Abstract
Nonimmunogenic antigens can be efficiently rendered immunogenic by targeting them to antigen-presenting cells via differentially expressed chemokine receptors. For example, self-tumor or HIV antigens genetically fused with proinflammatory chemoattractants elicit potent immune responses and protective antitumor immunity in mice. Herein we demonstrate that the mechanism by which chemokine fusions elicit responses is efficient uptake, processing, and presentation of antigens via the major histocompatibility complex class II pathway. Experiments with inhibitors of intracellular trafficking suggest that chemoattractant fusion proteins, but not antigen alone, were processed and presented through early/late endosomal and Golgi compartments and stimulated antigen-specific CD4+ T cells both in vitro and in vivo. Chemokine fusion also facilitated the presentation of antigen by dendritic cells to an autologous human tumor-specific CD4+ T-cell line. Taking advantage of chemokine redundancy, viral chemokine fusions were equally potent in inducing protective immunity in vivo, providing a possible strategy to circumvent hypothetical, vaccine-induced antihost chemokine autoimmunity, for example, by use of viral chemoattractants in humans.
Introduction
Cell trafficking is regulated by differential expression of heterotrimeric Gi protein coupled 7-transmembrane–domain chemokine receptors (GPCRs).1 Sentinel antigen-presenting cells (APCs), the immature dendritic cells (DCs), preferentially express CCR1, CCR2, CCR5, and CCR6.2-4 Upon ligand binding the receptor is phosphorylated and endocytosed through clathrin-coated vesicles using β-arrestin adaptors,5-7 although some viral chemokine receptors, such as US28, are endocytosed independently of β-arrestins.8 The internalized receptors may then be dephosphorylated and recycled back to the cell surface or targeted for degradation.5,9 CCR5 is transported to early endosomes and subsequently recycled to the cell surface, bypassing the Golgi apparatus and late endosomes, and this process does not involve protein synthesis.5 Upon chemokine receptor binding, the chemokine ligand is also internalized although its fate is not known and presumed to be degraded. Moreover, the fate of the internalized receptor and the bound ligand may be regulated by the strength of the ligand-induced signaling or the nature of the ligand itself. For example, CCR5 is endocytosed through clathrin-coated vesicles on binding to RANTES or AOP-RANTES (aminooxypentane regulated-on-activation normal T-expressed and secreted), although the latter drives CCR5 to a degradation pathway, whereas RANTES-bound CCR5 is recycled to the cell surface.5,10 The internalized receptors are degraded by proteosomes, which are considered as major regulators of cytokine receptor expression.11-13
Active immunotherapy based on the targeting of idiotypic antigen (Id), expressed by malignant B cells, is one of the most promising human cancer vaccine approaches.14 Recently, we have demonstrated that effective adaptive immunity against weakly immunogenic tumor antigens could be induced by targeted delivery of such antigens to chemokine receptors on professional APCs by linkage to their chemoattractant ligands (β-defensins or chemokines). Mice immunized with chemoattractants fused with nonimmunogenic lymphoma Id or sFv elicited potent anti-idiotypic responses and were protected from challenge with a lethal dose of syngeneic lymphoma cells.15,16 Moreover, we demonstrated that protective and therapeutic antitumor immunity depended on the ability of the vaccine to target immature, but not mature DCs, in vivo. The vaccine did not require use of any adjuvants; immune responses were elicited from injections of recombinant proteins alone or DNA constructs encoding fusion proteins. However, it was essential that tumor antigen was fused physically with a functionally active chemokine because immunizations with unlinked free chemokine plus antigen did not induce immune responses. Moreover, fusion constructs lacking the ability to bind chemokine receptors were unable to elicit any immune responses in vivo.15 These data suggested that antigens are efficiently taken up, processed, and presented by APCs when they are delivered to chemokine receptors via chemoattractant carriers.
Herein, we report that chemokine receptors can indeed facilitate uptake and processing of tumor antigens to elicit major histocompatibility complex (MHC) class II–restricted antigen presentation. We demonstrate that APCs incubated with functionally active, but not mutant, chemoattractants fused with model lymphoma antigens, single-chain antibody, and VL chain of MOPC315 tumor, or human lymphoma-derived Id induce efficient antigen-specific cellular responses both in vitro and in vivo. Our data suggest that chemokine receptors targeted with chemoattractant fusion proteins were internalized to early endocytic compartments and used the MHC class II antigen-processing pathway. Furthermore, the approach not only is potent and does not require any adjuvants, but also xenogeneic chemokines, such as the viral broad-range chemokine antagonist viral macrophage inflammatory protein II (vMIP-2), which binds to multiple chemokine receptors, can be used to reduce the possibility of vaccine-induced antihost chemokine autoimmunity.
Materials and methods
Fusion gene cloning and plasmid construction
Cloning of sFv from 38C13 has been reported previously.15 The same strategy was applied to clone VH and VL fragments from MOPC315 plasmacytoma (American Type Culture Collection [ATCC], Manassas, VA) and arrange them as sFv315 using the following primers: for VH chain, PRMOPC315VH-1, AAACATATGCTCGAGGACGTGCAGCTGCAGGAGTCT, and PRMOPC315VH-R1, TGTCGACGCCGCCGCCAGAACCACCACCACCTGAGGAGACTGTGAGAGT; for VL chain, PRMOPC315VL-2, AAACTCGAGGGTGGCGGTGGGAGCCAGGCTGTTGTGACTCAGGAA, and PRMOPC315VL-R2, ATAAGATCTTCCCGGGCCTAGGACAGTGACCTTGGT. Genes for mature murine β-defensin 2 and chemokine MIP-3α, or human MIP-3α, or viral chemokines vMIP-2 and MC148 were cloned in frame with sFv or VL fragments using reverse transcription–polymerase chain reaction (RT-PCR) as described previously15-17 ; a cDNA for vMIP-218,19 was cloned by RT-PCR from total RNA extracted from cells infected with human herpes virus 8 (HHV-8; BCBL-1), and MC14820 was recloned from the plasmid kindly provided by Bernard Moss (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). The following pairs of primers were used for vMIP-2: PRvMIP2-L1, TAAGCTTCACCATGGACACCAAGGGCATCCTGCTCGT, and PRvMIP2-R1, TGAATTCGCGAGCAGTGACTGGTAATTGCTGCAT; for MC148: PRMC148-L1, AAAGCTAGCACCATGAGGGGCGGAGACGTCTTC, and PRMC148-R1, AAAGAATTCCAGAGACTCGCACCCGGACCATAT. For bacterial expression, chemokines were cloned without signal sequences using the following pairs of primers: PRvMIP2M-2, ACCATGGGAGCGTCCTGGCATAGA, and PRvMIP2-R1; and PRMC148M-2,AACATATGCTCGCGAGACGGAAATGTTGTTTGAAT, and PRMC148-R1, respectively. Point-mutated vMIP-2 and MC148 genes were generated by PCR replacing the first cysteine with a serine to abrogate receptor binding.16,21 All constructs were verified by the DNA sequencing method (Amersham, Arlington Heights, IL) and purified using a plasmid purification kit (Qiagen, Valencia, CA).
Recombinant fusion proteins production
Fusion proteins were purified as inclusion bodies after overnight induction in SuperBroth (Digene Diagnostics, Beltsville, MD) with 0.8 mM isopropyl β-d-thiogalactoside as described previously15 and refolded according to Buchner et al.22 The integrity and purity (> 90%) of recombinant proteins were tested by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and Western blot hybridization with 9E10 anti–c-myc monoclonal antibody (mAb; Sigma, St Louis, MO). For endotoxin removal, endotoxin removal gel (Acticlean Etox, Sterogene Bioseparations, Carlsbad, CA) was used according to manufacturer's instructions for 3 consecutive rounds. The endotoxin content of fusion proteins was assessed by Limulus Amebocyte Lysate assay using a commercially available kit (Biowhittaker, Walkersville, MD) according to the manufacturer's recommendations.
Cell lines
The carcinogen-induced, C3H 38C-13 B-cell lymphoma23 was a gift from Dr Ronald Levy (Stanford, CA). MOPC315 plasmacytoma cells were purchased from ATCC. The 7A10B2 T-cell line, which recognizes the murine plasmacytoma MOPC315 immunoglobulin (amino acids 91-101), presented by the MHC class II molecule I-Ed,24 was a gift from Dr B. Bogen (Oslo, Norway). The murine epidermis-derived DC line XS52,25 displaying an immature phenotype, was kindly provided by Dr A. Takashima (Dallas, TX).
Isolation of murine bone marrow-derived DCs
Cells were isolated by the method described by Fields et al.26 Briefly, bone marrow was collected from tibias and femurs of 4- to 6-month-old BALB/c mice. Cells were cultured in DC medium (RPMI 1640 containing 5% heat-inactivated fetal bovine serum, 1% penicillin, streptomycin, 1% l-glutamine, and 5 × 10–5 2-mercaptoethanol [2-ME]) containing 10 ng/mL each of murine interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ). Immature DCs (iDCs) at day 4 to 5 of cultivation were in general CD11+ (69%), B7.2+ and I-Ab+ (21%), B7.2– and I-Ab+ (18%), CD40+ (27%). On maturation, the DCs were CD11c+ (87%), B7.2+ and I-Ab+ (62%), B7.2– and I-Ab+ (3%), CD40+ (87%).
Generation of human DCs
Monocyte-derived iDCs were generated from cryopreserved peripheral blood mononuclear cells (PBMCs), as previously described,27 with some modifications. Briefly, PBMCs were enriched for monocytes by depletion of T cells with CD3 microbeads over a magnetic column (Miltenyi Biotec, Auburn, CA) using the manufacturer's protocol. The T cell–depleted PBMCs were plated in serum-free AIM-V medium (Invitrogen, Carlsbad, CA) at 1 × 106 /mL. After 2 hours of incubation at 37° C in 5% CO2 in air, nonadherent cells were discarded and cells were cultured for 7 days in AIM-V medium with IL-4 (500 U/mL) and GM-CSF (800 U/mL; PeproTech). Phenotypic characterization of the iDCs revealed moderate expression of CD11c, HLA DR, and CD86 and low expression or absence of CD14, CD80, and CD83.27
In vitro chemotaxis assay
The migration of DCs was assessed using a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD) with a 5-μm polycarbonate filter (Osmonics, Livermore, CA) as described.28,29 Cells were incubated at 37° C with 5% CO2 for 1.5 hours. DCs migrating across the filter were counted using a Bioquant semiautomatic counting system (Bioquant Image Analysis, Nashville, TN). The results (as the mean ± SE of triplicate samples) are presented as chemotactic index (CI) defined as the fold increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration (in the absence of test factors). MIP-3α and MIP-3β were from PeproTech.
Chemokine receptor-binding assays
Chemokine binding was performed with HEK293 cells transfected with CCR5 or CXCR4. The cells (1 × 106) in 100 μL RPMI 1640 (1% bovine serum albumin [BSA]) were incubated with 1 ng/mL radioiodinated chemokine (New England Biosciences, Boston, MA) in the presence of increasing concentrations of unlabeled fusion proteins or human MIP-1β (PeproTech) for 20 minutes at room temperature. The cells were filtered through a 10% sucrose/phosphate-buffered saline (PBS) cushion and then were measured for γ emission. The rate of inhibition of binding was calculated by the formula: % inhibition = 1 – (cpm in the presence of unlabeled ligands/cpm in the presence of radiolabeled ligand alone) × 100%.
MOPC315 Id-specific T-cell line stimulation
The BALB/c mouse CD4+ T-cell clone 7A10B2 specifically recognizes an idiotypic peptide from the light chain of the murine plasmacytoma MOPC315 immunoglobulin (amino acids 91-101), presented by the MHC class II molecule I-Ed,24 BALB/c bone marrow dendritic cells (BMDCs) were incubated with endotoxin-free fusion proteins overnight, washed extensively with cold PBS, irradiated (2000 rad), and placed in culture with 7A10B2 T cells in 96-well round-bottom plates at a 1:1 ratio (2 × 104 cells each) for 48 hours. Supernatants were assessed for interferon γ (IFN-γ) by enzyme-linked immunosorbent assay (ELISA). Control DCs were matured by overnight treatment with lipopolysaccharide (LPS; 10 ng/mL) before peptide pulsing with 0.2 μg/mL specific 91-101 peptide, or 10 μg/mL of an irrelevant peptide. The second control, iDCs, were also pulsed with 10 μg/mL of the 91-101 peptide at the same time DCs were treated with fusion proteins.
Human idiotype-specific T-cell line
An idiotype-specific T-cell line was generated by repeated stimulation and rest cycles as described elsewhere30 from a patient with follicular lymphoma who had received Id-KLH vaccine.14 Briefly, after vaccination, PBMCs from patient LE were first stimulated in vitro with autologous Id protein (100 μg/mL). During subsequent restimulations, irradiated (3300 rad) autologous prevaccine PBMCs were used as APCs. The Id-specific T-cell line, LE-1, consisted of more than 99% CD3+CD4+ T cells and they recognized autologous Id in an HLA class II–associated manner.31 The T cells were generally used between 10 and 15 days following previous antigen stimulation.
Cytokine induction assay for human idiotype-specific T-cell line
iDCs were irradiated to 2000 rads and plated in triplicate at 1 × 104 cells/100 μL/well in a 96-well U-bottom plate. DCs were cultured for 4 hours in the presence or absence of autologous idiotype protein (100 μg/mL), irrelevant idiotype protein (100 μg/mL), hMIP3sFvLF (10/100/1000 ng/mL), MIP3sFv38 (10/100/1000 ng/mL), sFvLF (100/1000 ng/mL), or LPS (10 ng/mL). The antigen was removed after 4 hours of incubation by washing the DCs twice with complete medium, and Id-specific LE-1 T cells (1 × 105/well) were added to the DCs in 200 μL complete medium. Supernatants were harvested and pooled from replicate wells after 72 hours of incubation. Cytokine production (IFN-γ and GM-CSF) was measured by ELISA using Quantikine kits (R&D Systems, Minneapolis, MN).
Intracellular trafficking and processing of fusion proteins
BALB/c mouse splenocytes were incubated overnight with 100 ng/mL chemokine fusion proteins (MIP-3αVL315) or Id (sFv315) alone (Figure 3) or with various inhibitors of intracellular trafficking and processing, such as brefeldin A (BrefA; 500 μM), and monensin (Mon; 0.67 μg/mL), leupeptin (Leu; 5 μg/mL), chloroquine (Chlor; 50, 10, and 1 μM), and lactocystin (Lact; 50, 10, and 1 μM). T-cell stimulation (IFN-γ production) was assessed as described (see “MPOC315 Id-specific T-cell line stimulation”).
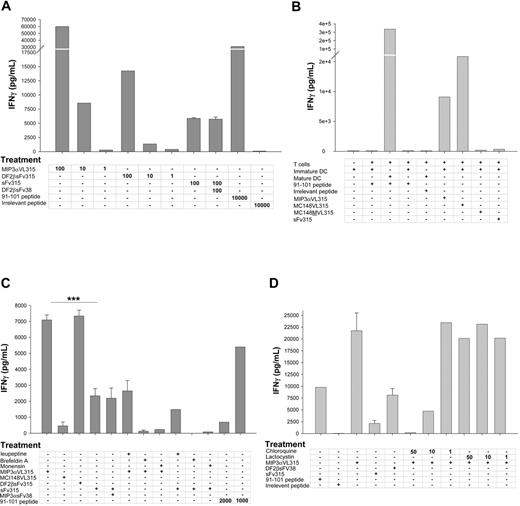
Chemokine or defensin fusion proteins are taken up, processed, and presented by APCs in vitro via chemokine receptor. Titrated amounts of protein (shown in ng/mL), 91-101 peptide, or an irrelevant peptide derived from A20 lymphoma VL chain were incubated with BALB/c mice splenocytes (A,C-D). APCs were then washed, irradiated, and placed in culture with epitope-specific 7A10B2 T-cell line for 48 hours, and IFN-γ was assayed in culture supernatants. (B) The same assay as in panel A, except BM-derived iDCs were treated with recombinant proteins (100 ng/mL). Control treatment groups were iDCs or matured by overnight treatment with LPS (10 ng/mL). DCs were pulsed with 0.2 μg/mL 91-101 peptide or with 10 μg/mL irrelevant peptide. (C-D) Assessment of antigen presentation pathway of chemokine fusion proteins. Splenocytes were treated with 2 and 10 μg/mL 91-101 peptides or 100 ng/mL recombinant proteins alone or together with various inhibitors of intracellular trafficking and processing, such as brefeldin A (500 μM), and monensin (0.67 μg/mL), leupeptin (5 μg/mL) and chloroquine (50, 10, and 1 μM; Figure 3D), and lactocystin (50, 10, and 1 μM, Figure 3D). Representative of 5 (A), 6 (B), and 4 (C-D) consecutive experiments performed in duplicate wells. Results are presented as pg/mL IFN-γ ± SEM. ***P < .002 as compared MIP3αVL315 versus MIP3α+ sFv315 or mDF2βsFv315 versus sFv315, respectively (C).
Chemokine or defensin fusion proteins are taken up, processed, and presented by APCs in vitro via chemokine receptor. Titrated amounts of protein (shown in ng/mL), 91-101 peptide, or an irrelevant peptide derived from A20 lymphoma VL chain were incubated with BALB/c mice splenocytes (A,C-D). APCs were then washed, irradiated, and placed in culture with epitope-specific 7A10B2 T-cell line for 48 hours, and IFN-γ was assayed in culture supernatants. (B) The same assay as in panel A, except BM-derived iDCs were treated with recombinant proteins (100 ng/mL). Control treatment groups were iDCs or matured by overnight treatment with LPS (10 ng/mL). DCs were pulsed with 0.2 μg/mL 91-101 peptide or with 10 μg/mL irrelevant peptide. (C-D) Assessment of antigen presentation pathway of chemokine fusion proteins. Splenocytes were treated with 2 and 10 μg/mL 91-101 peptides or 100 ng/mL recombinant proteins alone or together with various inhibitors of intracellular trafficking and processing, such as brefeldin A (500 μM), and monensin (0.67 μg/mL), leupeptin (5 μg/mL) and chloroquine (50, 10, and 1 μM; Figure 3D), and lactocystin (50, 10, and 1 μM, Figure 3D). Representative of 5 (A), 6 (B), and 4 (C-D) consecutive experiments performed in duplicate wells. Results are presented as pg/mL IFN-γ ± SEM. ***P < .002 as compared MIP3αVL315 versus MIP3α+ sFv315 or mDF2βsFv315 versus sFv315, respectively (C).
In vivo uptake of chemokine fusion proteins
BALB/c mice (3/group) were subcutaneously immunized with 25 μg endotoxin-free MIP-3αVL315, DF2βsFv315, or sFv315 each. After 10 and 48 hours, lymph node cells were harvested, irradiated (2000 rad), and mixed directly (without any additional protein stimulation/incubation) with 7A10B2 T cells in 96-well round-bottom plates at a 1:1 ratio (2 × 104 cells each) for 48 hours. At the end of culture period, IFN-γ production was assessed by ELISA in culture supernatants.
In vivo immunization and tumor protection
Animal care was provided in accordance with the procedures outlined in a Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 86-23, 1985). Six- to 9-week-old female C3H/HeNCrlBR mice (Charles River Laboratories, Frederick, MD) were immunized using the Helios Gene Gun System (Bio-Rad, Hercules, CA) with 1 to 2 μg plasmid DNA 3 times every 2 weeks as described.15 Two weeks after the last immunization, mice were challenged intraperitoneally with 2 × 103 38C13 cells and followed for survival. Differences in survival between groups were determined by nonparametric log-rank test (BMDP statistical software, Los Angeles, CA). P values refer to comparison with the group immunized with DNA expressing the same chemokine or defensin fused with an irrelevant sFv, or sFv fused with mutant chemokine, unless otherwise specified.
Results
Chemoattractant fusion proteins retain their functional activity
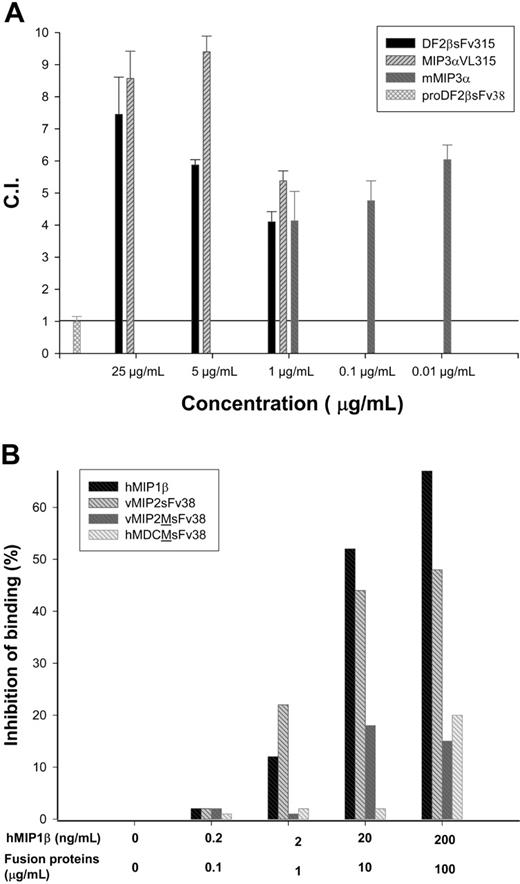
First, we produced a number of chemoattractant fusion proteins with idiotypic (Id) fragments isolated from MOPC315 plasmacytoma cells (Figure 1) by purifying them from bacterial inclusion bodies using cobalt affinity columns under denaturing conditions, followed by a refolding process and heparin-Sepharose affinity chromatography.16 Purity was on average above 95%, and endotoxin content was reduced (≤ 0.1 EU/μg protein) using Acticlean etox resins (Sterogene Bioseparations). Chemoattractant fusion proteins retained functional activity after being fused with various immunoglobulin fragments, namely, single-chain antibody fragments (sFv) or VL from MOPC315 Igλ chain, such as MIP-3αVL315 (murine MIP-3α fused with VL315) or DF2βsFv315 (murine β-defensin 2 fused with sFv315) chemoattracted CCR6 transfected HEK293 cells in a dose-dependent manner (Figure 2A). No chemotaxis was detected in fusions that contained a point mutation of the first cysteine residue in the chemokine moiety (not shown), or when Id (sFv) was fused with the natural, inactive form of β-defensin, pro-β-defensin 2 (proDF2βsFv38; Figure 2A). Fusion proteins with wild-type chemokine moieties were also able to bind the respective chemokine receptors. For example, viral chemokine antagonist peptide vMIP-2 fusion protein (vMIP2sFv38; Figure 2B) efficiently competed with human MIP-1β (hMIP-1β; Figure 2B) for CCR5. Control proteins with mutated (m) vMIP-2 or macrophage-derived chemokine (MDC) moieties were not able to bind CCR5 (vMIP2M-sFv38 and hMDCM-sFv38, respectively; Figure 2B). Thus, these data and our previous report15 suggest that the fusion proteins retain chemoattractant functions and are able to bind the respective chemokine receptors.
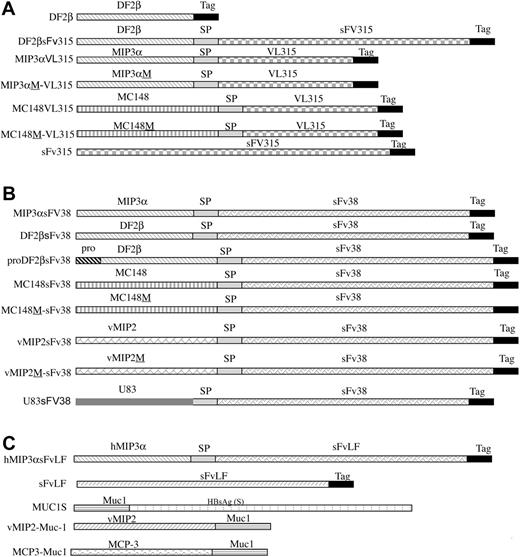
Schema of constructs used in the study. Genes for mature sequences of murine MIP-3α and DF2β, or viral chemokines vMIP2, MC148, and US83 were fused in-frame with DNA encoding either sFv38 from 38C13 or VL from MOPC315 mouse B-cell tumors. B-cell lymphoma patient LF's sFv (sFvLF) was fused with human MIP-3α (hMIP3αsFvLF). Control constructs encoded chemokines with point mutation, which abrogated chemokine receptor binding (MIP3αM-VL315, MC148M-VL315, MC148M-sFv38, hMDCM-sFv3816 and vMIP2M-sFv38, respectively), or chemokine genes fused with an irrelevant antigen, human breast cancer Muc1 (vMIP2-Muc1 and MCP3-Muc115,16), or inactive pro–β-defensin 2 (proDF2βsFv3816). To enable purification and detection, c-myc and His peptide tags were fused to COO end of constructs (Tag). For DNA vaccines (not shown), fusion constructs were in-frame fused with signal sequence (SL) from murine IP-10 gene or contained native SL (vMIP2 and MC148 constructs only) to enable protein secretion, and constructs were cloned in pcDNA3.1 (Invitrogen).15,16 Spacer fragment (SP) was inserted between chemoattractant and antigen moieties to enable proper folding of the proteins. Recombinant proteins are depicted for (A) MOPC315 plasmacytoma construct; (B) 38C-lymphoma construct; and (C) human lymphoma or other constructs.
Schema of constructs used in the study. Genes for mature sequences of murine MIP-3α and DF2β, or viral chemokines vMIP2, MC148, and US83 were fused in-frame with DNA encoding either sFv38 from 38C13 or VL from MOPC315 mouse B-cell tumors. B-cell lymphoma patient LF's sFv (sFvLF) was fused with human MIP-3α (hMIP3αsFvLF). Control constructs encoded chemokines with point mutation, which abrogated chemokine receptor binding (MIP3αM-VL315, MC148M-VL315, MC148M-sFv38, hMDCM-sFv3816 and vMIP2M-sFv38, respectively), or chemokine genes fused with an irrelevant antigen, human breast cancer Muc1 (vMIP2-Muc1 and MCP3-Muc115,16), or inactive pro–β-defensin 2 (proDF2βsFv3816). To enable purification and detection, c-myc and His peptide tags were fused to COO end of constructs (Tag). For DNA vaccines (not shown), fusion constructs were in-frame fused with signal sequence (SL) from murine IP-10 gene or contained native SL (vMIP2 and MC148 constructs only) to enable protein secretion, and constructs were cloned in pcDNA3.1 (Invitrogen).15,16 Spacer fragment (SP) was inserted between chemoattractant and antigen moieties to enable proper folding of the proteins. Recombinant proteins are depicted for (A) MOPC315 plasmacytoma construct; (B) 38C-lymphoma construct; and (C) human lymphoma or other constructs.
Integrity of chemokine-fused proteins. (A) MIP3αVL315 and DF2βsFv315, but not proDF2βsFv38, fusion proteins induce chemotaxis of murine CCR6-transfected HEK293 cells. Protein concentration used is shown in μg/mL. Representative data from 6 independent experiments are presented as chemotactic index (CI ± SEM of triplicate samples), defined by the fold increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration. Murine MIP-3α (mMIP3α; PeproTech) was used as control. (B) vMIP2sFv38, but not vMIP2M-sFv38 or hMDCM-sFv38, binds to CCR5. Titrated amounts of proteins (0-100 μg/mL) were used to inhibit binding of 0 to 200 ng/mL human radiolabeled human MIP-1β (PeproTech) to hCCR5-transfected HEK293 cells. Unlabeled human MIP-1β (hMIP1β; PeproTech) was used as control. Data are from 2 independent experiments.
Integrity of chemokine-fused proteins. (A) MIP3αVL315 and DF2βsFv315, but not proDF2βsFv38, fusion proteins induce chemotaxis of murine CCR6-transfected HEK293 cells. Protein concentration used is shown in μg/mL. Representative data from 6 independent experiments are presented as chemotactic index (CI ± SEM of triplicate samples), defined by the fold increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration. Murine MIP-3α (mMIP3α; PeproTech) was used as control. (B) vMIP2sFv38, but not vMIP2M-sFv38 or hMDCM-sFv38, binds to CCR5. Titrated amounts of proteins (0-100 μg/mL) were used to inhibit binding of 0 to 200 ng/mL human radiolabeled human MIP-1β (PeproTech) to hCCR5-transfected HEK293 cells. Unlabeled human MIP-1β (hMIP1β; PeproTech) was used as control. Data are from 2 independent experiments.
Chemoattractant fusion proteins can be taken up, processed, and presented to antigen-specific T cells
Previously, we reported that immunizations with chemokines fused with Id, a weakly immunogenic lymphoma antigen, elicited effector CD8+ T cell–dependent antitumor immunity15 and hypothesized that the mechanism was chemokine receptor-mediated uptake. To elucidate this, we tested whether antigen uptake by APCs would be augmented by chemokine fusion, and, if so, whether the internalized antigens would be efficiently processed and presented to T cells. To this end, the CD4+ T-cell clone 7A10B2, which specifically recognizes an Id peptide from the λ chain of the murine plasmacytoma MOPC315 immunoglobulin (amino acids 91-101), presented by the MHC class II molecule I-Ed,24 was stimulated with irradiated immature BMDCs or splenocytes from BALB/c mice, pretreated with titrated amounts of fusion proteins overnight, and thoroughly washed. Compared with unfused sFv, significant IFN-γ production was detected after 48 hours of incubation only in groups treated with as little as 100 ng/mL MIP-3α or β-defensin 2 fused with MOPC315 Id fragments (MIP-3αVL315 and DF2βsFv315, respectively; Figure 3A). All 3 types of APCs tested, splenocytes (Figure 3A,C-D), iDCs (Figure 3B), and an epidermis-derived immature DC cell line XS52 (not shown), were able to efficiently stimulate 7A10B2 cells. Viral chemokine antagonists also induced internalization of chemokine receptors and may be used to target antigen. For example, DCs treated with a viral antagonist MC148 fusion protein stimulated 7A10B2 cells (MC148VL315; Figure 3B). The process optimally required chemokine receptor engagement because DCs or splenocytes incubated with sFv315 alone (Figure 3A-D) or unlinked mixtures of free sFv315 with MIP-3α (MIP-3αsFv38 + sFv315; Figure 3C), β-defensin 2 (Figure 3A), or chemokine antagonist MC148 (not shown) stimulated T cells only weakly. Moreover, fusion proteins containing mutated chemokines (MC148MVL315: Figure 3B), which were unable to bind its respective receptor and elicit chemotaxis,16 failed to stimulate T cells compared with antigen alone. The DCs used had an immature phenotype and expressed low levels of MHC class II. Such DCs directly pulsed with MOPC315 Igλ peptide (amino acids 91-101) at concentrations up to 10 μg/mL inefficiently stimulated 7A10B2 T cells (Figure 3B), unless they were preactivated and matured. For example, DCs matured by overnight treatment with LPS before pulsing stimulated 7A10B2 T cells with as little as 0.2 μg/mL of the peptide (Figure 3B). Taken together, these data suggest that uptake of Id antigen is facilitated by binding chemokine receptors expressed on BMDCs and splenic APCs and that such receptor-targeted antigens are processed and presented to specific CD4 T cells.
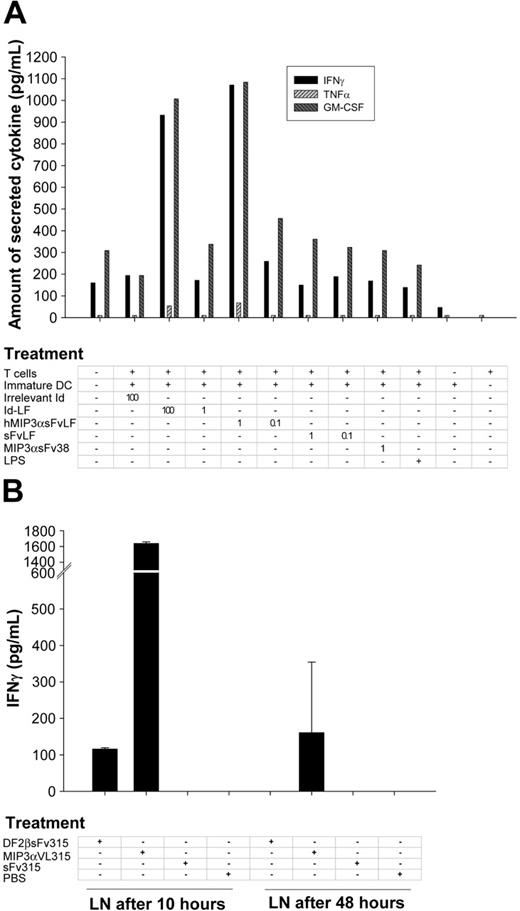
This observation was further confirmed using human fusion proteins; specifically, chemokine fusion proteins facilitated the uptake and presentation of a human lymphoma sFv and stimulated Id-specific T cells from the same patient. For example, patient DCs, treated with human MIP-3α fused with the patient's sFv (hMIP-3αsFvLF, 1 μg/mL), and mixed with an autologous T-cell line induced production of IFN-γ and GM-CSF (Figure 4A). This stimulation was greater than DCs treated with sFvLF alone or MIP-3α fusion protein with an irrelevant sFv (MIP-3αsFv38; Figure 4A). In contrast, a much higher concentration of intact lymphoma immunoglobulin (100 μg/mL, Id-LF; Figure 4A) was required to elicit a comparable response.
Chemokine fusion improves protein up take in vivo and in vitro. (A) hMIP3αsFvLF, MIP3αsFv38, or sFvLF fusion proteins at 2 different concentrations (1 μg/mL and 0.1 μg/mL) were incubated for 4 hours with the patient's PBMC-derived DCs. Then, DCs were thoroughly washed, irradiated, and mixed with the patient-derived Id-specific T-cell lines. After 72 hours, IFN-γ, GM-CSF, and TNF-α production was assayed in culture supernatants. Control DCs were incubated with 100 μg/mL and 1 μg/mL of the patient B-cell lymphoma-derived IgM (Id-LF) or LPS (10 ng/mL). Representative of 2 experiments performed in duplicate wells. (B) BALB/c mice (3/group) were immunized subcutaneously with 25 μg endotoxin-free MIP3αVL315, DF2βsFv315, or sFv315 each, or mock injected with PBS. After 10 and 48 hours, lymph node (LN) cells had been produced, irradiated (2000 rad), and mixed directly (without any additional protein stimulation) with 7A10B2 T cells in 96-well round-bottom plates at a 1:1 ratio (2 × 104 cells each) for 48 hours. IFN-γ production was assessed by ELISA in culture supernatants. Representative of 2 consecutive experiments performed in duplicate wells are shown. Error bars depict standard error of the mean of 3 mice per group.
Chemokine fusion improves protein up take in vivo and in vitro. (A) hMIP3αsFvLF, MIP3αsFv38, or sFvLF fusion proteins at 2 different concentrations (1 μg/mL and 0.1 μg/mL) were incubated for 4 hours with the patient's PBMC-derived DCs. Then, DCs were thoroughly washed, irradiated, and mixed with the patient-derived Id-specific T-cell lines. After 72 hours, IFN-γ, GM-CSF, and TNF-α production was assayed in culture supernatants. Control DCs were incubated with 100 μg/mL and 1 μg/mL of the patient B-cell lymphoma-derived IgM (Id-LF) or LPS (10 ng/mL). Representative of 2 experiments performed in duplicate wells. (B) BALB/c mice (3/group) were immunized subcutaneously with 25 μg endotoxin-free MIP3αVL315, DF2βsFv315, or sFv315 each, or mock injected with PBS. After 10 and 48 hours, lymph node (LN) cells had been produced, irradiated (2000 rad), and mixed directly (without any additional protein stimulation) with 7A10B2 T cells in 96-well round-bottom plates at a 1:1 ratio (2 × 104 cells each) for 48 hours. IFN-γ production was assessed by ELISA in culture supernatants. Representative of 2 consecutive experiments performed in duplicate wells are shown. Error bars depict standard error of the mean of 3 mice per group.
Chemoattractant fusion proteins are also taken up, processed, and presented to T cells in vivo
Next, we tested whether APCs could uptake and present Id when targeted with chemoattractant fusion proteins in vivo. The 7A10B2 T cells were mixed with irradiated draining lymph node cells from BALB/c mice removed 10 and 48 hours after subcutaneous injection with 25 μg fusion proteins. Significant IFN-γ secretion was observed from T cells stimulated with lymph node cells from mice given injections 10 hours previously with MIP-3α or β-defensin fusion proteins (MIP-3αVL315 and DF2βsFv315, respectively; Figure 4B). The response was much less apparent with lymph node cells obtained 48 hours after injection. By contrast, lymph node cells removed from control mice injected with sFv315 alone failed to stimulate T cells (Figure 4B). These results suggest that APCs can uptake, process, and present antigen to T cells in vivo, when their chemokine receptors are targeted with chemokine fusion proteins.
Mechanism of T-cell processing of chemoattractant fusion proteins
To further elucidate the mechanism of chemokine receptor-mediated antigen processing, various inhibitors of intracellular trafficking and processing were tested. Brefeldin A is a fungal metabolite that disassembles the Golgi apparatus and inhibits vesicle transport of newly synthesized MHC class II molecules between endoplasmic reticulum (ER) and Golgi32 ; monensin is a sodium/potassium/proton ionophore that blocks Golgi transport and prevents acidification of intracellular compartments and internalization of CCR5 receptors.33 These reagents were assessed for their effects on chemokine fusion protein processing and presentation by APCs. Both inhibitors completely abrogated T-cell stimulation by splenocytes pulsed with MIP-3αVL315 (Figure 3C). Similarly, T-cell stimulation was also significantly reduced by treatments with agents that affect antigen processing within endosomal-lysosomal compartments, such as the serine and cysteine protease inhibitors leupeptin (Figure 3C) and chloroquine (Figure 3D). In contrast, splenocytes treated with MIP-3αVL315 together with a specific proteosomal inhibitor lactocystin (Figure 3D) were able to induce IFN-γ secretion from 7A10B2 T cells at the same level as APCs incubated with MIP-3αVL315 alone (Figure 3D). These data suggest that chemoattractant receptors on APCs can efficiently internalize chemokine-fused antigens, which are then processed through the early/late endocytic compartments using the MHC class II presentation pathway. This process does not require proteosomal activity.
Viral chemokine antagonist fusions as candidate vaccines for clinical development
Protein or DNA immunizations with lymphoma-derived Id and its fragments alone, particularly from 38C13 lymphoma, fail to induce immunity in syngeneic mice.15 However, as we reported recently, this nonimmunogenic antigen can be rendered immunogenic by vaccinating with fusion constructs with various syngeneic chemokines.16 However, use of host chemokine carriers may elicit antihost chemokine autoimmunity, which may hamper their future clinical use. Therefore, to circumvent this potential problem we tested whether xenogeneic ligand, viral chemokine antagonists vMIP-2 and MC148, would elicit anti-Id responses. Syngeneic mice were immunized with plasmids encoding vMIP-2 or MC148 fused with sFv38 (pvMIP2sFv38 and MC148sFv38, respectively) and challenged with a lethal dose of 38C13 lymphoma. Control mice were immunized with DNA constructs encoding sFv fused with mutated chemokines (pvMIP2M-sFv38) or prototypic protein vaccine consisting of lymphoma-derived Id chemically cross-linked with KLH (Ig38-KLH), currently being tested in a phase 3 clinical trial.14 No survival was observed in control groups of mice immunized with PBS (Figure 5A,E) or plasmids encoding sFv38 fused with inactive mutant viral chemokine constructs pvMIP2M-sFv38 (Figure 5A) or pMC148M-sFv38 (not shown). In contrast, significant protective immunity was elicited in mice immunized with both fusion constructs pvMIP2sFv38 or pMC148sFv38 (logrank P < .0001 compared with pvMIP2M-sFv38; Figure 5A). The protection elicited with both constructs was comparable to that induced by Ig38-KLH (Figure 5A). DNA vaccinations with fusion constructs with viral chemokines generated mostly significant levels of anti-Id IgG1 antibodies (Figure 5B,C). In contrast, no antibodies were produced in mice immunized with mutant constructs pvMIP2M-sFv38 and pMC148M-sFv38 (not shown), which were unable to bind the respective chemokine receptors. Similarly, as we reported for other host chemokines,15 no antibody was generated in mice immunized with DNA expressing a mixture of plasmids containing unlinked sFv and vMIP-2 (not shown), suggesting the importance of physical linkage between chemoattractant and antigen.
Viral chemokine fusion proteins as vaccine carrier. (A) Ten C3H/HeN mice per group were immunized with DNA constructs pvMIP2sFv38 or pMC148sFv38. Control groups of mice received PBS or were immunized with pvMIP2M-sFv38. As positive control, mice were immunized intraperitoneally with tumor-specific immunoglobulin conjugated to KLH (Ig38-KLH, 50 μg).15,16 The log-rank P value is for comparison of pvMIP2sFv38 with control pvMIP2M-sFv38. (B-C) To test effects of preexisting anticarrier immunity, mice initially were immunized with pvMIP2-Muc1 to establish anti-vMIP2 antibody responses. Then the mice were immunized with pvMIP2sFv38 ([pvMIP2-Muc1[pvMIP2sFv38) or control pvMIP2-Muc1 ([pvMIP2-Muc1]pvMIP2-Muc1). The serum levels of anti-Id38 IgG (B, C) or anti-vMIP2 IgG (D) tested after 2 weeks after the last vaccination. (E) A survival plot of representative 4 independent experiments with 10 mice/group, challenged intraperitoneally with a lethal dose of 38C13 tumor cells. The log-rank P value is for comparison of pvMIP2sFv38 with PBS. (F) Chemokine coadministration reduced levels anti-Id38 IgG. Data from pooled sera from 5 mice per group vaccinated with pvMIP2sFv38 vaccine together with competing chemokine construct (pvMIP2-Muc1), or irrelevant chemokine plasmid (pMCP3-Muc1) or antigen (pMucS). The P value is a comparison between groups pMucS + pvMIP2sFv38 and pvMIP2-Muc1 + pvMIP2sFv38. Error bars depict the standard error of the mean of 5 mice per group.
Viral chemokine fusion proteins as vaccine carrier. (A) Ten C3H/HeN mice per group were immunized with DNA constructs pvMIP2sFv38 or pMC148sFv38. Control groups of mice received PBS or were immunized with pvMIP2M-sFv38. As positive control, mice were immunized intraperitoneally with tumor-specific immunoglobulin conjugated to KLH (Ig38-KLH, 50 μg).15,16 The log-rank P value is for comparison of pvMIP2sFv38 with control pvMIP2M-sFv38. (B-C) To test effects of preexisting anticarrier immunity, mice initially were immunized with pvMIP2-Muc1 to establish anti-vMIP2 antibody responses. Then the mice were immunized with pvMIP2sFv38 ([pvMIP2-Muc1[pvMIP2sFv38) or control pvMIP2-Muc1 ([pvMIP2-Muc1]pvMIP2-Muc1). The serum levels of anti-Id38 IgG (B, C) or anti-vMIP2 IgG (D) tested after 2 weeks after the last vaccination. (E) A survival plot of representative 4 independent experiments with 10 mice/group, challenged intraperitoneally with a lethal dose of 38C13 tumor cells. The log-rank P value is for comparison of pvMIP2sFv38 with PBS. (F) Chemokine coadministration reduced levels anti-Id38 IgG. Data from pooled sera from 5 mice per group vaccinated with pvMIP2sFv38 vaccine together with competing chemokine construct (pvMIP2-Muc1), or irrelevant chemokine plasmid (pMCP3-Muc1) or antigen (pMucS). The P value is a comparison between groups pMucS + pvMIP2sFv38 and pvMIP2-Muc1 + pvMIP2sFv38. Error bars depict the standard error of the mean of 5 mice per group.
Next, we tested whether preexisting antichemokine immunity would affect anti-Id responses elicited by pvMIP2sFv38. Ten mice per group were first immunized twice with vMIP-2 constructs fused with an irrelevant antigen, human breast cancer antigen Muc1 (pvMIP2-Muc1). Then mice were immunized 3 more times with pvMIP2sFv38. Mice preimmunized with pvMIP2-Muc1 generated significant levels of anti-vMIP2 IgG antibody (pvMIP2-Muc1/pvMIP2-Muc1 and pvMIP2-Muc1/pvMIP2sFv38; Figure 5D). Nevertheless, these same mice that generated anti-vMIP2 antibody also produced idiotype-specific antibody when they were immunized with the specific pvMIP2sFv38 construct ((pvMIP2Muc1)pvMIP2sFv38; Figure 5B,C). Both groups of mice, naive or anti-vMIP2 antibody producer, immunized with pvMIP2sFv38 generated comparable levels of anti-Id antibody (P > .9; Figure 5C). However, only naïve mice immunized with pvMIP2sFv38 were clearly protected from tumor challenge (log-rank P < .03 compared with PBS; Figure 5E). In contrast, pvMIP2sFv38 immunizations of mice with existing anti-vMIP2 antibody elicited lower tumor protection in 2 of 2 experiments with 10 mice/group (Figure 5E, although not statistically significant). Tumor protection was not due to nonspecific effects of chemokine carriers because mice immunized with vMIP-2 fused to the irrelevant antigen (pvMIP2-Muc1; Figure 5E) were not protected.
Overall, these data suggest that viral chemokine antagonists vMIP-2 and MC148 can be used to render a nonimmunogenic tumor antigen immunogenic and elicit protective antitumor immunity, even for a very aggressive lymphoma, 38C13, which kills all control mice within 20 days after challenge. To further test the idea that the immune response was a chemokine receptor-mediated process, we tried to inhibit immunity by coinjection of the competing ligand. Mice were immunized with either pvMIP2sFv38 mixed with DNA encoding an irrelevant chemokine (pMCP3-Muc1), or antigen (pMucS), or with vMIP-2 fused with irrelevant antigen (pvMIP2-Muc1; Figure 5F). Sera of mice immunized with pvMIP2sFv38 together with an irrelevant chemokine or antigen-expressing plasmid contained an amount of idiotype-specific IgG antibodies ranging between 100 and 150 μg/mL (pMCP3-Muc1 + pvMIP2sFv38, and pMucS + pvMIP2sFv38, respectively; Figure 5F). However, mice receiving coinjections of plasmids encoding pvMIP2sFv38 and competing pvMIP2-Muc1 generated significantly lower levels (up to 40 μg/mL) of anti-Id antibody (pvMIP2-Muc1+ pvMIP2sFv38; Figure 5F). Similarly, coinjection of competing plasmids pvMIP2-Muc1 and pvMIP2sFv38 also reduced tumor protection. Whereas 30% of mice immunized with pvMIP2sFv38 together with either an irrelevant chemokine fusion construct pMC3sFv38 or an irrelevant antigen plasmid pMucS were tumor free after 100 days after challenge, all mice given coinjections with a mixture of pvMIP2sFv38 and pvMIP2-Muc1 died by day 25 (not shown). Therefore, these data further support the view that immunity to nonimmunogenic tumor antigens fused with viral chemokines also depends on their ability to engage chemokine receptors.
Discussion
Herein, we expand our previous observation that chemokines are able to render a model self-tumor antigen, lymphoma idiotype,15 immunogenic by efficiently delivering antigen to APCs via chemokine receptors. These vaccines require chemokine receptor signaling because Id-specific antibody and protective antitumor immunity were elicited only in mice immunized with sFv physically linked with functionally active chemoattractant moieties. Moreover, immunizations with fusion constructs encoding mutant chemoattractants, which could not bind to respective chemokine receptors, failed to elicit any immune responses. Furthermore, similarly to other receptor-mediated phenomena,16 the immune responses elicited by viral chemokine-fused antigens can be efficiently abrogated by coinjection of a competing ligand. For example, both antibody (Figure 5F) and antitumor protection from pvMIP2sFv38 vaccine was abrogated by coinjection of vMIP-2–expressing constructs, but not an irrelevant chemokine MCP-3 or antigen. Thus, these data further support the idea that the viral chemokine-based vaccines also require chemokine receptor targeting to deliver and render fused antigens immunogenic and that immunity was not due to generation of an immunogenic neoantigen. The induction of local chemotaxis to the vaccine site alone is not sufficient to break nonresponsiveness to self-tumor antigens, probably due to inefficient antigen uptake by infiltrating cells.16
Our in vitro data support the hypothesis that chemokine-fused antigens are efficiently taken up and processed in early endocytic compartments of the APCs because T-cell stimulation by APCs treated with MIP-3α fusion antigens was significantly reduced by coincubation with leupeptin or chloroquine, agents that affect antigen processing within endosomal-lysosomal compartments. Furthermore, the antigen was transported to late endosomes and Golgi because monensin, an inhibitor of acidification of intracellular compartments and pathways dependent on clathrin-coated pits, and brefeldin A, which blocks transport between ER and Golgi, also suppressed T-cell stimulation (Figure 3C). Thus, these data suggest that chemokine fusion antigens are taken up via chemokine receptors, processed in early/late endocytic compartments and presented in the context of MHC class II, although precise data on colocalization and trafficking will require use of more direct techniques.
At present, we do not know whether chemokine-fused antigens can also use the MHC class I presentation pathway. However, we have previously reported data that mice immunized with HIV Env fused with MCP-3 or β-defensin elicited efficient systemic and mucosal CD8 cytotoxic T-lymphocyte (CTL) responses,34 and antitumor therapeutic and protective immunity induced by DNA vaccinations with proinflammatory chemokine fusion antigens was dependent on effector CD8 cells.15 These data suggest indirectly that chemokine may also deliver antigens for MHC class I presentation. Moreover, proteosomes, an essential part of MHC class I processing and presentation,35 play an important role in internalization and down-regulation of several cell surface receptors, including cytokine receptors.11-13 It has been also reported that chemokine receptor CCR5 is constitutively associated with the ζ subunit of proteosome,36 and CD4 receptor degradation induced with HIV Env also depends on proteosomes.37 However, a specific proteosomal inhibitor, lactocystin,38 did not affect MIP-3α–mediated antigen presentation (Figure 3D), despite the fact that it completely inhibits MIP-1β or stromal cell-derived factor 1 (SDF-1α)–induced down-regulation of CCR5 and CXCR4, respectively.36 Therefore, these data suggest that internalization of MIP-3αVL315 mediated by CCR6 may use an alternative proteosome-independent pathway. It is tempting to hypothesize that chemokine fusion proteins, once internalized, are processed or degraded using both MHC class I and class II pathways. The existence of an alternative mechanism of internalization is indirectly supported by the fact that the same IL-8 regulates differentially the internalization of CXCR1 and CXCR2 receptors,39 and endocytosis of viral chemokine receptor US28 occurs via a clathrin-mediated and β-arrestin independent mechanism.40 Activated CCR5 is transported to endosomes via clathrin-coated pits and then either is recycled back to the cell surface or transported and degraded in endosomes and lysosomes.41,42
It is noteworthy that chemoattractant-fused antigens do not require adjuvants and are able to stimulate immune responses in vivo16,34 and in vitro with relatively low doses. As we demonstrated, murine DCs and splenocytes treated with as little as 100 ng/mL fusion proteins with MIP-3α or β-defensin 2 efficiently stimulated the syngeneic mouse CD4+ T-cell clone, which specifically recognizes an MOPC315 plasmacytoma light-chain epitope.24 Similarly, T cells from a patient with B-cell lymphoma required about 100-fold smaller amounts of Id (hMIP3αsFvLF), when it was provided to DCs as fusion protein with MIP-3α, compared with Id-LF alone. APCs treated with unlinked chemoattractant and Id or Id alone or fused with mutant and inactive chemoattractants were not able to efficiently stimulate T cells, thus ruling out the possibility of nonspecific in vitro effects.
Overall, the use of chemokines as vaccine carriers is an efficient and simple strategy to elicit both humoral and cellular responses. Because of chemokine and chemokine receptor redundancy, human chemokine carriers may be effectively replaced by xenogeneic analogues, an important consideration in clinical trials to circumvent possible autoimmunity against host chemokines. Although, vMIP2-based vaccines elicited protective antitumor immunity against syngeneic B-cell lymphoma, not every viral chemokine is a carrier, because we could not get any immune responses in mice immunized with constructs expressing HHV-6–derived chemokine agonist U83 despite its chemotactic properties for the monocytic cell line, THP-143 (pU83SPsFv38, not shown). It is not clear whether its ineffectiveness is due to the receptor's inability to be internalized or poor stability of the fusion protein. The fate of the internalized receptor is thought to be controlled by the nature of the ligand and the strength of signaling, because it was observed during cross-desensitization of CXCR1 and CCR1, CXCR4 and CCR5, and inhibition of HIV-1 entry,44 and preferential inhibition of CCR5 recycling by AOP-RANTES.10 Selection of separate chemokines also enables controlled induction of humoral or cellular immune responses.16 For example, some T helper (Th) type 2–specific chemokines, such as MDCs, failed to elicit CD8+ CTLs, but generated superior levels of antibody.34 The broad-range viral antagonist vMIP-2 also has been classified as a Th2-specific chemoattractant, and its potency to elicit Th1-cellular responses has yet to be studied.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2004-02-0637.
Supported in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Orville C. Bowersox and Wanhua Gong for technical assistance; Elena Klyushnenkova for help with isolation of murine BM-derived DCs; Dr Akira Takashima for the gift of XS52 cells, Dr Bjarne Bogen for the gift of 7A10B2 cells, and Dr Joshua Farber for the gift of mCCR6/HEK293; Dr Dan Longo for helpful comments and suggestions.

![Figure 5. Viral chemokine fusion proteins as vaccine carrier. (A) Ten C3H/HeN mice per group were immunized with DNA constructs pvMIP2sFv38 or pMC148sFv38. Control groups of mice received PBS or were immunized with pvMIP2M-sFv38. As positive control, mice were immunized intraperitoneally with tumor-specific immunoglobulin conjugated to KLH (Ig38-KLH, 50 μg).15,16 The log-rank P value is for comparison of pvMIP2sFv38 with control pvMIP2M-sFv38. (B-C) To test effects of preexisting anticarrier immunity, mice initially were immunized with pvMIP2-Muc1 to establish anti-vMIP2 antibody responses. Then the mice were immunized with pvMIP2sFv38 ([pvMIP2-Muc1[pvMIP2sFv38) or control pvMIP2-Muc1 ([pvMIP2-Muc1]pvMIP2-Muc1). The serum levels of anti-Id38 IgG (B, C) or anti-vMIP2 IgG (D) tested after 2 weeks after the last vaccination. (E) A survival plot of representative 4 independent experiments with 10 mice/group, challenged intraperitoneally with a lethal dose of 38C13 tumor cells. The log-rank P value is for comparison of pvMIP2sFv38 with PBS. (F) Chemokine coadministration reduced levels anti-Id38 IgG. Data from pooled sera from 5 mice per group vaccinated with pvMIP2sFv38 vaccine together with competing chemokine construct (pvMIP2-Muc1), or irrelevant chemokine plasmid (pMCP3-Muc1) or antigen (pMucS). The P value is a comparison between groups pMucS + pvMIP2sFv38 and pvMIP2-Muc1 + pvMIP2sFv38. Error bars depict the standard error of the mean of 5 mice per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/7/10.1182_blood-2004-02-0637/6/m_zh80190467330005.jpeg?Expires=1767758622&Signature=ILgZx32RtVt59oH-v0ixFBrlG0IHqt-MSZlMJ0ucfXE5aDOxtkBgbEsvFdTjodE1ByDT4F3oYXjq81cqsbQhxetEwTaNUfq8m0ujJN8jSnW4ETVlRcMwHHpSx3DRXD0yXpCNmQzjdfxItyj8lYyF8zoCwOCaGsFcAaQHxo2RfaIQ8hYDjbRpZkKQ3eJkpheoRtMWrgCRgd-fIIn3hotyD~bt2cg~xh26bvD3zZ1Kh-zo~0K1lH~6z8p3dzsLoovJcENv77dtBXmx0CZduEm0eMlCpnMe~KOLEWQuHCMTHDVLL5wB6YH44bUae6hiGiCRGazzItqgQH5walW2sPpDUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal