Comment on Zauli et al, page 2044
Zauli et al are the first to report that the TNF family member TRAIL can inhibit osteoclastogenesis, thus suggesting a greater potential role for TRAIL in the treatment of osteolytic cancers.
Bone remodeling is an essential process for healthy maintenance of skeletal bone. However, skeletal bone is a complex system in which osteoblasts are forming bone while the osteoclasts, multinucleated cells derived from fusion of monocyte/macrophages in blood, are continually resorbing it. This process allows for the periodic replacement of bone for such purposes as removing microfissures that occur with aging and support of the kidney regulating calcium homeostasis. Therefore, it is not surprising that many pathways that lead to the production and function of 1 cell type are often controlled by the other cell type. One of the best examples of this is the tumor necrosis factor (TNF) family, which plays an integral part of osteoclast differentiation. The soluble factor RANKL (receptor activator of nuclear factor kappa B ligand) is expressed by osteoblasts and stromal cells and binds to its cognate receptor RANK on preosteoclastic cells in the local circulation, leading to osteoclast differentiation.1 However, when the soluble receptor osteoprotegerin is present, it binds RANKL and effectively abolishes osteoclastogenesis and bone resorption.2
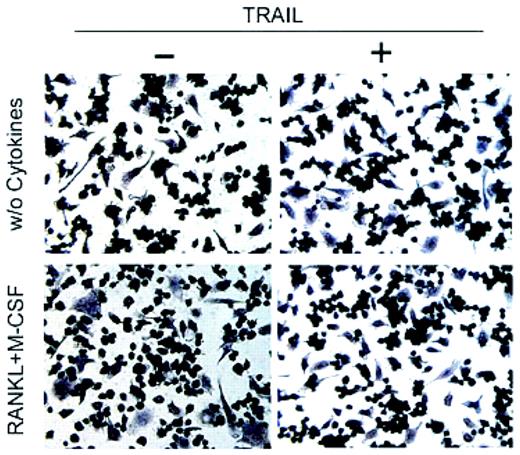
If things weren't complicated enough, in this issue of Blood, Zauli and colleagues demonstrate the ability of another member of the TNF family, TNF-related apoptosis-inducing ligand (TRAIL)/Apo-2 ligand (L), to inhibit osteoclastogenesis. In this report, they demonstrate the ability of recombinant human TRAIL protein to block RANKL + macrophage–colony-stimulating factor (M-CSF)–stimulated osteoclast differentiation in primary human monocytes, as well as the murine monocyte/macrophage cell line RAW264.7, thus blocking the production of the multinucleated cells and totally abolishing their bone resorptive activity.
This study is one of the first to identify a negative regulatory role for one of the TNF family members in osteoclastogenesis and bone resorption. TRAIL is best characterized for its role in inducing apoptosis in tumor cells while displaying minimal or no toxicity in normal cells.3 Currently, no physiologic role in normal cellular processes has yet been assigned to TRAIL other than its known role in tumor protection, and mice deficient in TRAIL showed no abnormal developmental defects,4 thus continuing the mystery. Therefore, the potential physiologic role of TRAIL as a negative regulator of osteoclastogenesis is extremely exciting, although the lack of phenotype in the gene-deficient mice suggests that there may be other factors that also can function in this capacity. The group further demonstrated that TRAIL blocked the phosphorylation of the p38/mitogen-activated protein kinase (MAPK). Since this pathway is activeFIG1 during osteoclastogenesis,5 presumably this pathway is a regulatory target of TRAIL.
Effect of TRAIL on differentiation of RAW264.7 cells into functional osteoclasts. See the complete figure in the article beginning on page 2044.
Effect of TRAIL on differentiation of RAW264.7 cells into functional osteoclasts. See the complete figure in the article beginning on page 2044.
The significance of this study is, perhaps, its potential positive secondary effect of recombinant TRAIL in the treatment of osteolytic lesions such as multiple myeloma. In multiple myeloma, there is an increase in osteoclasts at the sight of lesion and a significant increase in bone resorption.6 This uncoupling of remodeling leads to rapid bone loss and a bone degrading lesion. Thus, recombinant TRAIL may be capable of killing the tumor cells as well as attenuating the bone resorption, providing an effective treatment for this disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal