We read with special interest the article of Busiello and colleagues in Blood.1 They described atypical features of familial hemophagocytic lymphohistiocytosis (FHLH) in a patient/family presenting 2 different perforin gene alterations: an already known homozygous A91V and a novel heterozygous R231H exchange. Since the identification of this gene, which is responsible for the disease in a subgroup of patients,2 several groups have reported on the identification of novel mutations.3-6 Small deletions and nonsense and missense mutations were described and scattered both coding exons of the gene. In this family, the homozygous A91V and the heterozygous R231H exchange was detectable in both twins, one of them presenting typical signs of hemophagocytic lymphohistiocytosis (HLH) at the age of 11 years with a rapidly fatal course of the disease. The second twin, with the same perforin mutation pattern, had yet no signs of HLH, including a normal natural killer (NK) cell activity. The 272C>T transition was further detectable at the heterozygous level in the remaining healthy family members (father, mother, and 2 sisters). The R231H exchange was found in the father and, as mentioned before, also in the healthy twin. The authors conclude that due to the identical genetic pattern in the second twin, a late onset of the disease may still be possible in this child.
From our studies, we present evidence that the A91V exchange represents a polymorphism in the perforin gene not causative of the HLH phenotype. We analyzed exon 2 in a series of 86 control DNA samples from healthy unrelated Caucasian individuals by denaturing high-performance liquid chromatography (DHPLC) and found a heterozygous 272C>T transition in 15 cases (17.5%). Additionally, Feldmann et al reported on a homozygous A91V in a nonaffected subject.3 Finally, Molleran Lee et al confirmed the observation of a polymorphism at this nucleotide in the perforin gene by analyzing a large cohort of controls with a heterozygosity of 3% (7 out of 202 investigated cases).7 In contrast to these data, Clementi et al described a family including 2 brothers with late onset of the disease and a compound heterozygous pattern of mutations in the perforin gene.8 In parallel to a W374X mutation leading to a premature stop, the heterozygous A91V exchange was found in both twins. NK cell activity and perforin expression were markedly reduced in both patients. Taken together, the A91V transition has been described either as polymorphism (Feldmann et al,3 Molleran Lee et al,7 and our own observations) or as disease causing mutation in 2 families including 4 patients with late onset of the disease.1,8 The frequency of this transition differed between the geographic or ethnic origin of the samples. With the assumption of a pathologic role of A91V and an allelic frequency of about 9% in our healthy population, the incidence of HLH should be much higher than observed in Germany. However, the real disease prevalence is not yet determined exactly because of a possible underestimation of the diagnosis due to atypical phenotypic presentations. A reduced perforin expression may also occur in heterozygous carriers or may be due to additional genetic defects in the regulatory region of the gene (eg, exon 1).
The presence of a noncausative A91V polymorphism described in the paper by Busiello et al is underlined by the fact that the healthy twin, who has genetically the same mutation pattern as his affected sister, has a completely healthy phenotype, including a normal NK cell activity. This supports our findings that the described genotype is not responsible for the onset of the disease. In conclusion, we show very strong evidence that A91V represents a polymorphism rather than a relevant mutation. This should be taken into account for further genetic counseling in affected families.
Role of A91V mutation in perforin gene in hemophagocytic lymphohistiocytosis
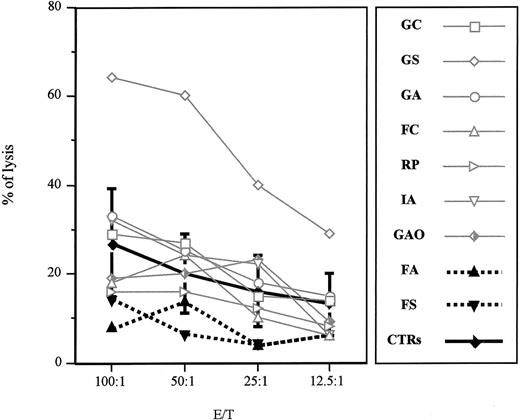
The letter by zur Stadt et al addresses an important issue concerning the pathogenic role of the A91V mutation in the perforin (PRF1) gene for the familial form of hemophagocytic lymphohistiocytosis (FHLH). This mutation has previously been detected in association with FHLH in a few families from Southern Italy.1,2 The authors report on the molecular evaluation of the PRF1 gene in a cohort of healthy white German subjects showing an allelic frequency of the mutation of 9%. The authors conclude that the A91V mutation represents a common polymorphism, thus supporting the previous observation of healthy subjects carrying the same mutation. Our previously published data, in which the mutation was described in 2 twin sisters, one affected with FHLH and one with a completely healthy phenotype, led us to hypothesize that along with the A91V mutation other factors may interfere in the clinical expression of the disease.2 Hence, we asked the same question raised by zur Stadt et al, and to address the issue we looked for more research subjects carrying the A91V mutation at the heterozygous status among relatives of affected probands from our area. In these research subjects, we examined cytolytic activity and biochemical features. All research subjects were asymptomatic, but one who was affected 2 years earlier with a manifest and severe form of FHLH and is currently in a stable remission phase. These research subjects cleared normally from common virus infections. Actually, all the research subjects have normal biochemical parameters, including fibrinogen, triglycerides, and ferritin values. As displayed in Figure 1, 7 of 9 research subjects carrying the A91V mutation at the heterozygous status showed a normal natural killer (NK) activity. One research subject (PN 09) had a very low CD56+CD16+ cell number (1.1%) and proportionately reduced NK activity. Finally, PN 08 had a normal NK cell number and borderline NK activity. Of note, PN 02 is the patient with FHLH in remission who showed absent NK activity in the acute phase of the disease.
Natural killer activity of A91V heterozygous individuals. Nonadherent peripheral blood lymphocytes isolated from heterozygous subjects and healthy controls (PNs 01-09 and controls, respectively) were incubated for 4 hours at 37° C with 51Cr-pulsed K562 cell line. Specific lysis was measured in a triplicate assay performed with 5 × 103 target cells mixed with effector cells at different effector-target ratios (E/T). The percentage of specific lysis was calculated as follows: 100 × (specific release – spontaneous release)/(total release – spontaneous release). Mean and SDs were calculated on 10 control individuals.
Natural killer activity of A91V heterozygous individuals. Nonadherent peripheral blood lymphocytes isolated from heterozygous subjects and healthy controls (PNs 01-09 and controls, respectively) were incubated for 4 hours at 37° C with 51Cr-pulsed K562 cell line. Specific lysis was measured in a triplicate assay performed with 5 × 103 target cells mixed with effector cells at different effector-target ratios (E/T). The percentage of specific lysis was calculated as follows: 100 × (specific release – spontaneous release)/(total release – spontaneous release). Mean and SDs were calculated on 10 control individuals.
On the basis of the 9% allelic frequency of the A91V mutation, zur Stadt and coworkers argue that the expectance of the disease in Germany should be much higher than that observed. However, the real disease prevalence is not easily determined, because of the underestimation of the diagnosis due to the variable phenotypic presentation. Moreover, the evaluation of cytolytic activities and the analysis of perforin expression are not performed on a routine basis in all patients, adults or children, who have an unusually severe and rapidly progressive clinical course of a common viral infection.
In conclusion, we propose that A91V may represent a molecular alteration that is not, per se, causative of the disease and not sufficient to impair the cytolytic activity. Beside this, A91V seems to play a role in the pathogenesis of the disease conferring a genetic susceptibility in the development of FHLH.
In the future, it might be helpful to definitively clarify the role of the A91V alteration in the pathophysiology of the disease in order to examine the distribution of this mutation in populations originating from different geographic areas and compare the genetic results.
Supported by the Grants “Ministero della Salute Regione Campania, Legge 502,” MURST-PRIN 2002.
Correspondence: Claudio Pignata, Department of Pediatrics, Unit of Immunology, “Federico II” University, via S. Pansini 5-80131, Naples, Italy; e-mail: pignata@unina.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal