Abstract
Nuclear factor κB (NF-κB) activation plays a critical role in oncogenesis by human T-cell lymphotrophic virus type I (HTLV-I), the etiologic agent of adult T-cell leukemia (ATL), and is indispensable for maintenance of the malignant phenotype. In T lymphocytes, Tax-mediated p53 inhibition is dependent on Tax activation of the NF-κB pathway and is linked to p53 phosphorylation. We now report that blocking NF-κB transcriptional activation in HTLV-I–transformed cells restores p53 activity. Further, using mouse embryo fibroblast (MEF) null cells and antisense oligonucleotides to inhibit expression of NF-κB family members, we demonstrate that the p65 subunit of NF-κB is uniquely involved in p53 inhibition. Coimmunoprecipitation assays demonstrate an interaction between p65 and p53 in HTLV-I–transformed cells. In transient transfection assays, we demonstrate that Tax induces the p53-p65 interaction. Phosphorylation of p53 at serines 15 and 392 is critical for complex formation. Importantly, Tax-mediated p53 inhibition correlates with p65 and p53 interaction. By using chromatin immunoprecipitation (ChIP) assays, we find that in HTLV-I–transformed cells p53 and p65 form a complex on the inactive, p53-responsive murine double minute 2 (MDM2) promoter. Consistent with reduced transcriptional activity, transcription factor IID (TFIID) binding is not observed. These studies identify a unique mechanism for p53 regulation by the p65/RelA subunit of NF-κB.
Introduction
The nuclear factor κB (NF-κB) family of eukaryotic transcription factors plays an important role in the regulation of immune responses, embryo and cell lineage development, apoptosis, cell cycle progression, inflammation, and oncogenesis. There are 5 members of the NF-κB family that have been identified and cloned, including NF-κB1 (p50/p105), NF-κB2 (p52/p100), relA (p65), relB, and c-Rel.1 All of the family members share a highly conserved Rel homology domain that is responsible for sequence-specific DNA binding, dimerization, and interaction with IκBα, the intracellular inhibitor of NF-κB.1-4 The C-terminal domains of relA, relB, and c-Rel contain a transcriptional activation domain that is important for NF-κB–mediated gene activation. The C-terminal domains of the precursor molecules for p50 and p52, p105 and p100, respectively, contain multiple copies of the ankyrin repeat motif, which is found in IκB family members: IκBα, IκBβ, IκBϵ, Bcl3, and Drosophila cactus.1
Of the IκB proteins, IκBα is the most abundant inhibitory protein for NF-κB.5 Like all IκB proteins, IκBα contains 2 conserved serine residues within its N-terminal domain. Phosphorylation of these conserved serines in response to inducers leads to the polyubiquitination and subsequent degradation by the 26S proteasome.6,7 This liberates NF-κB and allows its translocation to the nucleus where it activates transcription. When these serine residues are mutated to alanines, IκBα can no longer be phosphorylated; it remains bound to NF-κB and acts as a dominant-negative inhibitor of NF-κB activity.8
A wide range of stimuli has been shown to activate NF-κB. One such activator is the human T-lymphotropic virus type I (HTLV-I) transcriptional activator protein Tax.9-11 HTLV-I is the etiologic agent of the aggressive and fatal malignancy of CD4+ T lymphocytes called adult T-cell leukemia (ATL). HTLV-I has also been associated with a variety of inflammatory diseases, including HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP), uveitis, and infectious dermatisis.12-17
Because of the low incidence of developing ATL and the long latency before its onset, leukemogenesis is thought to be a multistep process.18 Although it is unclear as to the exact mechanism of leukemogenesis, Tax has been shown to play a key role in disease development. Tax induces leukemia and neurofibromas in transgenic mice, immortalizes rat embryo fibroblasts, and cooperates with ras in cellular transformation.19 Tax expression allows factor-independent growth of established murine T-cell lines20 and can immortalize human T lymphocytes by transduction in a Herpes virus saimiri or retroviral vectors.21,22 Tax not only transactivates the viral long terminal repeat (LTR) but also transactivates or transrepresses the expression or function of a wide number of cellular genes, including cytokines, growth factors, cellular receptors, cell cycle regulators, DNA repair proteins, or proteins that regulate apoptosis.23-26
One protein whose function is inhibited by Tax is the tumor suppressor p53. p53 Functions as an integrator of stress response signals by activating or repressing the transcription of genes that regulate cell cycle progression and/or apoptosis.27-30 The importance of p53 function is underscored by the observation that p53 is mutated in about 60% of human cancers. However, in most HTLV-I–infected cells, p53 is wild type in sequence.31-33 Nevertheless, p53 is functionally inactive, and, more specifically, we and others have shown that Tax inhibits p53 function.34-38 In lymphocytes, the primary target for HTLV-I, p53 inhibition is linked to Tax-mediated NF-κB activation and a novel pathway that does not function through squelching of the coactivator p300/CBP (CREB binding protein) but is linked to altered p53 phosphorylation.37
In this report, we show that blocking NF-κB transcriptional activation in HTLV-I–transformed cells restores p53 activity. We have further investigated the contribution of individual subunits to NF-κB, including p50, p65, and c-Rel in Tax-mediated p53 inhibition. Although antisense oligonucleotides to p50, c-Rel, and p65 all inhibited Tax-mediated NF-κB activation, only antisense to p65 recovered p53 activity in the presence of Tax. Consistent with these results, Tax cannot inhibit p53 activity in p65–/– mouse embryo fibroblasts (MEFs).37 Coimmunoprecipitation assays demonstrate an interaction between p53 and p65 in HTLV-I–transformed cells. Further, when Tax and p65 constructs are coexpressed in p65–/– MEFs, a direct correlation between the ability of p65 to bind to p53 and Tax-mediated inhibition was observed. Importantly, chromatin immunoprecipitation (ChIP) assays on HTLV-I–transformed cells indicate that p53-p65 interaction occurs on p53-responsive promoters. We find weak transcription factor IID (TFIID) and polymerase II (polII) binding at the promoter, suggesting a failure to recruit the basal transcriptional machinery, resulting in inhibited p53 transcriptional activity.
Materials and methods
Cell lines and transfections
HTLV-I–transformed cells C81, MT2, and Hut102 and T-lymphocytic cell lines Jurkat and Molt4 were grown in RPMI supplemented with 10% fetal calf serum and 2 mM l-glutamine. MEFs p65–/–, NF-κB–inducing kinase–/– (NIK–/–), p50–/–, and wild type (WT) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% calf serum and 2 mM l-glutamine. p53–/–, murine double minute 2–/– (MDM2–/–) MEFs were grown in DMEM supplemented with 10% fetal calf serum and 2 mM l-glutamine. HTLV-I–transformed cells were transfected by using Transfast reagent (Promega, Madison, WI) as described by the manufacturer. MEFs were transfected by using Lipofectamine plus reagent (Invitrogen, Carlsbad, CA) or Effectene reagent (Qiagen, Valencia, CA) as described by the manufacturer.
NF-κB antisense oligonucleotides and transfections
Antisense phosphorothioate oligodeoxynucleotides (ASOs) were synthesized by Lofstrand (Gaithersburg, MD). Antisense sequences were 5′-TCGTCTGCCATGGTGAAGAT-3′ for p50, 5′-AAACAGATCGTCCATGGTCA-3′ for p65, and 5′TCGGTACCGGAGGCCACGCAT-3′ for c-Rel. Jurkat cells were transfected with Superfect transfection reagent (Qiagen), and ASOs (100 nM) were added as complexes with p53 and pcTax DNA. Cells were harvested 24 hours after transfection.
TNF-α and PMA treatment of cells
Jurkat cells were treated with 10 nM phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) or 10 ng/mL tumor necrosis factor α (TNF-α; R & D System, Minneapolis, MN) for 1 hour before transfection.
Plasmids and luciferase assay
The reporter constructs PG13-Luc (p53 reporter construct), 4 × NF-κB–Luc, and HTLV-I–LTR-Luc have been described previously.38 The wild-type p65, p50, and c-Rel constructs and pcTax were kindly provided by Gary Nabel (Howard Hughes Medical Institute, University of Michigan Medical Center), Neil Perkins (University of Dundee, United Kingdom), and Warner Greene (University of California at San Francisco). The p65 deletion constructs were provided by Dr Paul T. van der Saag (Netherlands Institute for Developmental Biology). Cell lysates were prepared 16 to 24 hours after transfection following the Promega Dual Luciferase and Tropix GalactoLight assay kit instructions. All transfections included the control plasmid cytomegalovirus (CMV)–βgal, Rous sarcoma virus (RSV)–βgal, or RLTK-Luc (as indicated in the text) to control for transfection efficiency.
Coimmunoprecipitation assays
For analysis of the interaction between p53 and p65, 1 mg whole-cell extracts or 0.5 mg nuclear extracts was used. Whole-cell and nuclear extracts were prepared as described previously.37 Cell extracts were immunoprecipitated with anti-p53 (Ab1 or Ab6; Oncogene Research Product, Boston, MA) or anti-p65 (Ab2; Oncogene Research Product). Immunoprecipitates were denatured, and proteins were separated by electrophoresis on 8% Tris (tris(hydroxymethyl)aminomethane)–glycine gels (Novex, San Diego, CA). The proteins were then transferred to polyvinylidene diflouride (PVDF) membranes (Immobilon, Billerica, MA), analyzed for anti-p53 (Ab1 or Ab7; Oncogene Research Product) or anti-p65 (CT) (Upstate) or anti-p65(A) (Santa Cruz Biotechnology, Santa Cruz, CA).
RNA Isolation
Total RNA was isolated with use of RNA-Bee reagent (TelTest, Friendswood, TX). Reverse transcription polymerase chain reaction (RT-PCR) was performed with use of the Ambion (Austin, TX) RT-PCR system following manufacturers' instructions. Primer sequences are as follows: MDM2 forward, 5′-CAGCTTCGGAACAAGAGACC-3′; reverse, 5′-GTCCGATGATTCCTGCTGAT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GCCAGTGGACTCCACGAC-3′; and GAPDH reverse, 5′-CAACTACATGGTTTACATGTTC-3′.
ChIP assay
Genomic DNA and proteins were cross-linked by addition of formaldehyde (1% final concentration) to cells (1 × 108) and incubated for 10 minutes at room temperature. Glycine was then added to a final concentration of 0.125 M, and cells were incubated an additional 5 minutes at room temperature. Chromatin preparation, immunoprecipitations, and DNA isolation were preformed as described previously.39 Antisera to p53, p65, TFIID, polII, or control immunoglobulin G (IgG) were used. DNA (5 μL) was used as a template for PCR amplification to examine the MDM2 promoter region containing the p53-responsive element. PCR was performed with use of the following primer pair: MDM2 promoter forward, 5′-CTCGTTGCTGGGTCCAGGAGGTGA-3′, and MDM2 promoter reverse, 5′-CTGCGGAAACGGGGCAGCGTTTAAAT-3′.
Results
Expression of IκBα-DN in HTLV-I–transformed cells restores p53 activity
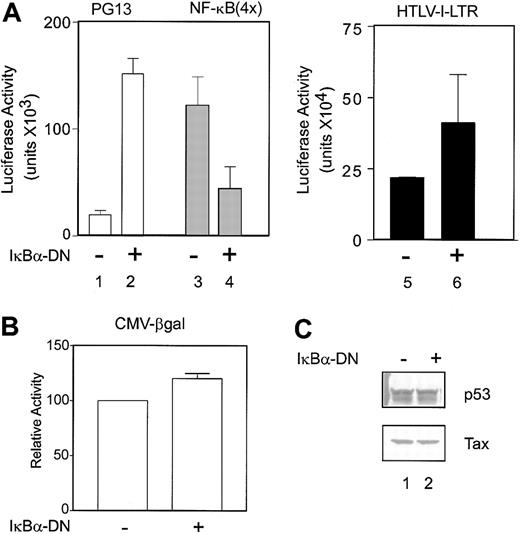
Our previous studies demonstrated that activation of NF-κBbyTax was required for Tax-mediated p53 inhibition in transiently transfected lymphocytes.37 In contrast, others have reported that the cyclic AMP response element-binding protein (CREB) activation pathway is important for p53 inhibition.35 To test whether NF-κB activation is critical for p53 inhibition in HTLV-I–transformed cells, we used a phosphorylation mutant of IκBα which inhibits NF-κB activation in a dominant-negative manner (IκBα-DN).8 IκBα-DN was cotransfected with the HTLV-I–LTR-, NF-κB–, or p53-luciferase reporter construct into the HTLV-I–transformed cell line C81. As expected, expression of IκBα-DN inhibited NF-κB activation (Figure 1A lanes 3 and 4). In contrast, IκBα-DN had little effect on Tax activation of the viral LTR (< 2-fold), a CREB-dependent activation pathway (Figure 1A lanes 5-6). Interestingly, an 8- to 9-fold increase in p53 transcriptional activity, measured on the p53 reporter PG13-Luc, was observed when the NF-κB pathway was blocked (Figure 1A lanes 1-2). The increase in p53 activity does not reflect an overall increase in transcription as a result of IκBα-DN expression, because the control reporter CMV-βgal showed no significant change in the presence or absence of the IκBα-DN construct (Figure 1B). Western blot analysis demonstrated that the levels of p53 and Tax were not significantly changed by expression of the IκBα-DN protein (Figure 1C). These results indicate that, consistent with results of transient transfection of T lymphocytes, activation of NF-κB is critical for p53 inhibition in HTLV-I–transformed cells.
IκBα-DN mutant restores p53 transcriptional activity in C81 cells. (A) The HTLV-I–transformed cell line C81 was cotransfected (Transfast; Promega) with 2 μg reporter (lanes 1-2, PG13-Luc; lanes 3-4, 4 × NF-κB–Luc; lanes 5-6, HTLV-I–LTR-Luc) and either control (lanes 1, 3, and 5) or 2 μg IκBα-DN (lanes 2, 4, and 6) DNA. Cells were harvested 24 hours after transfection, and luciferase activity was measured. Luciferase values were adjusted for transfection efficiency by using RSV-βgalactosidase. The values represent luciferase activity from 3 independent experiments. Error bars indicate SD. (B) Transfections were performed as for panel A, but the reporter construct CMV-βgalactosidase was used (CMV-βgal). Error bars indicate SD. (C) Western blot analysis for p53 and Tax levels from transfected cells using Ab6 (Oncogene Research Product) and Tab172, respectively, were performed.
IκBα-DN mutant restores p53 transcriptional activity in C81 cells. (A) The HTLV-I–transformed cell line C81 was cotransfected (Transfast; Promega) with 2 μg reporter (lanes 1-2, PG13-Luc; lanes 3-4, 4 × NF-κB–Luc; lanes 5-6, HTLV-I–LTR-Luc) and either control (lanes 1, 3, and 5) or 2 μg IκBα-DN (lanes 2, 4, and 6) DNA. Cells were harvested 24 hours after transfection, and luciferase activity was measured. Luciferase values were adjusted for transfection efficiency by using RSV-βgalactosidase. The values represent luciferase activity from 3 independent experiments. Error bars indicate SD. (B) Transfections were performed as for panel A, but the reporter construct CMV-βgalactosidase was used (CMV-βgal). Error bars indicate SD. (C) Western blot analysis for p53 and Tax levels from transfected cells using Ab6 (Oncogene Research Product) and Tab172, respectively, were performed.
Ability to inhibit p53 activity is specific to the Tax-induced NF-κB pathway
Jurkat cells were transfected with Tax or treated with PMA or TNF-α. Tax, PMA, and TNF-α all caused a significant increase in the level of NF-κB activity (Figure 2A). Tax and TNF-α induced NF-κB activity by approximately 30-fold. PMA increased the NF-κB reporter activity by approximately 100-fold. Indeed, Arima et al40 have shown by electrophoretic mobility shift and UV cross-linking experiments that similar NF-κB DNA-binding complexes are induced.
Tax, but not PMA or TNF-α, induction of NF-κB inhibits p53 activity. (A) Jurkat lymphocytes were transfected by using Superfect reagent (Qiagen) with 4 × NF-κB–Luc reporter plasmid and pcTax (2 μg) or control plasmid (pcDNA3, 2 μg). (B) p53 activity was measured by cotransfection of Jurkat cells with the p53-responsive MDM2-Luc reporter construct and p53 (0.4 μg) in the absence or presence of pcTax (2 μg). TNF-α (10 ng/mL) or PMA (10 nM) was added to cells 1 hour prior to transfection. Cells were harvested 24 hours after transfection, and luciferase activity was measured. Activity is represented as relative percentages from at least 3 independent experiments. Values in panel A are expressed as percentage of activity in which Tax activity is set at 100%. Measurements in panel B are expressed as percentage of repression whereby the control is set at 1. Error bars indicate SD.
Tax, but not PMA or TNF-α, induction of NF-κB inhibits p53 activity. (A) Jurkat lymphocytes were transfected by using Superfect reagent (Qiagen) with 4 × NF-κB–Luc reporter plasmid and pcTax (2 μg) or control plasmid (pcDNA3, 2 μg). (B) p53 activity was measured by cotransfection of Jurkat cells with the p53-responsive MDM2-Luc reporter construct and p53 (0.4 μg) in the absence or presence of pcTax (2 μg). TNF-α (10 ng/mL) or PMA (10 nM) was added to cells 1 hour prior to transfection. Cells were harvested 24 hours after transfection, and luciferase activity was measured. Activity is represented as relative percentages from at least 3 independent experiments. Values in panel A are expressed as percentage of activity in which Tax activity is set at 100%. Measurements in panel B are expressed as percentage of repression whereby the control is set at 1. Error bars indicate SD.
Interestingly, although Tax, PMA, and TNF-α all efficiently induced NF-κB, only Tax was able to inhibit p53 activity (Figure 2B). This finding suggests that NF-κB activation alone is not sufficient to inhibit p53 activity. Indeed, previous reports suggest that TNF-α treatment does not induce p53 phosphorylation on serine 15.41 Combined with previous results about the importance of the NF-κB pathway in Tax-mediated p53 inhibition, these results suggest that something specific to the Tax NF-κB activation pathway is essential to p53 inhibition.
Comparison of the ability of Tax to inhibit p53 activity in p50–/–, p65–/–, NIK–/–, and WT MEFs
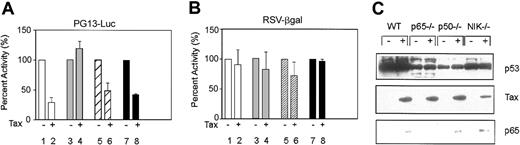
We have previously shown that the ability of Tax to inhibit p53 transcription function is impaired in p65–/– MEFs.37 To determine whether other components of NF-κB were required for Tax inhibition activity, we tested additional knock-out MEFs p50–/– or NIK–/–, which have been characterized previously.42-44 These cells, along with WT MEFs and the control p65–/– cells, were transfected with the p53 reporter plasmid PG13-Luc in the presence or absence of Tax. Twenty-four hours after transfection, cells were harvested, and p53 activity was determined. Consistent with previous results, Tax was unable to inhibit p53 activity in the absence of p65 (Figure 3A lanes 3-4). In contrast, Tax was found to inhibit p53 activity in WT, p50–/–, and NIK–/– cells (Figure 3A lanes 1-2 and 5-8). The control reporter, RSV-βgal showed no significant change in the presence or absence of Tax (Figure 3B). To ensure that the difference in Tax inhibition was not due to differences in protein expression, Western blot analysis for p53 and Tax was performed. The results presented in Figure 3C demonstrate that similar levels of Tax were expressed in the transfected cells. The p65–/– and p50–/– cells have lower levels of p53 than WT or NIK–/– cells, consistent with the studies of Wu and Lozano45 that showed NF-κB stimulates p53 expression in mouse cells. In addition, Tax induced p65 nuclear translocation in p50–/–, NIK–/–, and WT MEFs. These results suggest that p65 is of unique importance in p53 inhibition by Tax.
p53 Activity in MEF cells. (A) Mouse embryo fibroblasts (WT, □; p65–/–, ▦ ; p50–/–, ▨ ; and NIK–/–, ▪) were transfected with use of Effectene reagent (Qiagen) to measure p53 activity on the reporter construct PG13-Luc (1.5 μg) in the absence (–; lanes 1, 3, 5, and 7) or presence (+; lanes 2, 4, 6, and 8) of Tax (0.3 μg). The RSV-βgalactosidase (RSV-βgal, 0.2 μg) construct was included to control for transfection efficiency. The amount of p53 activity in the absence of Tax was set at 100%. The relative amounts represent averages of at least 2 independent experiments. Error bars indicate SD. (B) Transfections were performed as described in panel A. Values for the RSV-βgal are given as percentage of activity. Error bars indicate SD. (C) Western blot analyses of nuclear extracts indicate the expression level of endogenous p53 (top panel, Ab7; Oncogene Research Product), transfected Tax (middle panel, Tab172), or p65 (lower panel; Santa Cruz Biotechnology) as visualized by chemiluminescence.
p53 Activity in MEF cells. (A) Mouse embryo fibroblasts (WT, □; p65–/–, ▦ ; p50–/–, ▨ ; and NIK–/–, ▪) were transfected with use of Effectene reagent (Qiagen) to measure p53 activity on the reporter construct PG13-Luc (1.5 μg) in the absence (–; lanes 1, 3, 5, and 7) or presence (+; lanes 2, 4, 6, and 8) of Tax (0.3 μg). The RSV-βgalactosidase (RSV-βgal, 0.2 μg) construct was included to control for transfection efficiency. The amount of p53 activity in the absence of Tax was set at 100%. The relative amounts represent averages of at least 2 independent experiments. Error bars indicate SD. (B) Transfections were performed as described in panel A. Values for the RSV-βgal are given as percentage of activity. Error bars indicate SD. (C) Western blot analyses of nuclear extracts indicate the expression level of endogenous p53 (top panel, Ab7; Oncogene Research Product), transfected Tax (middle panel, Tab172), or p65 (lower panel; Santa Cruz Biotechnology) as visualized by chemiluminescence.
Antisense oligonucleotides specifically interfere with the ability of Tax to inhibit p53
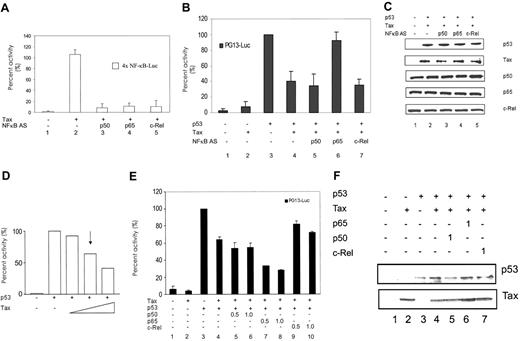
Another approach used to determine which subunit of NF-κB was important for Tax inhibition of p53 activity was to inhibit the individual p50, p65, and c-Rel subunits by using ASOs. Jurkat cells were transfected with Tax and p53 in the presence or absence of ASOs to p50, p65, and c-Rel. Twenty-four hours after transfection, cell extracts were prepared and analyzed for NF-κB and p53 activity. The results presented in Figure 4A demonstrate that the p50, p65, and c-Rel ASOs inhibited Tax-mediated NF-κB activity in the Jurkat cells. To note, because of transfection efficiency we do not see a decrease in p65, p50, or c-Rel proteins in response to ASOs, but a 90% inhibition of NF-κB activity was observed. In contrast, only the p65 ASO inhibited the ability of Tax to negatively regulate p53 activity (Figure 4B). In the absence of p53, Tax did not significantly affect reporter activity, because experimental variation fell within the error bars (Figure 4B lanes 1-2). As demonstrated previously, Tax inhibited p53 transcriptional activity (Figure 4B lanes 3-4). Tax-mediated p53 inhibition was maintained in the presence of both p50 and c-Rel ASOs. However, less than 5% inhibition was observed in the presence of p65 ASOs. Western blot analysis demonstrated that the cells contained equivalent levels of p53 and Tax (Figure 4C). These results provide further evidence that p65 is uniquely involved in the Tax inhibition pathway.
Antisense oligonucleotides to p65, but not p50 or c-Rel, abrogate Tax inhibition of p53. Jurkat lymphocytes were transfected with use of Superfect reagent with 4 × NF-κB–Luc (A) or PG13-Luc (B) and Tax (+; 2 μg) and p53 (+; 0.4 μg) in the presence or absence of the indicated antisense oligonucleotide (AS; 100 nM). Cells were harvested 24 hours after transfection, and activity was measured. Results represent an average of at least 3 independent experiments. Error bars indicate SD. (C) The expression levels of p53, Tax, p50, p65, and c-Rel are shown. (D) A titration of Tax plasmid was performed in Jurkat cells. Cells were transiently transfected with PG13-Luc reporter plasmid in the absence or presence of p53 plasmid. Increasing amounts of Tax plasmid (0.5, 1.0, 2.0 μg) were cotransfected, and the luciferase activity was measured. The arrow indicates the concentration of Tax plasmid used in other Jurkat transfections. (E) Jurkat cells were transiently cotransfected with PG13-Luc reporter construct, p53 (+), and Tax (+) in the absence (–) or presence of increasing amounts of p50, p65, or c-Rel (0.5 μgor1.0 μg). Cells were harvested 24 hours after transfection, and luciferase activity was measured. Activity is indicated as percentage of p53 activity and represents results from at least 3 independent experiments. Error bars indicate SD. (F) Western blot analysis indicates the protein expression levels for Tax (Tab172) and p53 (DO-1).
Antisense oligonucleotides to p65, but not p50 or c-Rel, abrogate Tax inhibition of p53. Jurkat lymphocytes were transfected with use of Superfect reagent with 4 × NF-κB–Luc (A) or PG13-Luc (B) and Tax (+; 2 μg) and p53 (+; 0.4 μg) in the presence or absence of the indicated antisense oligonucleotide (AS; 100 nM). Cells were harvested 24 hours after transfection, and activity was measured. Results represent an average of at least 3 independent experiments. Error bars indicate SD. (C) The expression levels of p53, Tax, p50, p65, and c-Rel are shown. (D) A titration of Tax plasmid was performed in Jurkat cells. Cells were transiently transfected with PG13-Luc reporter plasmid in the absence or presence of p53 plasmid. Increasing amounts of Tax plasmid (0.5, 1.0, 2.0 μg) were cotransfected, and the luciferase activity was measured. The arrow indicates the concentration of Tax plasmid used in other Jurkat transfections. (E) Jurkat cells were transiently cotransfected with PG13-Luc reporter construct, p53 (+), and Tax (+) in the absence (–) or presence of increasing amounts of p50, p65, or c-Rel (0.5 μgor1.0 μg). Cells were harvested 24 hours after transfection, and luciferase activity was measured. Activity is indicated as percentage of p53 activity and represents results from at least 3 independent experiments. Error bars indicate SD. (F) Western blot analysis indicates the protein expression levels for Tax (Tab172) and p53 (DO-1).
Overexpression of p65 facilitates Tax inhibition of p53 function
From the above-mentioned studies, it was predicted that overexpression of p65 might facilitate Tax inhibition of p53 function. To test this hypothesis, Tax was transfected with p53 and either p50, p65, or c-Rel. A titration of Tax plasmid was performed to determine the concentration at which cooperation between Tax and NF-κB subunits could be detected (Figure 4D). An arrow indicates the concentration of Tax plasmid used in subsequent transfections. The results of these studies demonstrate that the addition of exogenous p65 increased the level of p53 inhibition in the presence of Tax protein an additional 30% (Figure 4E lane 8). In contrast, overexpression of p50 and c-Rel did not facilitate Tax inhibition of p53 (Figure 4E, lanes 5, 6, 9, and 10). These results provide further evidence that p65 uniquely facilitates Tax-mediated p53 inhibition. The levels of Tax and p53 proteins are shown (Figure 4E lower panel).
Interaction of p53 and p65 in HTLV-I–transformed cells
Several groups have shown that Tax does not directly interact with p53.35,36,46 However, one mechanism for p53 inhibition could be through direct interaction of p65 with p53. Nuclear extracts prepared from control or HTLV-I–infected cells were immunoprecipitated with either control IgG or anti-p53 antibody. Our results demonstrate the presence of the p53-p65 complex in all HTLV-I cell lines tested, including MT2, C81, and Hut102 cells (Figure 5A). p65 was detected in the anti-p53, but not control IgG, immunoprecipitates from the HTLV-I–transformed cell lines. The p53-p65 complex was not detected in control Molt4 and p53-negative Jurkat lymphocytes, or U937 cells that contained a similar level of p65 protein, demonstrating that the p53 antibody did not nonspecifically immunoprecipitate p65 (Figure 5A lower panel).
p53 And p65 interact in HTLV-I–transformed cells. (A) Nuclear extracts were prepared from a number of lymphocytic cell lines as indicated and p53 immunocomplexes were isolated. Western blot analysis was then performed to assess the level of associated p65 protein (top panel). IgG antibody was used as a control for specificity. The middle panel indicates the amount of p53 immunoprecipitated. The lower panel indicates the amount of p65 in each extract. (B) Nuclear extracts from HTLV-I–infected cells C81, Hut102, and MT2 as well as the control cell line Molt4 were immunopreciptiated with anti-p53 sera and washed extensively, and proteins were separated on 10% Tris-glycine gels. Western blot analyses for p65, p50, c-Rel, and p53 were performed. (C) Western blot analysis of nuclear extracts (25 μg) from cells used in panel B indicates the level of expression of p65, p50, c-Rel, p53, and Tax. (D) Nuclear extracts from Molt4 cells 4 hours after treatment with 8 Gy γ-irradiation, MG132 (50 μM), or untreated or from MT2 cells were prepared, and immunocomplexes were isolated with control IgG or p53 (Ab6) antibodies and separated on 10% Tris-glycine gels (Invitrogen). Proteins were transferred to PVDF membranes, and p65 levels were visualized (top panel) with anti-p65(A) (Santa Cruz Biotechnology). The lower panel indicates the amount of p53 immunoprecipitated (Ab7; Oncogene Research Product).
p53 And p65 interact in HTLV-I–transformed cells. (A) Nuclear extracts were prepared from a number of lymphocytic cell lines as indicated and p53 immunocomplexes were isolated. Western blot analysis was then performed to assess the level of associated p65 protein (top panel). IgG antibody was used as a control for specificity. The middle panel indicates the amount of p53 immunoprecipitated. The lower panel indicates the amount of p65 in each extract. (B) Nuclear extracts from HTLV-I–infected cells C81, Hut102, and MT2 as well as the control cell line Molt4 were immunopreciptiated with anti-p53 sera and washed extensively, and proteins were separated on 10% Tris-glycine gels. Western blot analyses for p65, p50, c-Rel, and p53 were performed. (C) Western blot analysis of nuclear extracts (25 μg) from cells used in panel B indicates the level of expression of p65, p50, c-Rel, p53, and Tax. (D) Nuclear extracts from Molt4 cells 4 hours after treatment with 8 Gy γ-irradiation, MG132 (50 μM), or untreated or from MT2 cells were prepared, and immunocomplexes were isolated with control IgG or p53 (Ab6) antibodies and separated on 10% Tris-glycine gels (Invitrogen). Proteins were transferred to PVDF membranes, and p65 levels were visualized (top panel) with anti-p65(A) (Santa Cruz Biotechnology). The lower panel indicates the amount of p53 immunoprecipitated (Ab7; Oncogene Research Product).
To determine whether other NF-κB family members were present in the p53-p65 complex, p53 protein was immunoprecipitated from C81, Hut102, and MT2 cells as well as the control cells Molt4. Western blot analysis was performed to detect the presence of p65, p50, or c-Rel in the p53 complex (Figure 5B). As seen earlier (Figure 5A), p65 specifically associated with p53 in the HTLV-I cells. Interestingly, significant levels of c-Rel and p50 were not found in p53 complexes. These results are consistent with antisense experiments in which p50 and c-Rel did not appear to play a role in Tax-mediated p53 inhibition.
p53 is stabilized in HTLV-I cells. To determine whether stabilization played a role in p53-p65 interaction, Molt4 cells, which contain a wild-type p53 protein, were treated with the proteasome inhibitor, MG132 or 8 Gy ionizing radiation, and nuclear extracts were prepared 4 hours after treatment. MG132 will stabilize p53 without inducing posttranslational modifications, whereas ionizing radiation induces stabilization and transcriptional activation of p53 by way of extensive posttranslational modifications of N- and C-terminal residues. Although equivalent amounts of p53 protein were immunoprecipitated from all extracts, a p65-p53 complex was observed only in MT2 cells (Figure 5D). Immunoprecipitation of extracts with an antibody specific for p65 also demonstrated an interaction of p53 and p65 in MT2 cells (Figure 5D). Of interest, a small amount of p53-p65 complex was detected in γ-irradiated Molt4 cells (Figure 5D).
Interaction of p53 and p65 correlates with inhibition of p53 activity in the presence of Tax
To test what domain of p65 was important for Tax-mediated p53 inhibition, transfection studies were carried out with the p65 deletion mutants in p65–/– cells (Figure 6A). Tax was able to inhibit p53 in the presence of WT p65 or a deletion mutant containing amino acids 1 to 521 (Figure 6B). In contrast, Tax did not inhibit p53 in the presence of a p65 deletion mutant containing amino acids 1 to 305. These results indicate that the region from 305 to 521 is important for Tax-mediated p53 inhibition.
The domain of p65 between amino acids 305 to 521 is important for Tax-mediated p53 inhibition. (A) A schematic diagram of the p65 constructs used in transfection studies in p65–/– cells is shown. (B) p65–/– cells were transiently cotransfected with use of Effectene reagent (Qiagen) with the PG13-Luc reporter construct and control (con), wild-type p65 (WT, 0.1 μg), or p65 deletion constructs 521 or 305 in the absence (□) or presence (▪) of Tax (0.3 μg). A representative experiment is shown in which the p53 activity in the absence of Tax is set at 100%. The RLTK-Luc construct was included in the transfections to equilibrate for transfection efficiency. (C) Protein expression levels of the p65 constructs were determined by Western blot analysis (p65 Ab2; Oncogene Research Product). (D) p65–/– cells were transfected (Lipofectamine plus reagent; Invitrogen) with WT, 521, or 305 p65 constructs or control DNA in the presence (+) or absence (–) of Tax. Nuclear extracts were prepared, and p65 complexes were isolated with use of anti-p65 antibody (Ab2; Oncogene Research Product). Immunocomplexes were separated on 8% Tris-gylcine gels and transferred to PVDF membranes, and the levels of associated p53 protein were visualized by chemiluminescence with an anti-p53 antibody (Ab1; Oncogene Research Product). (E) Western blot analysis was performed on 50 μg nuclear extract from transfected p65–/– cells to determine the levels of Tax and p53. (F) Wild-type (WT; 0.4 μg) or serine-to-alanine mutants (Ser15Ala, Ser37Ala, Ser392Ala, Ser15, 392Ala, or Ser15, 37Ala) of p53 were cotransfected in the absence (–) or presence (+) of Tax (2 μg) and the PG13-Luc reporter (1 μg) in p53–/–, MDM2–/– MEF cells. Luciferase activity was measured 24 hours after transfection. The graph indicates percentage of activity whereby the p53 activity for each construct was set to 100%. (G) Nuclear extracts were prepared 48 hours after transfection. Immunocomplexes were isolated from 0.5 mg nuclear extract with use of p53 (Ab6; Oncogene Research Product) antibody, separated on 10% Tris-glycine gels (Invitrogen), and transferred to PVDF membranes (Immobilon), and p65 was visualized with anti-p65 antisera (CT; Upstate). The lower panel indicates the amount of p53 immunoprecipitated from each extract (Ab7; Oncogene Research Product).
The domain of p65 between amino acids 305 to 521 is important for Tax-mediated p53 inhibition. (A) A schematic diagram of the p65 constructs used in transfection studies in p65–/– cells is shown. (B) p65–/– cells were transiently cotransfected with use of Effectene reagent (Qiagen) with the PG13-Luc reporter construct and control (con), wild-type p65 (WT, 0.1 μg), or p65 deletion constructs 521 or 305 in the absence (□) or presence (▪) of Tax (0.3 μg). A representative experiment is shown in which the p53 activity in the absence of Tax is set at 100%. The RLTK-Luc construct was included in the transfections to equilibrate for transfection efficiency. (C) Protein expression levels of the p65 constructs were determined by Western blot analysis (p65 Ab2; Oncogene Research Product). (D) p65–/– cells were transfected (Lipofectamine plus reagent; Invitrogen) with WT, 521, or 305 p65 constructs or control DNA in the presence (+) or absence (–) of Tax. Nuclear extracts were prepared, and p65 complexes were isolated with use of anti-p65 antibody (Ab2; Oncogene Research Product). Immunocomplexes were separated on 8% Tris-gylcine gels and transferred to PVDF membranes, and the levels of associated p53 protein were visualized by chemiluminescence with an anti-p53 antibody (Ab1; Oncogene Research Product). (E) Western blot analysis was performed on 50 μg nuclear extract from transfected p65–/– cells to determine the levels of Tax and p53. (F) Wild-type (WT; 0.4 μg) or serine-to-alanine mutants (Ser15Ala, Ser37Ala, Ser392Ala, Ser15, 392Ala, or Ser15, 37Ala) of p53 were cotransfected in the absence (–) or presence (+) of Tax (2 μg) and the PG13-Luc reporter (1 μg) in p53–/–, MDM2–/– MEF cells. Luciferase activity was measured 24 hours after transfection. The graph indicates percentage of activity whereby the p53 activity for each construct was set to 100%. (G) Nuclear extracts were prepared 48 hours after transfection. Immunocomplexes were isolated from 0.5 mg nuclear extract with use of p53 (Ab6; Oncogene Research Product) antibody, separated on 10% Tris-glycine gels (Invitrogen), and transferred to PVDF membranes (Immobilon), and p65 was visualized with anti-p65 antisera (CT; Upstate). The lower panel indicates the amount of p53 immunoprecipitated from each extract (Ab7; Oncogene Research Product).
To determine whether there was a correlation between p53 inhibition and p53-p65 interaction, coimmunoprecipitation experiments were done in p65–/– MEFs by using p65 WT and deletion mutants. These results demonstrate that Tax induces the interaction of p53 and p65, because the complex was seen only in the presence of Tax (Figure 6D, compare lanes 3 and 4). Deletion mutants of p65 mapped the interaction site with p53 to amino acids 305 to 521 of p65 and correlated with Tax-mediated p53 inhibition. Although p53 interacted with both full-length and a deletion mutant containing the N-terminal 521 amino acids, no interaction was seen with the p65 deletion mutant containing only the first 305 amino acids (Figure 6D). Equivalent amounts of p53, Tax, and p65 were present in the transfected cells (Figure 6C,E).
We have also tested the ability of specific p53 phosphorylation site mutants to interact with p65. In previous experiments, we have shown that p53 mutation at serines (Ser) 15 and 392 to alanines (Ala) abrogated the ability of Tax to inhibit p53 activity.37 Interestingly, mutation of either Ser15 or Ser392 alone as well as the double mutation at Ser15 and 37 (Ser15, 37Ala) did not affect Tax inhibition (Figure 6F). When a series of p53 mutants were analyzed for their ability to interact with p65, we found that WT, Ser15Ala, Ser37Ala, and Ser392Ala p53 proteins all interact with p65 in the presence of Tax protein (Figure 6G). Of importance, the p53 mutant that was not inhibited by Tax also did not interact with p65 (Figure 6G lane 7). Thus, with use of p65 deletion mutants and p53 site-specific mutants, we observe a complete correlation between the binding of p53 and p65 and Tax-mediated p53 inhibition.
The p53-p65 complex is associated with the inactive, p53-responsive MDM2 promoter in HTLV-I–transformed cells
It was of importance at this point to demonstrate that the p53-p65 complex was actually associated with an inactive p53-responsive promoter. For these studies, we chose to use the well-characterized MDM2 promoter. We assayed MDM2 expression in Hut102 and γ-irradiated Molt4 cells. RNA was isolated, and the relative mRNA expression levels were analyzed by RT-PCR (Figure 7). The results of these studies confirm that MDM2 transcription in the HTLV-I cells is low and is not induced by γ-irradiation, consistent with the presence of an inactive p53 protein (Figure 7A lanes 1-2). In contrast, although the level of MDM2 expression was low in untreated Molt4 cells, it was induced by γ-irradiation (Figure 7A lanes 3-4).
p65-p53 complexes are associated with the inactive MDM2 promoter in HTLV-I–transformed cells. Following treatment of Hut102 and Molt4 cells with 8 Gy ionizing radiation (IR), total RNA (A) was extracted from time 0 (–) or 4 hours after irradiation (+) with use of RNA-Bee (TelTest) as described by the manufacturer. Total RNA (2 μg) was then subjected to reverse transcription and PCR, as described in “Materials and methods,” for amplification of MDM2 or GAPDH mRNA. Reactions were run on 2% agarose gels to visualize the products. (B) Chromatin immunoprecipitation (ChIP) assays were performed on untreated Hut102 cells or Molt4 cells treated with 8 Gy ioinizing radiation (Molt4-IR) with use of the indicated antibodies. PCR amplification was performed with primers specific for the MDM2 promoter. (C) ChIP assays were performed on Hut102 cells to determine the association of p65 and p53 on the MDM2 promoter. Samples were immunoprecipitated with either control IgG sera or anti-p53 or anti-p65 sera (lanes 2, 3, and 5, respectively). Supernatants from anti-p53 and anti-p65 precipitations were then subjected to a second round of precipitation with the anti-p65 or anti-p53 sera, respectively (lanes 4 and 6). DNA was then PCR amplified with primers specific for the MDM2 promoter.
p65-p53 complexes are associated with the inactive MDM2 promoter in HTLV-I–transformed cells. Following treatment of Hut102 and Molt4 cells with 8 Gy ionizing radiation (IR), total RNA (A) was extracted from time 0 (–) or 4 hours after irradiation (+) with use of RNA-Bee (TelTest) as described by the manufacturer. Total RNA (2 μg) was then subjected to reverse transcription and PCR, as described in “Materials and methods,” for amplification of MDM2 or GAPDH mRNA. Reactions were run on 2% agarose gels to visualize the products. (B) Chromatin immunoprecipitation (ChIP) assays were performed on untreated Hut102 cells or Molt4 cells treated with 8 Gy ioinizing radiation (Molt4-IR) with use of the indicated antibodies. PCR amplification was performed with primers specific for the MDM2 promoter. (C) ChIP assays were performed on Hut102 cells to determine the association of p65 and p53 on the MDM2 promoter. Samples were immunoprecipitated with either control IgG sera or anti-p53 or anti-p65 sera (lanes 2, 3, and 5, respectively). Supernatants from anti-p53 and anti-p65 precipitations were then subjected to a second round of precipitation with the anti-p65 or anti-p53 sera, respectively (lanes 4 and 6). DNA was then PCR amplified with primers specific for the MDM2 promoter.
To determine whether p65-p53 complexes were present at p53-responsive promoters, proteins were cross-linked to DNA with the use of formaldehyde. Following chromatin fragmentation, ChIP assays were performed with use of antibodies against p53, p65, the basal transcription factor TATA binding protein (TBP) (TFIID) and polII. The inactive p53-responsive MDM2 promoter was specifically immunoprecipitated with anti-p53 and anti-p65 antibodies in Hut102 cells (Figure 7B). Of interest, TFIID and polII were not detected in the MDM2 promoter complex, suggesting that the p53-p65 complex is blocked in its ability to recruit TFIID and polII (Figure 7B lanes 3-4). As a control for these studies, we have also included a ChIP analysis of Molt4 cells that have been γ-irradiated to activate the p53-response pathway. The results of this analysis demonstrate that p53, TFIID, and polII are associated with the transcriptionally active MDM2 promoter in Molt4 cells (Figure 7B). No p65 is found associated with the active p53 transcription complex.
To determine whether p65 and p53 are associated in the same complex on the MDM2 promoter in Hut102 cells, a depletion experiment was performed (Figure 7C). Cross-linked and sonicated Hut102 extracts were immunoprecipitated with anti-p53 or anti-p65 sera; supernatants were harvested and then re-precipitated with either anti-p65 or anti-p53 sera, respectively. Although control IgG sera did not precipitate the MDM2 promoter, anti-p53 and anti-p65 sera specifically complexed with the promoter. The results indicate that p53 and p65 are associated with each other because depletion of either p53 or p65 significantly reduced association of the opposing protein with the promoter (compare Figure 7C lanes 3 and 5 with lanes 4 and 6).
Discussion
The tumor suppressor p53 is known to function in maintaining genomic integrity at both the G1/S and G2/M phases of the cell cycle.30,47-49 We and others have demonstrated that Tax inhibits p53 transcriptional activation function.35,50-52 Of interest, with use of a Tax transgenic mouse model, Portis et al53 suggest that this functional inhibition of p53 contributes to late-stage tumor progression. In this report we extend our previous findings that the ability of Tax to activate NF-κB was critical for p53 inhibition in lymphocytes and did not involve squelching of the coactivator p300.36 Importantly, we demonstrate here that the p65 subunit of NF-κB directly binds p53 in HTLV-1–transformed cells and this binding directly correlates with Tax-mediated p53 inhibition.
The NF-κB family of transcription factors contains 5 members whose regulation occurs at several levels, including upstream kinase activation, cytoplasmic-nuclear shuttling, and modulation of its transactivation function.54 Although antisense to c-Rel, p50, and p65 all reduced NF-κB activation, only expression of antisense to p65 allowed recovery of p53 transactivation. This finding indicates that p65 is critical for Tax-mediated p53 inhibition. Further, Tax-mediated activation of NF-κB is specific for p53 inhibition because NF-κB activation by PMA and TNF-α did not inhibit p53 transactivation function. Additionally, we find an association between p65 and p53 in the presence of Tax. It is important to note that, although we do not find a role for p50 or c-Rel in Tax-mediated p53 inhibition, we have not ruled out the possibility that NF-κB2 may be associated with the complex.
The domain of p65 from amino acids 305 to 521 is critical for Tax-mediated p53 inhibition and p53-p65 interaction. Thus, the interaction of p65 and p53 correlated with functional inactivation by Tax. An association between p65 and p53 had been reported previously.55 However, the Rel homology domain of p65 was implicated to be important for interaction with p53. These results suggest the interesting possibility that Tax could induce a novel interaction between p53 and p65.
In previous studies, we demonstrated that p53 existed as tetramers that could bind DNA in a sequence-specific manner.37 Further, p53 in the transformed cells failed to interact with the basal transcription factor TFIID, which correlated with phosphorylation of p53 at serine 15.37 Here, we find by ChIP assays that p53 is bound to the inactive p53-responsive MDM2 promoter. p65 is bound to p53 at the promoter specifically in HTLV-I–transformed cells and not in the γ-irradiated Molt4 cells in which p53 is active and the MDM2 gene is induced. Binding of the p53-p65 complex, along with the phosphorylation of p53 at serine 15 apparently abrogates recruitment of the basal transcription machinery to the promoter as demonstrated by reduced levels of TFIID on the inactive MDM2 promoter in Hut102 cells, resulting in blocked expression of p53-responsive genes.
Posttranslational modifications of p53 have been shown to play a major role in its regulation and association with other proteins.27,56,57 In transient transfection experiments of Jurkat cells or p53–/–, MDM2–/– MEFs, we found a strong link between the ability to phosphorylate p53 at serines 15 and 392 with the ability of Tax to inhibit p53 transactivation of both reporter constructs and endogenous genes.36 In addition, phosphorylation of p53 at serine 15 alone inhibits p53-TFIID binding.37,58 Thus, we are currently investigating what modifications of p53 and p65 affect their interaction and p53 inhibition in Tax-expressing cells.
It is interesting to speculate that re-activation of p53 in HTLV-I–infected cells would allow the protein to perform its role as a tumor suppressor. Indeed, we find that expression of the IκBα-DN restores p53 activity in HTLV-1–transformed cells. In addition, reports have shown that inhibition of NF-κB activation with Bay 11-7082 or arsenic trioxide treatment of HTLV-I–infected cells results in apoptosis.59,60 Although no change in p53 stability or phosphorylation was observed in those studies, we propose that because of decreased binding of p65 to p53, p53 may be functionally active in these cells. It cannot be ruled out at this point that additional events may be important for full activation of p53.
Given its importance in immune-cell function, it is not surprising that a link exists between NF-κB activation and human leukemia and lymphoma. Genetic alterations have been associated with a variety of B- and T-cell lymphomas, including chronic lymphocytic leukemia, multiple myeloma, and cutaneous B- and T-cell lymphomas. Constitutive NF-κB activation has also been associated with breast, ovarian, prostate, and colon cancers.61-70 In addition, multiple families of viruses, including HIV, HTLV-I, hepatitis B virus, hepatitis C, Epstein-Barr virus (EBV), and influenza, have been shown to activate NF-κB to promote viral replication, prevent virus-induced apoptosis, and mediate the immune response.9 It will be important to determine whether constitutive NF-κB activation, specifically p65 expression, is a general mechanism for inhibition of p53 activity.
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2003-12-4174.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal