Comment on Teeling et al, page 1793
A collaboration between investigators in basic science and biotechnology has produced a new set of human mAbs which may have superior efficacy in the immunotherapy of B-cell lymphoma.
Teeling and colleagues have demonstrated that slower may be better when the issue is the relationship between the rate of dissociation of anti-CD20 monoclonal antibodies (mAbs) from B cells and complement-dependent cytotoxicity (CDC). Based on the notable but only partial success of anti-CD20 mAb rituximab (RTX) in B-cell lymphoma treatment, the authors developed candidates for the next generation of anti-CD20 mAbs. Clinical investigations and basic science studies with RTX have raised important questions regarding its cell-killing mechanism(s) and the reasons why RTX is effective for only about half of patients with non-Hodgkin lymphoma (NHL), with less success in treating chronic lymphocytic leukemia (CLL).1,2 Complete elucidation of the RTX in vivo mechanism should facilitate design of optimized treatment paradigms for mAb-based targeting of B-cell-associated CD20.FIG1
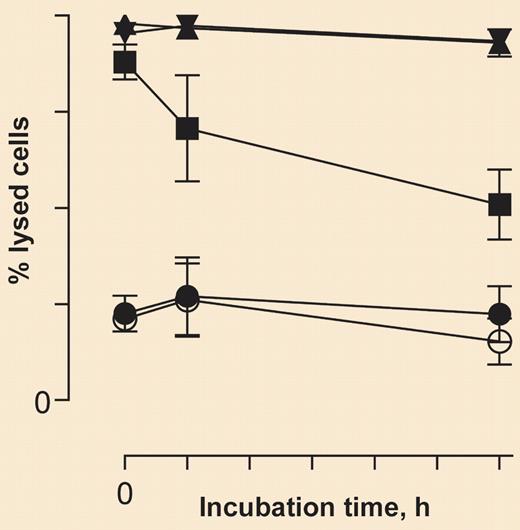
Caption to come. See the complete figure in the article beginning on page 1793.
Caption to come. See the complete figure in the article beginning on page 1793.
Most CLL patient B cells are resistant to RTX-mediated CDC, presumably due to the combination of enhanced expression of complement regulatory proteins and relatively low CD20 expression compared with levels in NHL.1,2 Teeling et al provide compelling evidence that 2 fully human high-affinity anti-CD20 mAbs effectively promote CDC in a variety of B cells, many of which are resistant to RTX. In the presence of complement, immunoglobulin G1 (IgG1) mAbs 2F2 and 7D8 lyse B-cell lines and isolated patient CLL cells.
Cell-bound mAbs 2F2 and 7D8 apparently induce greater CDC than RTX because they dissociate more slowly from targeted cells, thus increasing their biologically active concentration on the cell surface. Therefore, the cell-bound mAbs can more effectively engage and activate C1q and interact with downstream-activated complement components including C4b and C3b. The authors recognize that, based on steady-state considerations, 2 different mAbs may bind to B cells at the same CD20 epitopes with comparable levels of saturation, but the slower dissociating mAb will activate complement more effectively because there will be more time for mAb aggregates (2 or more IgGs) to interact with C1q. Covalent deposition of C3b should occur in close proximity to cell-bound mAbs3,4 ; although the authors did not evaluate C3 fragment deposition, in whole blood these mAbs should facilitate deposition of 100 000 or more C3b/iC3b molecules per cell.3 Therefore, the mAbs may also enhance antibody-dependent cellular cytotoxicity by promoting synergy between Fc receptors and complement receptor 3, which binds iC3b, the breakdown product of C3b.4
Increasing evidence argues that complement plays an important role in the RTX mechanism,5 and therefore, mAbs 2F2 and 7D8 may be more effective clinically than RTX. However, several caveats are in order. Rapid complement activation and cell lysis may induce serious side effects,1 and careful consideration of clinical and laboratory parameters will be required to optimize the treatment dose of mAb as well as its infusion rate. Moreover, the usual high dose of RTX (375 mg/m2), when used in CLL treatment, induces acute loss of CD204 ; therefore, whether the new anti-CD20 mAbs promote CD20 loss in CLL must be evaluated.
The mAbs developed and characterized by Teeling et al provide a powerful and reasoned extension in mAb-based immunotherapy. The simple and elegant paradigms set forth in the paper may ultimately be applicable to other tumor-associated antigens.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal