Abstract
Violacein, a pigment isolated from Chromobacterium violaceum in the Amazon River, presents diverse biologic properties and attracts interest as a consequence of its antileukemic activity. Elucidation of the molecular mechanism mediating this activity will provide further relevant information for understanding its effects on the cellular physiology of untransformed cells and for considering its possible clinical application. Here, we show that violacein causes apoptosis in HL60 leukemic cells but is ineffective in this respect in other types of leukemia cells or in normal human lymphocytes and monocytes. Violacein cytotoxicity in HL60 cells was preceded by activation of caspase 8, transcription of nuclear factor κB (NF-κB) target genes, and p38 mitogen-activated protein (MAP) kinase activation. Thus, violacein effects resemble tumor necrosis factor α (TNF-α) signal transduction in these cells. Accordingly, infliximab, an antibody that antagonizes TNF-α–induced signaling abolished the biologic activity of violacein. Moreover, violacein directly activated TNF receptor 1 signaling, because a violacein-dependent association of TNF receptor-associated factor 2 (TRAF2) to this TNF receptor was observed in coimmunoprecipitation experiments. Hence, violacein represents the first member of a novel class of cytotoxic drugs mediating apoptosis of HL60 cells by way of the specific activation of TNF receptor 1.
Introduction
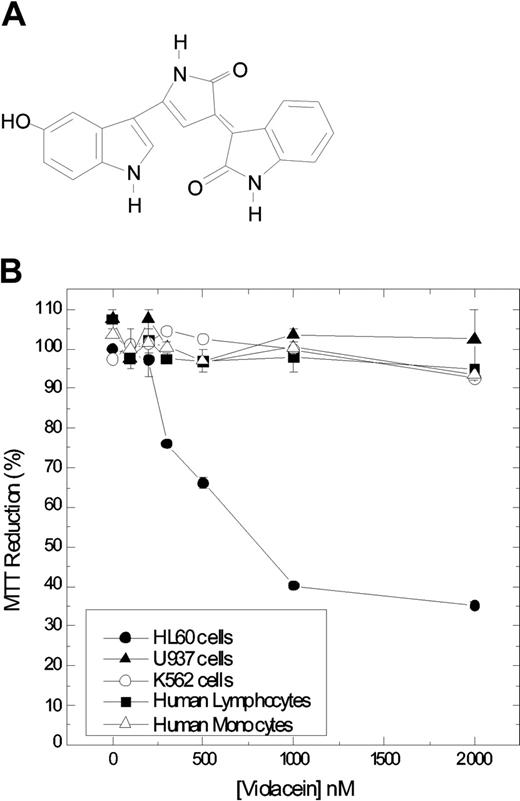
Violacein (Figure 1A), a purple-colored pigment produced by one of the strains of Chromobacterium violaceum found in the Amazon River, Brazil, is an indole derivative characterized as 3-(1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydene)-1,3-dihydro-2H-indol-2-one.1 The biosynthesis and biologic properties of this pigment have been extensively studied; in particular, its antitumoral, antibacterial, antiulcerogenic, antileishmanial, and antiviral activities are of interest.1-6 Its activity on acute myeloblastic leukemia (HL60) cells is of special interest because the compound effectively induces cell death in these cells.
Chemical structure of violacein and cytotoxicity in leukemic cell lines and normal human peripheral blood cells. Chemical structure of violacein (A). The cells were treated with different concentrations of violacein for 24 hours (B). In the absence of compounds, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) reduction was considered as 100%. The experiment was performed in a 24-well plate. Results represent the means ± standard error of 3 experiments run in triplicate (P < .05).
Chemical structure of violacein and cytotoxicity in leukemic cell lines and normal human peripheral blood cells. Chemical structure of violacein (A). The cells were treated with different concentrations of violacein for 24 hours (B). In the absence of compounds, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) reduction was considered as 100%. The experiment was performed in a 24-well plate. Results represent the means ± standard error of 3 experiments run in triplicate (P < .05).
The development of violacein as a possible novel therapeutic avenue for the treatment of leukemia, however, depends on the establishment of the molecular basis for this cytotoxicity. Evidently, a possible future application of violacein as a therapeutic agent critically depends on its ability to target leukemia cells more effectively than normal blood cells. Hence, research comparing the cytotoxicity of violacein in transformed cells and the corresponding untransformed ones is urgently called for. In addition, identification of the molecular details mediating violacein effects on leukemic cells will provide insight into the possible benefit of this compound in comparison to existing therapeutics.
The above-mentioned considerations prompted us to investigate the effects of violacein in HL60 cells. This cell line is generally accepted as a valid model for studying myeloid leukemia biology.7,8 Melo et al6 showed earlier that HL60 cells react to violacein with both increased cell death and diminished cellular proliferation. Hence, this cell type is attractive for studying the molecular mechanism of violacein, and we compared its effects to those observed in peripheral lymphocytes and monocytes of healthy donors and to its effects in cell types representing other types of leukemia. We used the histiocytic lymphoma cell line U937, which is less differentiated than HL609,10 and the K562 cell line which is representative of chronic myelogenic leukemia.11,12 The results show that violacein mediates its cytotoxic effects on HL60 cells by way of activation of tumor necrosis factor (TNF) receptor signaling and that this effect is specific to these cells because it is not observed in U937 or K562 cell lines or in untransformed cells. Hence, violacein represents the first member of a novel class of cytotoxic drugs, which mediate apoptosis by ways of the specific activation of TNF receptor 1 signal transduction.
Materials and methods
Cell line and reagents
HL60 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The polyclonal antibodies against antiphospho-p38 mitogen-activated protein kinase (MAPK), antiphospho-p42/44, antiphospho–c-jun NH2-terminal protein kinase (JNK), antiphospho–MAPK/ERK kinase 1 (MEK1), CREB (cyclic adenosine 5′-monophosphate [AMP] response element binding protein), antirabbit and antimouse peroxidase-conjugated antibodies were from Cell Signaling Technology (Beverly, MA). Antibodies against c-Jun, nuclear factor κB (NF-κB) p50 and p65, caspase 8, caspase 3, p21, signal transducer and activator of transcription 1 (STAT1) and 2, inhibitor of apoptosis 1 (IAP1), TNF receptor 1 (TNFR1), TNF receptor-associated factor 1 (TRAF1) and 2, TNF-α receptor-associated death domain (TRADD) and TNFR1 were from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal antibody against the p85 fragment of poly(adenosine 5′-diphosphate [ADP]–ribose) polymerase (PARP) was from Promega (Madison, WI). TNF-α was obtained from Biosource (Etten-Leur, The Netherlands). Infliximab was obtained from Centocor (Leiden, The Netherlands). Violacein was extracted from C violaceum (CCT 3496) and purified according to Rettori and Durán.2
Cell culture
HL60, U937, and K562 cells were routinely grown in suspension in RPMI 1640 medium containing glutamine (0.200 g/L), antibiotics (penicillin 100 IU/mL; streptomycin 100 μg/mL) and supplemented with 10% heat-inactivated fetal bovine serum, in a 5% CO2 humidified atmosphere at 37° C. In all experiments 3 × 105 cells/mL were seeded, and after 72 hours the cells were treated with violacein for 24 hours. Violacein dissolved in dimethyl sulfoxide (DMSO) was added to the culture medium and adjusted to a final DMSO concentration of 0.1% (vol/vol).
Lymphocytes and monocytes cultures
The blood was collected from healthy donors, and lymphocytes and monocytes were isolated by Ficoll/Hypaque gradient centrifugation. Lymphocytes were cultured at the same conditions described for HL60 cells, the only difference was the addition of 5 μg/mL phytohemagglutinin in each well. Cells were plated at density of 1 × 106 plating/mL in a 24-well plate. The medium was removed 48 hours after cell seeding and replaced with medium containing violacein. Monocytes were isolated from peripheral blood mononuclear cells by an adhesion step. After 4 hours of plating the cells were washed 5 times with RPMI medium and treated with violacein for 24 hours.
Cell viability
Cell viability was assessed by the trypan blue dye exclusion and the MTT reduction assays as previously reported.13
Western blotting
Cells (3 × 107) were lysed in 200 μL cell lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 7.4], 1% Tween 20, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA (ethylene glycol tetraacetic acid), 1 mM O-Vanadate, 1 mM NaF, and protease inhibitors [1 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM 4-(2-amino-ethyl)-benzolsulfonyl-fluorid-hydrochloride]) for 2 hours on ice. Protein extracts were cleared by centrifugation, and the protein concentration was determined using Lowry method.14 An equal volume of 2 × sodium dodecyl sulfide (SDS) gel loading buffer (100 mM Tris-HCl [pH 6.8], 200 mM dithiothreitol [DTT], 4% SDS, 0.1% bromophenol blue, and 20% glycerol) was added to samples and boiled for 10 minutes. Cell extracts, corresponding to 3 × 105 cells, were resolved by SDS-polyacrylamide gel (12%) electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 1% fat-free dried milk or bovine serum albumin (2%) in Tris-buffered saline (TBS)–Tween 20 (0.05%) and incubated overnight at 4° C with appropriate primary antibody at 1:1000 dilution. After washing in TBS-Tween 20 (0.05%), membranes were incubated with antirabbit or antimouse horseradish peroxidase–conjugated secondary antibodies, at 1:2000 dilutions (in all Western blotting assays), in blocking buffer for 1 hour. Detection was performed by using enhanced chemiluminescence (ECL).
NF-κB translocation
Briefly, 2 × 107 cells were harvested and washed twice with ice-cold phosphate-buffered saline (PBS) and resuspended in 0.2 mL ice-cold cell extract buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]–KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KC1, 0.5 mM DTT, and 0.2 mM phenylmethysulfonyl fluoride [PMSF]). The cells were kept on ice for 10 minutes to allow them to swell, mixed by vortex for 10 seconds, and microfuged at 4° C at 14000g for 30 seconds. The supernatant was discarded, and the pellet was resuspended in 30 μL nuclear extraction buffer (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgC12, 0.2 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM DTT, and 0.2 mM PMSF), placed on ice for 20 minutes, and centrifuged at 4° C at 14000g for 2 minutes. The supernatant was saved as the nuclear extract and used in Western blotting assay.
Immunoprecipitation of TNFR1
After treatment of the cells with 1000 nM violacein for 24 hours, whole-cell lysates were prepared with lysis buffer (20 mM HEPES, pH 7.7; 2.5 mM MgCl2, 0.1 mM EDTA, 1% Nonidet-P40 [NP40], 20 mM p-nitrophenylphosphate, 1 mM O-Vanadate, 1 mM 4-(2-amino-ethyl)-benzolsulfonyl-fluorid-hydrochloride), 1 mM DTT, 10 μg/mL aprotinin, and 10 μg/mL leupeptin) and chilled on ice for 2 hours. After centrifugation, lysates were incubated overnight with anti-TNFR1 at 4° C and then rotated with Protein A-Sepharose at 4° C for 2 hours. The beads were washed 3 times with lysing buffer and twice with PBS. Next, the immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF membrane as described in “Western blotting.”
TNF-α assay
Cytokine level was measured in the cell culture supernatants by using enzyme-linked immunosorbent assay (ELISA) kits from Biosource.
Annexin V and 7-amino-actinomycin D assays
Control and violacein-treated cells were collected and resuspended in 1 × binding buffer (0.01 M HEPES/NaOH, pH 7.4, 0.14 mM NaCl, and 2.5 mM CaCl2) at a concentration of 1 × 106 cells/mL. Subsequently, 100 μL cell suspension was transferred to a 5-mL tube, and Annexin V fluorescein isothiocyanate (FITC; 5 μL) and 7-amino-actinomycin D (7-AAD; 10 μL) were added. The cells were incubated at room temperature for 15 minutes, after which 400 μL 1 × binding buffer was added, and apoptosis was analyzed by flow cytometry.
Statistical evaluation
The Western blots represent 3 independent experiments. Cell viability and TNF quantification data were expressed as the means ± standard errors of 3 independent experiments run in triplicate. Data for each assay were analyzed statistically by analysis of variance (ANOVA).
Results
Violacein-dependent cytotoxicity is specific to HL60 cells
To establish the specificity of violacein for leukemic cells as compared with untransformed cells, we determined in parallel the effects of violacein on cell viability for HL60, U937, and K562 cells and for human peripheral blood cells (lymphocytes and monocytes) using a MTT assay. In agreement with Melo et al,6 who observed earlier that violacein is proapoptotic for HL60 cells, we established that after 24 hours of incubation violacein strongly reduced mitochondrial activity in this cell type, displaying an IC50 (concentration that inhibits 50%) value around 700 nM (Figure 1B). Effects of violacein on untransformed cells and on U937 and K562 leukemia cells were much less pronounced. Thus, the cytotoxicity effects of violacein are more specific for HL60 cells in this assay.
Effect of violacein on MAPKs phosphorylation in HL60 cells
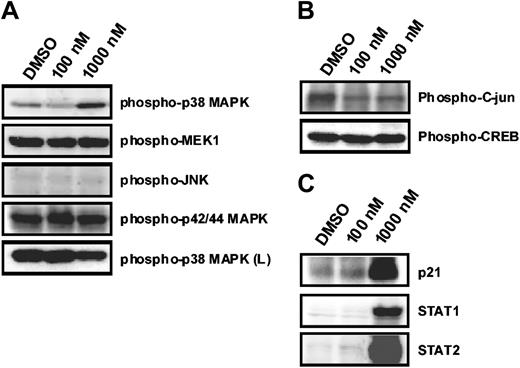
To obtain more insight into the molecular mechanisms mediating violacein effects on HL60 cells, we examined the phosphorylation state of a panel of signal transduction mediators in response to violacein in concentrations up to 1000 nM. The phosphorylation state remained unchanged of p42/p44 MAP kinase and its upstream activator, the mitogen-activated protein kinase 1, of JNK and its substrate c-jun as well as of cyclic adenosine 5′-monophosphate (AMP) response element binding protein (CREB) (Figure 2A-B). Thus, these elements are likely not involved in violacein cytotoxicity to HL60 cells. In contrast, p38 MAP kinase phosphorylation was strongly up-regulated in response to violacein (Figure 2A). This indicates that violacein action in HL60 cells is accompanied by the induction of a specific set of signal transduction events.
Effects of violacein on MAPKs phosphorylation and on p21, STAT1, and STAT2 expression in HL60. Cells were treated with violacein (100 and 1000 nM), and the phosphorylation of MAPKs (A); c-jun and CREB (B); and p21, STAT1, and STAT2 expression (C) were evaluated by immunoblotting. Soluble lysates were matched for protein content and analyzed on Western blot. The phosphorylation of p38 was also analyzed in human lymphocytes (L). One representative immunoblot of 3 independent experiments is presented.
Effects of violacein on MAPKs phosphorylation and on p21, STAT1, and STAT2 expression in HL60. Cells were treated with violacein (100 and 1000 nM), and the phosphorylation of MAPKs (A); c-jun and CREB (B); and p21, STAT1, and STAT2 expression (C) were evaluated by immunoblotting. Soluble lysates were matched for protein content and analyzed on Western blot. The phosphorylation of p38 was also analyzed in human lymphocytes (L). One representative immunoblot of 3 independent experiments is presented.
Up-regulation of p21, STAT1, and STAT2 by violacein
The effects of violacein on the expression of a selected set of proteins were investigated by Western blotting. In accordance with the described cell cycle arrest induced by violacein, we observed a marked up-regulation of p21 protein expression in HL60 cells after 24 hours of incubation (Figure 2C). Concomitantly, c-jun levels slightly decreased, but expression of signal transducers and activators of transcription (STAT1 and STAT2) was augmented (Figure 2B-C).
Violacein effects on the NF-κB pathway in HL60 cells
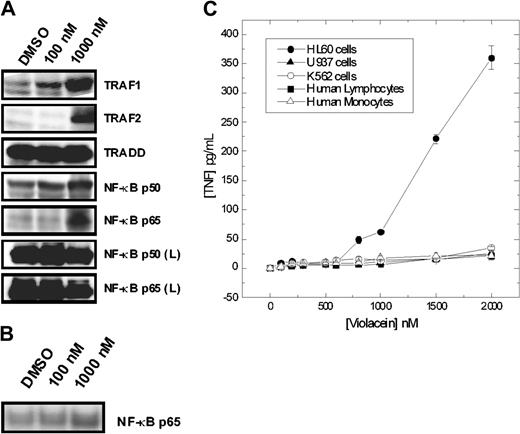
Importantly, a set of genes recognized as being downstream of NF-κB was up-regulated, including the p50 and p65 subunits of NF-κB, as well as TRAF2 (Figure 3A). The possible activation of NF-κB was confirmed by direct observation of the nuclear translocation of NF-κB as determined by Western blot analysis of nuclear extracts of HL60 cells (Figure 3B). Furthermore, in HL60 cells violacein led to the production of TNF-α, an event generally associated with the activation of NF-κB. Analogous to the cytotoxic effect, violacein-mediated NF-κB activation was specific to HL60 cells as evident by the lack of TNF-α production and up-regulation of NF-κB observed in the other cell types investigated in this study (Figures 3A-C). We concluded that the effects of violacein on gene expression resemble those induced by the activation of proinflammatory receptors in HL60 cells.
Effects of violacein on the expression of proteins involved in the TNFR1 signaling, NF-κB p65 translocation, and TNF-α production. (A) After incubation for 24 hours with violacein, equal amounts of protein (50 μg) of total lysates were subjected to immunoblot analysis with TRAF1 and 2, TRADD, NF-κB (p50 and p65) antibodies. The profile of NF-κB (p50 and p65) was also evaluated for samples from human lymphocytes (L). (B) Nuclear extracts were subjected to immunoblot analysis with NF-κB p65 antibody. (C) Cells were treated with different concentrations of violacein for 24 hours, and the TNF-α concentrations were determined in the supernatants by ELISA. Results represent the mean value ± standard error of the mean (SEM).
Effects of violacein on the expression of proteins involved in the TNFR1 signaling, NF-κB p65 translocation, and TNF-α production. (A) After incubation for 24 hours with violacein, equal amounts of protein (50 μg) of total lysates were subjected to immunoblot analysis with TRAF1 and 2, TRADD, NF-κB (p50 and p65) antibodies. The profile of NF-κB (p50 and p65) was also evaluated for samples from human lymphocytes (L). (B) Nuclear extracts were subjected to immunoblot analysis with NF-κB p65 antibody. (C) Cells were treated with different concentrations of violacein for 24 hours, and the TNF-α concentrations were determined in the supernatants by ELISA. Results represent the mean value ± standard error of the mean (SEM).
Violacein activates the TNF receptor 1
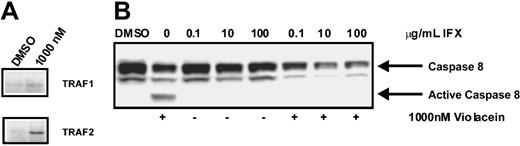
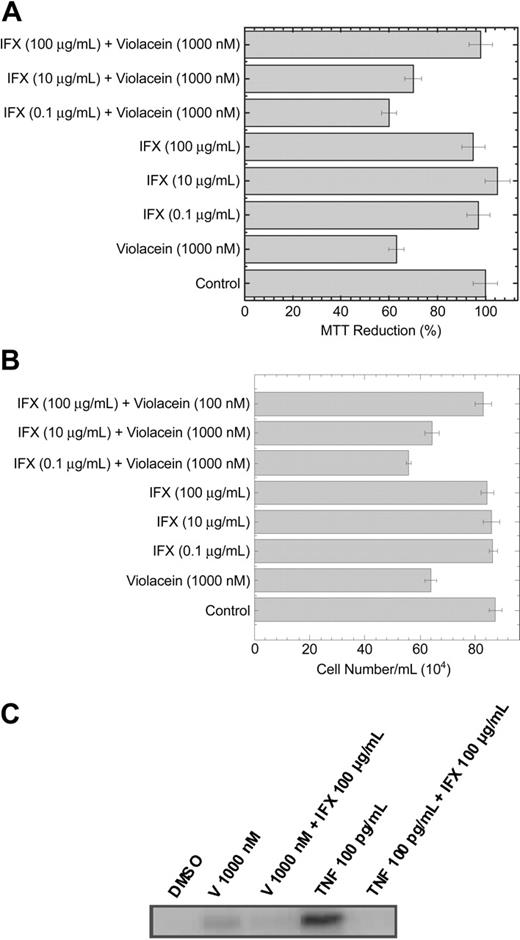
As the effects of violacein on HL60 cells (NF-κB activation and cell death) resemble those of TNF receptor 1 activation in these cells, we directly assessed the activation state of this receptor by using a coimmunoprecipitation assay. To this end, the TNF receptor 1 was immunoprecipitated, and, subsequently, the association of TRAF2 to the receptor was studied by using Western blotting. As TRAF2 association to the receptor is dependent on its activation, this assay allows direct assessment of TNF receptor 1 activation. As evident from Figure 4A, treatment of HL60 cells with violacein activated the TNF receptor. In agreement, caspase 8, which is a prominent effector of TNF receptor 1, was strongly activated by violacein (Figure 4B). Finally, preincubation for 1 hour with 100 μg/mL infliximab, an inhibitor of human TNF signaling,15 strongly reduced the violacein-induced cytotoxicity in HL60 cells (Figure 5A-B). Thus, effects of violacein on HL60 cells involve activation of the TNF receptor 1.
Scaffold assembly of TNFR1 complex and caspase 8 activation after violacein treatment. (A) TNFR1 was immunoprecipitated from HL60 cells and then lysates were subjected to Western blot to detect interacting proteins. (B) Cellular lysates were extracted from HL60 cells treated with 1000 nM violacein, infliximab (IFX; 0.1, 10, and 100 μg/mL) or violacein plus infliximab. Western blot analysis was performed to assess the processing of caspase 8.
Scaffold assembly of TNFR1 complex and caspase 8 activation after violacein treatment. (A) TNFR1 was immunoprecipitated from HL60 cells and then lysates were subjected to Western blot to detect interacting proteins. (B) Cellular lysates were extracted from HL60 cells treated with 1000 nM violacein, infliximab (IFX; 0.1, 10, and 100 μg/mL) or violacein plus infliximab. Western blot analysis was performed to assess the processing of caspase 8.
Cytotoxicity and activation of caspase 3 on HL60 cells by violacein is prevented by infliximab. The graphs show the effects of violacein and IFX on and MTT reduction (A) and cell number (B). In the absence of compounds, the MTT reduction was considered as 100%. All experiments were performed in a 24-well plate. Results represent the means ± standard error of 3 experiments run in triplicates (P < .05). (C) Cellular lysates were extracted from HL60 cells treated with 1000 nM violacein (V), V 1000 nM + IFX 100 μg/mL, TNF 100 pg/mL, or TNF 100 pg/mL + IFX 100 μg/mL. Western blot analysis was performed to assess the active caspase 3.
Cytotoxicity and activation of caspase 3 on HL60 cells by violacein is prevented by infliximab. The graphs show the effects of violacein and IFX on and MTT reduction (A) and cell number (B). In the absence of compounds, the MTT reduction was considered as 100%. All experiments were performed in a 24-well plate. Results represent the means ± standard error of 3 experiments run in triplicates (P < .05). (C) Cellular lysates were extracted from HL60 cells treated with 1000 nM violacein (V), V 1000 nM + IFX 100 μg/mL, TNF 100 pg/mL, or TNF 100 pg/mL + IFX 100 μg/mL. Western blot analysis was performed to assess the active caspase 3.
Activation of caspase 3 by violacein and TNF
To confirm that the activation of the TNF signaling pathway by violacein culminates in apoptosis of HL60 cells, we treated these cells with violacein (1000 nM), TNF (100 pg/mL), and their combinations with IFX (100 μg/mL) for 24 hours. The activation of caspase 3 was observed when the cells were treated with violacein or TNF, and this effect was prevented in the presence of infliximab (Figure 5C).
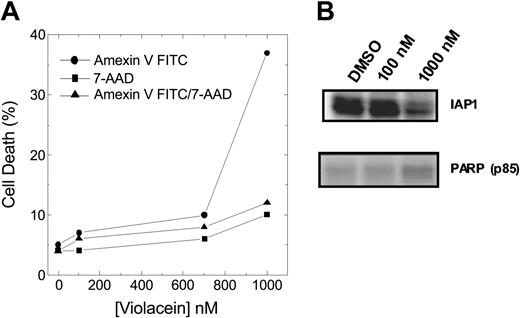
In addition, apoptosis induced by violacein was also detected through phosphatidylserine exposure, fragmentation of PARP, and down-regulation of IAP1 (Figure 6A-B), without accompanying necrosis. Hence, the specific induction of TNFR-mediated apoptosis is the predominant molecular mechanism in violacein-induced cytotoxicity.
Effect of violacein on HL60 cells apoptosis induction. (A) Cell samples were prepared as described in “Materials and methods,” and Annexin V–positive, 7-AAD–positive, and Annexin V/7-AAD–positive populations were analyzed by flow cytometry. (B) Western blot analysis was performed to assess IAP1 and fragmented PARP (p85) levels.
Effect of violacein on HL60 cells apoptosis induction. (A) Cell samples were prepared as described in “Materials and methods,” and Annexin V–positive, 7-AAD–positive, and Annexin V/7-AAD–positive populations were analyzed by flow cytometry. (B) Western blot analysis was performed to assess IAP1 and fragmented PARP (p85) levels.
Discussion
Natural products have been regarded as important sources of potential chemotherapeutic agents.16-19 Violacein could be an interesting candidate for cancer treatment because of its cytotoxic effect at low concentration on different transformed cell lines, and it seems especially promising with respect to leukemia.1,6 The development of violacein to treat cancerous disease is limited, however, by a lack of rational insight into the molecular mechanisms by which violacein affects leukemic cell physiology as well as the specificity of these mechanisms to HL60 cells in comparison to K562, U937, and untransformed cells. In an effort to determine the molecular mechanisms underlying the effects of violacein, we analyzed a variety of signal transduction events. The most prominent effects were the activation of p38 MAP kinase, the activation of caspase 8, and the increased levels of proinflammatory products of the NF-κB pathway. All these events are closely associated with the activation of TNF receptor 1 (Figure 7). Accordingly, direct assessment of the activation status of this receptor revealed that violacein resulted in activation of TNF receptor 1. Subsequent inhibition studies, using the clinical human TNF signaling inhibitor infliximab, showed that activation of TNF receptor 1 mediates at least in part violacein cytotoxicity in HL60 cells. We here demonstrate for the first time, that the mechanism of violacein cytotoxicity in HL60 cells is completely different when compared with other leukemia cell lines (U937 and K562) and with normal human blood cells (lymphocytes and monocytes). These data suggest that the induction of TNF production is a specific response of HL60 cells to violacein.
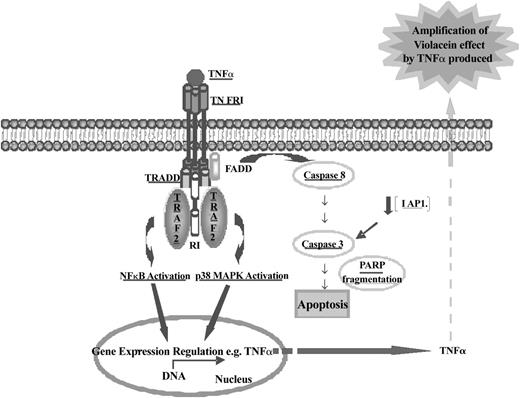
Schematic representation of the molecular mechanism of violacein-induced HL60 cell death. Data presented in this report revealed that violacein induces apoptosis in HL60 cells through activation of the TNF signaling cascade as demonstrated by up-regulation of TRAF2 and its coimmunoprecipitation with TNFR1. Trimeric TNF binds to and trimerizes TNFR1 molecules, leading to a recruitment of TRADD, which in turn can recruit TRAF2, receptor-interacting protein (RIP), and Fas-associated death domain (FADD). This event is involved in NF-κB, MAPK, and caspase 8 induction. p38 MAPK and NF-κB are translocated to the nucleus and activate some gene transcription (eg, TNF-α). Newly produced TNF-α will amplify the effect of violacein in neighboring cells. Active caspase 8 initiates the apoptotic cascade as demonstrated by activation of caspase 3 and PARP cleavage. TNFR1 can activate 2 signaling pathways, cell survival (through activation of NF-κB), or apoptosis. HL60 cells treated with violacein for 24 hours presented dominance of the apoptotic signaling cascade (which was also demonstrated by down-regulation of IAP1 and PARP cleavage). All proteins investigated in this work have been underlined.
Schematic representation of the molecular mechanism of violacein-induced HL60 cell death. Data presented in this report revealed that violacein induces apoptosis in HL60 cells through activation of the TNF signaling cascade as demonstrated by up-regulation of TRAF2 and its coimmunoprecipitation with TNFR1. Trimeric TNF binds to and trimerizes TNFR1 molecules, leading to a recruitment of TRADD, which in turn can recruit TRAF2, receptor-interacting protein (RIP), and Fas-associated death domain (FADD). This event is involved in NF-κB, MAPK, and caspase 8 induction. p38 MAPK and NF-κB are translocated to the nucleus and activate some gene transcription (eg, TNF-α). Newly produced TNF-α will amplify the effect of violacein in neighboring cells. Active caspase 8 initiates the apoptotic cascade as demonstrated by activation of caspase 3 and PARP cleavage. TNFR1 can activate 2 signaling pathways, cell survival (through activation of NF-κB), or apoptosis. HL60 cells treated with violacein for 24 hours presented dominance of the apoptotic signaling cascade (which was also demonstrated by down-regulation of IAP1 and PARP cleavage). All proteins investigated in this work have been underlined.
TNF receptor 1 signaling can simultaneously activate caspase 8 and the transcription factor NF-κB. Activation of caspase 8 is required for TNF-α–induced apoptosis, whereas NF-κB activation is associated with cell survival.20,21 Thus, violacein-dependent TNF receptor 1 activation will provide 2 opposing signals to the cell with respect to the decision to undergo programmed cell death (Figure 7). This may partly be explained by the fact that in leukemia cells various antiapoptotic pathways are constitutively activated. It has been demonstrated that the activation of IAP, X-linked inhibitor of apoptosis protein (XIAP), and Bcl-2 by NF-κB mediates resistance to several apoptotic stimuli in some cell types, and aberrant activation of these proteins has been described for leukemic cells.22-24 In HL60 cells, the net effect is clearly proapoptotic as evident from the strong effects of violacein on cell death. Hence, the activation of TNF-dependent proapoptotic mechanisms may gain dominance in relation to the TNF receptor 1-induced antiapoptotic pathways (Figure 7). The dominance of the proapoptotic pathway induced by TNF was confirmed by detecting caspase 3 activation when the cells were treated with violacein or TNF. In addition, this activity was abolished in the presence of infliximab. Therefore, the slight increase of activated caspase 3 caused by the treatment with violacein for 24 hours confirmed previous results, suggesting the early apoptosis induction by this pigment. In other words, the production of TNF stimulated by violacein may amplify the effect of this pigment in neighboring cells. In addition to the increase of activated caspase 3, apoptosis induction by treatment with violacein for 24 hours was demonstrated by down-regulation of IAP1, PARP cleavage, and exposure of phosphatidylserine. Interestingly, no accompanying necrosis was detected, highlighting that induction of TNFR signaling is the major effector of violacein signaling in leukemia cytotoxicity.
Our data also suggest that violacein-induced activation of p38 and TNF in HL60 cells leads to growth arrest. Violacein caused decrease of c-jun expression, which is implicated in the regulation of cell proliferation and cell cycle progression. C-Jun being part of activated protein 1 (AP-1) transcription factor complex functions as a proliferation-promoting gene and is involved in cell cycle progression.25 Consequently, the expression of c-Jun would be expected to change in response to a decreased proliferation and growth arrest of HL60 cells on action of violacein. In addition, we demonstrated a strong increase in p21 protein level. This protein is a typical inhibitor of cell cycle progression. Because HL60 cells are p53 (one of the activators of p21 transcription) negative of homozygous deletions,24 it is conceivable that p21 induction by violacein is p53 independent. Taken together, our data suggest that the increase in p38 activity induced by violacein accounts for the activation of p21 and/or induction of cell cycle arrest at G2/M. This hypothesis is supported by a recently published report demonstrating the requirement of p38 activation in G2/M arrest and an increase in p21 at G1/S phase by low doses of decitabine.26 In addition, it has been demonstrated that the p21 expression can be regulated through STATs, which mechanism is p53 independent and can occur in response to TNFR1 signaling.27 According to this observation and our results, we suggest that the p21 up-regulation in response to violacein could be also dependent on STATs.
An important question concerns the difference of violacein effects in the HL60, K562, U937, and the untransformed peripheral blood cells. We did not detect the same level of violacein-induced cytotoxicity or inflammatory gene transcription in the latter cell types. Although, obviously, this observation argues well for possible future clinical application of violacein, the mechanistic explanation for this observation is still unclear. Possible explanations include differences in levels of TNF receptor expression or different activity of the feedback mechanisms induced after TNF receptor activation. Alternatively, the violacein-dependent cell death of HL60 cells may be a consequence of violacein-induced TNF release and difference between the untransformed and transformed cells lies in their ability to react to violacein with TNF production. Future work is essential to distinguish between these various possibilities.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2004-02-0594.
Supported by a postdoctorate training fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (proc 02/03 842-8 [C.V.F.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Angelique Groot and Dennis Van Der Coelen for their assistance with the cytokine quantification and Jennie M. Pater for FACS analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal