated by gene targeting.18,19 A colony of hemophilic mice was created and used in the experiments described. Murine synovial tissue was isolated from the knee joints of animals killed at the indicated times following a controlled trauma to induce hemarthrosis.20 The joints were dissected, opened, and observed for the presence of blood. Synovial tissue from the right (injured) and left (uninjured) joints of 20 hemophilic mice was removed and the respective samples were then pooled together for further processing and analysis.
RNA extraction and isolation
Total RNA was extracted from samples of tissue and cells, using TRIzol reagent according to the manufacturer's instruction (Invitrogen, Carlsbad, CA). Briefly, samples were homogenized or lysed in one mL TRIzol. A quantity of 200 μL chloroform per one mL TRIzol was added and the mixture was shaken vigorously for 10 seconds, then incubated on ice for 2 to 3 minutes, clarified by centrifugation (12 000g for 15 minutes at 4°C), and the aqueous phase was transferred into a fresh tube. An equal volume of isopropanol was added and stored overnight at -70°C. The RNA was pelleted by centrifugation (12 000g for 15 minutes at 4°C) and the supernatant removed. The pellet was washed with one mL of 75% ethanol and dried. The RNA was dissolved in ddH2O and concentration was determined at an optical density (OD) of 260 nm. Isolated samples were stored at -70°C until used for reverse transcriptase-polymerase chain reaction (RT-PCR).
Human gene array analysis
Human apoptosis-2 GEArray (catalog no. 9 905 020; SuperArray, Bethesda, MD) was used to screen for changes in the expression of 23 genes involved in apoptosis and cell cycle control. Human synovial cells were maintained in culture in the presence of ferric citrate (one mM) or the same concentration of sodium citrate for 9 days. RNA was isolated according to the methods described in the previous section and cDNA was generated by reverse transcription. A 32p cDNA probe was synthesized and hybridized to the membrane, which was then exposed to x-ray film.
Mouse gene array
A GEArray Q Series Mouse Cancer Pathway Finder Gene Array (SuperArray) was used to identify changes in the expression of 96 genes associated with a mouse cancer pathway. All steps were performed according to manufacturer's recommendations. Briefly, total RNA was isolated as described in “RNA extraction and isolation,” then cDNA probes were synthesized with one mM Biotin-16-dUTP (Roche, Indianapolis, IN) using provided GEAprimer mix and buffer, 2 μg isolated total RNA, RNase inhibitor (Promega, Madison, WI), and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). The labeling reaction was performed at 42°C for 90 minutes. After labeling, the cDNA probe was denatured by heating at 94°C for 5 minutes and hybridized at 60°C overnight with continuous agitation. A GEArray Q Series membrane was washed twice with 2x standard saline citrate (SSC), 1% sodium dodecyl sulfate (SDS), and twice with 0.1x SSC, 0.5% SDS at room temperature with agitation then incubated with chemiluminescent substrate at room temperature for 2 minutes and exposed to x-ray film. Images were scanned and analyzed by Scanalyze software (Stanford University, Stanford, CA).
cDNA synthesis and semiquantitative RT-PCR analysis
The isolated total RNA served as the template for cDNA synthesis by reverse transcriptase (Promega). For each 50 μL reaction, one μg total RNA, 5 U avian myeloblastosis virus (AMV) reverse transcriptase, 0.4 mM deoxynucleoside triphosphate (dNTP) mix, 1x AMV reaction buffer, one mM MgSO4, 1.25 μg oligo(dT)15 primers, and 20 U recombinant RNasin ribonuclease inhibitor were used. Reverse transcription reaction was performed at 48°C for 45 minutes. Then, 2 μL RT reaction product (equivalent to 80 ng starting RNA from each sample) was amplified by PCR. The primers for mouse mdm2 were as follows: sense 5′-ATGTGCAATACCAACATCTCTGTGTC-3′ and antisense 5′-GCTGACTTACAGCCACTAAATTTC-3′ generating a 337-bp product. For mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) the primers were as follows: sense 5′-GGTATCGTGGAAGGACTCAT-3′ and antisense 5′-ACCACCTGGTGCTCAGTGTA-3′ generating a 350-bp product. The reaction was performed in 25 μL total volume using 1x HotMaster Taq Buffer with 25 mM Mg2+, 1.25 U HotMaster Taq DNA polymerase (Eppendorf, Hamburg, Germany), 0.2 mM dNTP mix, and one μM mdm2 primers. After initial denaturation at 94°C for 2 minutes, 40 cycles were performed as follows: 94°C for 20 seconds, 56°C for 10 seconds, 70°C for 35 seconds. Final extension was done at 72°C for 10 minutes. The final PCR products were resolved by gel electrophoresis using 1.5% agarose gel, then stained in 0.5 μg/mL ethidium bromide solution and photographed under UV transillumination.
Pathologic examinations
Mouse knee joints were dissected, fixed in formalin, and decalcified, and 5-μm paraffin-embedded sections from an equatorial plane were stained with hematoxylin-eosin. The sections were then examined by light microscopy. To enumerate the number of cell layers, 5 areas of the synovial membrane were chosen at random and the number of cell layers in the membrane was counted by 4 independent observers blinded to the experimental conditions. The results were pooled and the average and standard deviation calculated. Student t test was used to compare the differences between groups.
Results
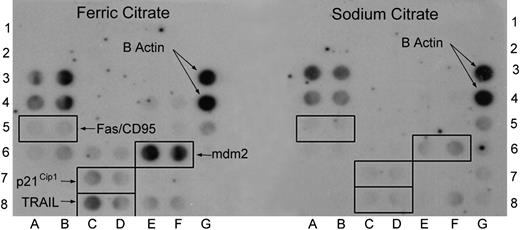
Iron induces mdm2 gene expression by normal human synovial cells. To determine if iron from erythrocytes in blood was responsible for the induction of mdm2 gene expression, we examined the effects of iron on mdm2 gene expression by cultured human synovial cells. To screen for changes in the expression of 23 genes involved in apoptosis and cell cycle control, the human apoptosis-2 GEArray was used. Following culture in the presence of ferric citrate (one mM) or the same concentration of sodium citrate for 9 days, RNA was isolated from HSFCs and cDNA was generated by reverse transcription. The one-mM concentration of iron in the form of ferric citrate used in vitro was derived from experiments performed by Nishiya.21 Furthermore, this concentration of iron was found to be optimal for in vitro experiments based on our previously published results.13 Results from a representative experiment are shown in Figure 1. The expression of β actin (positions 3G and 4G), a control gene shown for reference, was identical on both membranes. A marked increase in the expression of mdm2 (positions 6E and 6F) is evident. Using the Storm 860 Imaging System (Amersham, Piscataway, NJ), this was equivalent to an 8-fold increase in expression following HSFC culture with ferric citrate compared with the signal generated by cells cultured with sodium citrate. Other genes that were induced in the presence of ferric citrate but not sodium citrate include Fas/CD95 (average of 2 dot blots, 2-fold), p21CIP1 (2.5-fold), and TRAIL (3-fold). This experiment has been repeated twice and similar results were observed. The data are consistent with that from Gazitt et al17 who showed that iron induces aberrant proto-oncogene expression of p21CIP1.
Iron induces mdm2 gene expression by normal human synovial cells. Following incubation in the presence or absence of ferric citrate (1 mM) or the same concentration of sodium citrate for 9 days, RNA was isolated from normal human synovial cells and probed using a microarray (GEArray no. 9 905 020; SuperArray. The left membrane demonstrates the pattern of gene expression in cells cultured with ferric citrate, and the right membrane shows the pattern following exposure to sodium citrate. This experiment was repeated with similar results and consistent induction of these genes was observed.
Iron induces mdm2 gene expression by normal human synovial cells. Following incubation in the presence or absence of ferric citrate (1 mM) or the same concentration of sodium citrate for 9 days, RNA was isolated from normal human synovial cells and probed using a microarray (GEArray no. 9 905 020; SuperArray. The left membrane demonstrates the pattern of gene expression in cells cultured with ferric citrate, and the right membrane shows the pattern following exposure to sodium citrate. This experiment was repeated with similar results and consistent induction of these genes was observed.
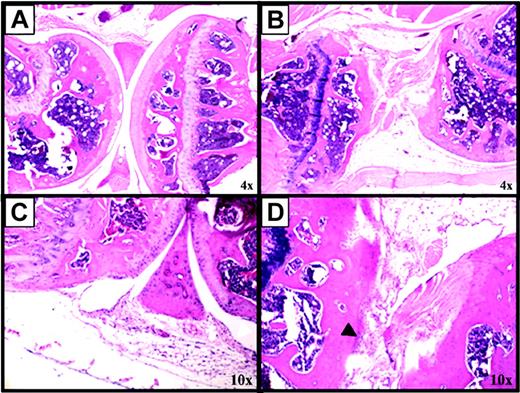
Blood induces synovitis and mdm2 expression in a murine model of human hemophilia. A standardized protocol to produce hemarthrosis in mouse knee joints was used to induce synovitis. Factor VIII knockout mice were injured 2 times (on days 0 and 7) or 3 times (on days 0, 7, and 14), then killed on day 14 or day 21, respectively. This protocol was chosen to simulate recurrent bleeding into a target joint with the development of HS. Figure 2 shows the pathologic changes induced following hemarthrosis. Figure 2B is a representative right knee joint from a mouse killed on day 14 following 2 episodes of hemarthrosis. There is loss of cartilage, destruction of the articular surface, and bony deformity with fibrosis on day 14 and the formation of a large pannus (Figure 2B), which is not observed at day 0 (Figure 2A) or in the sham-injured left control joint on day 14 (Figure 2C). Proliferation of synovial cells within the membrane is also apparent on day 21 (Figure 2D). The black arrowhead indicates an area of the membrane with 6 to 8 cell layers compared with one or 2 cell layers in the membrane of the left knee joint at day 14 (Figure 2C, white arrowheads). The cartilage and synovial reflections appear normal. Similar observations have been made in more than 20 mice, demonstrating the consistency of the results.
Blood-induced synovitis as a murine model of human HS. Factor VIII-deficient mice were subjected to a controlled blunt trauma to the right knee joint to induce 2 (days 0 and 7) or 3 (days 0, 7, and 14) episodes of hemarthrosis one week apart. Sham injury was performed on the left knee at each time point. (A) Normal appearance of a right knee joint from a mouse killed on day 0, not having had hemarthosis. (B) Abnormal appearance of a right knee joint from a mouse killed on day 14 following 2 episodes of hemarthrosis. (C) Normal appearance of the left knee of a mouse killed on day 14 after sham injury. (D) A mouse killed on day 21 after 3 episodes of hemarthrosis. Hematoxylin and eosin-stained sections of murine knee joints. More than 20 mice have been evaluated in 4 different experiments with similar results. Images were acquired using a CK2 microscope (Olympus, Melville, NY), objective lenses 4×, 0.10 (A-B) or 10×, 0.25 (C-D), and a DP12 microscope digital camera system with DP-Soft acquisition software (Olympus).
Blood-induced synovitis as a murine model of human HS. Factor VIII-deficient mice were subjected to a controlled blunt trauma to the right knee joint to induce 2 (days 0 and 7) or 3 (days 0, 7, and 14) episodes of hemarthrosis one week apart. Sham injury was performed on the left knee at each time point. (A) Normal appearance of a right knee joint from a mouse killed on day 0, not having had hemarthosis. (B) Abnormal appearance of a right knee joint from a mouse killed on day 14 following 2 episodes of hemarthrosis. (C) Normal appearance of the left knee of a mouse killed on day 14 after sham injury. (D) A mouse killed on day 21 after 3 episodes of hemarthrosis. Hematoxylin and eosin-stained sections of murine knee joints. More than 20 mice have been evaluated in 4 different experiments with similar results. Images were acquired using a CK2 microscope (Olympus, Melville, NY), objective lenses 4×, 0.10 (A-B) or 10×, 0.25 (C-D), and a DP12 microscope digital camera system with DP-Soft acquisition software (Olympus).
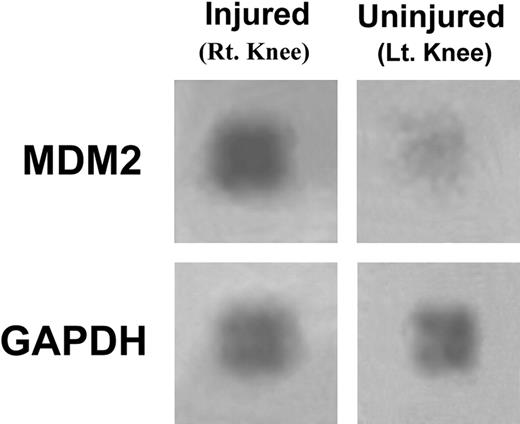
Synovial tissue was dissected from the knees of 10 mice and pooled, and then total RNA was isolated and probed using gene microarray analysis. A marked (8-fold) increase in expression of mdm2 in synovial tissue from the right injured knee is evident compared with that isolated from the left uninjured (control) joint (Figure 3). The signal generated by GAPDH, a regulatory gene, is shown for reference.
Hemarthrosis inducesmdm2expression in mouse synovium. Synovial tissue was isolated from the right and left knee joints of 10 hemophilic mice and the RNA isolated then pooled and probed using a gene expression microarray assay. The expression of mdm2 and GAPDH are shown in tissue from the injured (right) and uninjured (left) control joints. This experiment was repeated with similar changes in mdm2 gene expression.
Hemarthrosis inducesmdm2expression in mouse synovium. Synovial tissue was isolated from the right and left knee joints of 10 hemophilic mice and the RNA isolated then pooled and probed using a gene expression microarray assay. The expression of mdm2 and GAPDH are shown in tissue from the injured (right) and uninjured (left) control joints. This experiment was repeated with similar changes in mdm2 gene expression.
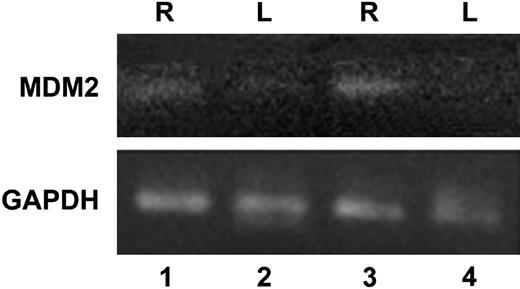
To confirm the results obtained using gene microarray analysis, we performed RT-PCR on samples of mouse synovium following injury to induce hemarthrosis. In Figure 4, RNA was used in RT-PCR with primers for mdm2 and GAPDH. RNA from tissue from the right (R, lanes 1 and 3) and left (L, lanes 2 and 4) knee joints after one (lanes 1 and 2) or 2 (lanes 3 and 4) episodes of hemarthrosis are shown. The expression of mdm2 in the synovium from the injured right knee (lanes 1 and 3) shows an increase compared with tissue from the left uninjured knee (lanes 2 and 4). As a reference, the expression of GAPDH is shown and was not changed following hemarthrosis. Similar changes were observed 3 days after a single episode of hemarthosis (day 3) and on the third day following a second episode of hemarthosis (day 10, data not shown). This result confirms the observation that the expression of mdm2 is increased following joint bleeding.
Expression ofmdm2by synovial cells using RT-PCR. Synovial tissue was isolated as described for Figure 3. RNA was used in RT-PCR with primers for mdm2 and GAPDH. RNA from tissue from the right (R, lanes 1 and 3) and left (L, lanes 2 and 4) knee joints after one (lanes 1 and 2) or 2 (lanes 3 and 4) episodes of hemarthrosis are shown. Similar results were obtained when tissue from day 3 or day 10 was analyzed. This experiment was repeated twice with similar results.
Expression ofmdm2by synovial cells using RT-PCR. Synovial tissue was isolated as described for Figure 3. RNA was used in RT-PCR with primers for mdm2 and GAPDH. RNA from tissue from the right (R, lanes 1 and 3) and left (L, lanes 2 and 4) knee joints after one (lanes 1 and 2) or 2 (lanes 3 and 4) episodes of hemarthrosis are shown. Similar results were obtained when tissue from day 3 or day 10 was analyzed. This experiment was repeated twice with similar results.
Hemarthrosis increases synovial cell proliferation
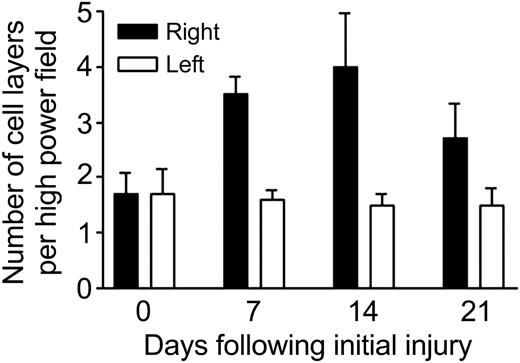
Since mdm2 is a powerful regulator of cell proliferation, we examined the numbers of synovial cell layers in mouse knee joints after one, 2, or 3 episodes of experimental hemarthrosis. On day 7 following a single hemarthrosis (induced on day 0), there were 3.5 ± 1.08 versus 1.6 ± 0.55 cell layers in the right versus left joints, respectively (Figure 5). This increase in synovial cell number persisted after 14 days and 2 episodes of hemarthrosis (on days 0 and 7): 4.0 ± 3.2 versus 1.5 ± 0.7. At day 21 following 3 episodes of hemarthrosis (days 0, 7, and 14) there were 2.7 ± 2.0 versus 1.5 ± 1.0 cell layers. These differences were statistically significant at all 3 time points compared with day 0 (P < .001).
Hemarthrosis increases synovial cell proliferation. The right knees of hemophilic mice were subjected to a controlled blunt trauma to induce hemarthrosis. The animals were killed on day 7 after the initial injury, on day 14 after 2 injuries on days 0 and 7, or on day 21 after 3 injuries on days 0, 7, and 14 to simulate recurrent target joint bleeding. Equatorial histologic sections through the knee joints were prepared and examined by light microscopy. Five areas of the synovial membrane were chosen randomly and the number of synovial cell layers counted by 4 independent observers blinded to the experimental conditions. The results were pooled and the average and standard deviation (shown by error bars) calculated. Student t test was used to compare the differences between groups.
Hemarthrosis increases synovial cell proliferation. The right knees of hemophilic mice were subjected to a controlled blunt trauma to induce hemarthrosis. The animals were killed on day 7 after the initial injury, on day 14 after 2 injuries on days 0 and 7, or on day 21 after 3 injuries on days 0, 7, and 14 to simulate recurrent target joint bleeding. Equatorial histologic sections through the knee joints were prepared and examined by light microscopy. Five areas of the synovial membrane were chosen randomly and the number of synovial cell layers counted by 4 independent observers blinded to the experimental conditions. The results were pooled and the average and standard deviation (shown by error bars) calculated. Student t test was used to compare the differences between groups.
Iron increases the expression of mdm2 by murine synovial cells
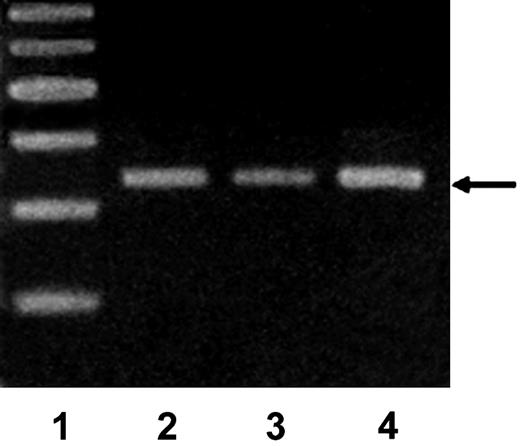
Since iron increased the proliferation of human synovial cells in culture13 and we observed a similar increase in vivo following hemarthrosis, we suspected that iron might be responsible for the molecular changes observed in vivo. Normal murine synovial cells were cultured in the presence of iron citrate (10 μM) for 7 days, after which RNA was isolated, subjected to RT-PCR, and then analyzed by gel electrophoresis with ethidium bromide and photographed under UV transillumination (Figure 6). In lane 3, the expression of mdm2 is increased 2.5-fold in murine synovial cells cultured in the presence of 10 μM ferric citrate for 7 days compared with control synovial cells maintained in standard culture medium without added salt (lane 2). Cells exposed to sodium citrate (lane 3) demonstrated reduced expression (0.4-fold) of mdm2 compared with control cells in medium alone. This result was reconfirmed in 5 additional independent experiments and suggests that iron is responsible for the proliferative and molecular changes observed in HS.
Iron increases the expression of mdm2 by murine synovial cells. Normal murine synovial cells were cultured in the presence of iron citrate (10 μM) for 7 days after which RNA was isolated, subjected to RT-PCR, and then analyzed by gel electrophoresis with ethidium bromide and photographed under UV transillumination. Lane 1, markers; lane 2, control synovial cells maintained in standard culture medium without added salt; lane 3, synovial cells exposed to sodium citrate; lane 4, cells cultured in the presence of 10 μM ferric citrate for 7 days. Arrow indicates mdm2. Data shown are representative of 6 total experiments performed.
Iron increases the expression of mdm2 by murine synovial cells. Normal murine synovial cells were cultured in the presence of iron citrate (10 μM) for 7 days after which RNA was isolated, subjected to RT-PCR, and then analyzed by gel electrophoresis with ethidium bromide and photographed under UV transillumination. Lane 1, markers; lane 2, control synovial cells maintained in standard culture medium without added salt; lane 3, synovial cells exposed to sodium citrate; lane 4, cells cultured in the presence of 10 μM ferric citrate for 7 days. Arrow indicates mdm2. Data shown are representative of 6 total experiments performed.
Discussion
The component(s) in blood responsible for the pathologic and molecular changes that result in HS are not known.22,23 Iron has long been suspected to be the culprit but direct evidence has been lacking. Previously, we showed that iron increased human synovial cell proliferation and induced c-myc expression.13 Here we show that intra-articular blood in a joint in vivo and iron in vitro result in increased expression of the p53-binding protein, mdm2.24 This molecular change may be the basis of the increase in cell proliferation and the development of HS.
Synovitis is the most common complication of hemophilia and can lead to joint destruction and eventually to a crippling arthritis at a relatively young age.2 Although it is clear that blood in the joint leads to this devastating complication of hemophilia, the pathobiology and molecular mechanisms associated with the disorder are poorly understood.8,25 Bleeding into the joint space exposes synovial fibroblasts to blood and all of its contents including iron. Morris proposed a central role for iron in HS.15 Roosendaal et al14 studied the relationship of iron to the catabolic properties in synovial tissue from patients with hemophilia. Iron deposits were present in the cytoplasm of the lining synovial cells as discrete granules but in subsynovial layers there were dense aggregates located intracellularly and extracellularly. Hemosiderotic tissues had diffuse lymphocyte infiltration and neovascularization in the deep subsynovial layers. When these iron-loaded tissues were cultured in vitro, they synthesized more proinflammatory cytokines, such as interleukin 6 (IL-6), IL-1, and tumor necrosis factor alpha (TNFα), compared with normal-appearing synovial tissue.
In canine26 and rabbit models of human hemophilia,27 one of the earliest observations following a joint hemorrhage is swelling of the joint capsule due to edema and infiltration of the tissues by neutrophils, lymphocytes, and monocytes. Villous hypertrophy and increased vascularity of the synovial tissue occur soon after the hemorrhage. Villous hypertrophy may be the result of promotion of cell growth and/or abrogation of cell death. The introduction of blood and its components including immune cells capable of producing cytokines, growth factors, and their natural antagonists into the joint space disrupts the natural homeostatic balance limiting synovial hypertrophy and vascularity. It has been postulated that blood and one or more of its components may disrupt this balance favoring villous hypertrophy and increased vascularity. The role of iron in synovial cell proliferation has been explored in vitro.13 In this recently published paper it was shown that exposure of HSFCs to 0.1 mM or one mM ferric citrate for 9 days resulted in a 2-fold increase in cell proliferation compared with control HSFCs: 0.17 ± 0.03 or 0.19 ± 0.01 versus 0.09 ± 0.01, respectively. The increase in cell number was evident after 6 days in the presence of iron citrate. Furthermore, cells cultured with one mM ferric citrate demonstrated prominent c-myc expression compared with cells exposed to one mM sodium citrate or control cells grown in standard medium without salt supplementation. These observations led us to speculate that iron may alter the expression of other oncogenes.
The p53 binding protein, mdm2, is an oncoprotein that was initially identified as an amplified gene on a murine double minute chromosome in the spontaneously transformed BALB/c 3T3 cells.28 The p53 tumor suppressor helps the cell to maintain genomic integrity and coordinates the cell's response to DNA damage by inducing cell cycle arrest or apoptosis. Inactivation of p53 is one of the most common events leading to neoplastic transformation. In approximately half of all cancers, p53 is inactivated by mutations or other genomic changes.29 In the majority of the remaining cases of cancer, p53 is functionally inactivated following interaction with mdm2 oncoprotein, which acts as a bridge over p53 and pRb, antagonizing the regulatory effects of p53.30
The cyclin-dependent kinase inhibitor p21(WAF1/CIP1) plays a central role as a regulator of the G1/S cell cycle checkpoint. Iron is required to support p21 expression.17 Cellular iron depletion results in a G1/S arrest. Almasan and Ashkenazi31 showed that iron chelators markedly up-regulated the mRNA levels of p21 but they paradoxically inhibited its translation. Myc-activation and exposure of ovarian carcinoma cells to proapoptotic ligands including Apo2 ligand or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) can induce H-ferritin expression, which binds to and reduces intracellular iron. Intracellular iron depletion triggered apoptosis.32 These data suggest a link between iron hemostasis and p21 and TRAIL.
Cytokines, including interferons, induce transcriptional activation of TRAIL. Members of the TNF receptor family signal apoptosis independently of the p53 tumor-suppressor gene.33 TRAIL induces apoptosis in many transformed cells but not in normal cells.31 TRAIL-induced cell death is characterized by activation of caspase-8 and -3, poly(ADP-ribose) polymerase cleavage, and DNA fragmentation.34 We examined a large panel of human malignant glioma cell lines and primary cultures of normal human astrocytes for their sensitivity to TRAIL. Resistant cell lines expressed higher levels of apoptosis-inhibitor phosphoproteins and had higher cell-surface expression of TRAIL decoy receptors, which compete for binding to the ligand and reduce activation of death signals mediated through TRAIL death receptors. Unlike the p53-target CDK-inhibitor p21WAF1/CIP1, the TRAIL death receptors are only induced in cells undergoing p53-dependent apoptosis and not cell cycle arrest.35 Since synovial fibroblasts are not transformed cells and express high levels of mdm2, p53-dependent apoptosis may be limited in these cells. Furthermore, increased expression of p21 may protect cells from apoptosis by mediating cell cycle arrest. The interplay between p53, mdm2, p21, and TRAIL was recently summarized in an elegant review by El-Deiry.36
In summary, we provide evidence that iron may be linked to multiple molecular changes (c-myc and mdm2) that result in the pathologic proliferative changes observed in HS. We speculate that iron is responsible for the aberrant gene expression. An increase in mdm2 expression decreases p53 activity, resulting in abrogation of synovial cell apoptosis and/or an increase in proliferation. The increase in c-myc further drives synovial cell proliferation. The identification of mdm2 as a key mediator involved in HS suggests that this molecule (or its pathway) may be a target for future therapeutic interventions to counter the devastating complications of joint bleeding in hemophilia.
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2003-12-4231.
Supported by a grant from Baxter Bioscience.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal