Abstract
Sepsis is an acute inflammatory disease characterized by dysfunctional blood flow and hypotension. Nitric oxide (NO) is elevated during sepsis and plays an integral role in the associated vascular pathology. However, precise mechanisms and functions of NO in sepsis remain unclear. In this study, we show that red blood cells (RBCs) are foci for nitrosative reactions during acute inflammation, resulting in the formation of cells that can promote systemic vascular relaxation in an uncontrolled manner. Specifically, using experimental models of endotoxemia and surgical sepsis, NO adducts were found in the RBCs, including S-nitrosohemoglobin (SNOHb). These RBCs, referred to as septic RBCs, spontaneously stimulated vasodilation in a manner consistent with elevated SNOHb concentrations. Moreover, relaxation was cyclic guanosine monophosphate (cGMP) dependent and was inhibited by RBC lysis and glutathione but not by the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5 tetramethylimidazoline 1-oxyl 3-oxide (C-PTIO). The potential mechanism of septic RBC–mediated vasorelaxation is discussed and may involve the intermediate, nitroxyl (HNO). Coupled with data showing that NO adducts in septic RBCs were dependent on the inducible nitric oxide synthase and correlated with plasma nitrite, these findings provide a novel framework to understand mechanisms underlying dysfunctional blood flow responses during sepsis. Specifically, the concept that RBCs directly mediate systemic hypotension through NO-dependent mechanisms is discussed.
Introduction
Sepsis is a complex systemic inflammatory disease and the leading cause of death in surgical intensive care units. Clinically, sepsis is defined as a systemic inflammatory response to infection that, if unstopped, may progress to septic shock. The latter is defined as sustained hypotension despite application of vasopressor therapy and is caused by dysfunction in mechanisms that control vascular resistance. The resulting hypotension and vaso-occlusion prevent adequate matching of oxygen delivery to tissue demand, leading to organ failure and death. Although the outcome of septic patients has improved in the past 10 years largely because of improved patient monitoring, the mortality rate of 40% to 50% remains high.1
The vascular abnormalities associated with septic shock include transition from an initial hyperdynamic phase followed by a hypodynamic phase characterized by decreased tissue perfusion.2 The underlying mechanisms that contribute to the development of the hypodynamic phase and the resultant loss of vascular homeostasis remain unclear. Contributing factors include hyporeactivity to catecholamines and vasoconstrictors such as endothelin 1 and increased concentrations of vasodilator peptides, including adrenomedulin and calcitonin gene–related peptide (CGRP).3,4
Nitric oxide (NO) is a critical modulator of vascular resistance and inflammation, with diverse roles for this free radical in sepsis reported.5,6 It is clear that elevated NO formation during sepsis by way of the inducible isoform of nitric oxide synthase (iNOS) is important and may contribute to the hypotension, refraction to vasopressors, and inflammatory tissue damage associated with formation of reactive nitrogen species.7,8 Consistent with these concepts, studies using iNOS-deficient animal models or inhibitors of iNOS activity have shown a protective effect.9 Moreover, studies targeted toward scavenging NO and inhibiting its function in animal models of sepsis have improved outcome and contribute to the model that iNOS-derived NO contributes to the pathology associated with sepsis.10,11 In contrast, however, other studies using NO-scavenging approaches or iNOS-deficient mice have failed to result in improvement for the treatment group and in some instances even increased mortality. These observations highlight potential beneficial roles of NO, including anti-inflammatory and antioxidant properties.12,13 The general consensus is that NO elicits a spectrum of effects in the course of sepsis and the subsequent septic shock that includes both protective and contributory roles.13,14 A detailed understanding of underlying molecular mechanisms is critical, therefore, to the formulation of therapies.
S-nitrosothiols have been investigated as endogenous or therapeutic storage forms of NO that can stimulate vascular signaling and modulate diverse functions, including blood flow, inhibition of platelet aggregation, and control of enzyme function.15-17 Over the past decade, much interest has surrounded S-nitrosohemoglobin (SNOHb) as a regulator of physiologic blood flow.18-20 This S-nitrosothiol, in which a specific cysteine residue on the hemoglobin (Hb) β-chain (β93cys) is S-nitrosated, is the central player in one model proposed to explain how NO can exert multiple vascular effects despite the presence of Hb, which rapidly reacts and inhibits NO function.21,22 On the basis of mechanisms and SNOHb concentrations, however, recent data indicate that SNOHb does not play a role in modulating physiologic blood flow.18,20,23-25 Clearly, however, SNOHb can stimulate vasodilation and NO signaling in the vessel wall. The mechanisms of this effect remain under investigation and are regulated by thiols, heme redox state and may involve distinct redox congeners of NO, including nitroxyl (HNO).21,23,26
Interestingly, formation of SNOHb and other circulating S-nitrosothiols (eg, S-nitrosoalbumin) has been documented in conditions associated with iNOS induction and elevated NO formation and suggests that the conditions in the vascular compartment during acute inflammation are conducive to nitrosative chemistry.27-29 A potential for SNOHb to mediate vascular blood flow during acute inflammation has been postulated but remains untested.30-32
A role for red blood cells (RBCs) in sepsis has been discussed in the context of altered RBC deformability that contributes to stasis, capillary plugging, and ensuing tissue ischemia.2,33 In this study we investigated the hypothesis that the RBC is a central player in the dysfunctional blood flow responses during sepsis and specifically contributes to hypotensive effects by way of the formation of SNOHb. Our data demonstrate that RBCs isolated from animals with sepsis or endotoxic shock are able to dilate vessels in an NO-dependent manner and reveal a novel mechanism through which the RBC contributes to dysfunctional blood flow in acute inflammation.
Materials and methods
Materials
S-nitrosogluathione (GSNO) was purchased from Alexis Biochemicals (San Diego, CA). All other reagents were purchased from Sigma Chemical (St Louis, MO). S-nitrosocysteine (SNOC) was synthesized as previously reported.34
Experimental model of endotoxemia
Male Sprague-Dawley rats 250 to 300 g (Harlan, Madison, WI) received either sterile saline or 5 mg/kg lipopolysaccharide (LPS) by way of intraperitoneal injection. Blood samples were collected by way of cardiac puncture by using ethylenediaminetetraacetic acid (EDTA) as anticoagulant at differing times as indicated. Similar procedures were used with wild-type (C57bl6) and iNOS-deficient (iNOS–/–) mice, except blood collection was performed by way of exposure and catheterization of the inferior vena cava distal to the renal vein.
Experimental model of surgical sepsis: cecal ligation and puncture (CLP)
Peritoneal sepsis was initiated by performing CLP as previously described.35,36 Briefly, a 2-cm midline incision is made in the abdomen of rats maintained in a fasting state overnight. The cecum is exposed, ligated with 3-0 silk suture distal to the ileocecal valve, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The incision is closed in layers, and the animal receives fluid resuscitation (3 mL isotonic saline per 100 g body weight).
Measurement of NO adducts in RBCs and plasma
NOx concentration profiles of RBC samples were performed by NO chemiluminescence analyzer (ANTEK Instruments, Houston, TX). Total NOx was determined by reduction of all NO-containing species to NO by using VCl3 (0.16 M in 2 M HCl, at 90° C under vacuum and bubbled with helium gas) and comparison to nitrate standards. Measurement of nitrite, S-nitrosothiols, and other nitroso species present in RBCs was performed by using an I3–-based reducing system as described previously29,31,37-39 and validated in our laboratory (not shown). Briefly, RBCs and plasma are separated by centrifugation (1000g, 10 minutes), washed 3 times with phosphate-buffered saline (PBS) + 100 μM diethylenetriaminepentaacetic acid (DTPA), 4° C. To prevent nitrite oxidation and S-nitrosothiol decomposition, RBC pellets (100 μL) or plasma (100 μL) were mixed with a “stabilization buffer” (final concentrations: 4 mM KCN, 12 mM K3Fe(CN)6, 100 μM DTPA, 10 mM N-ethlymaleimide (NEM), 1% vol/vol IGEPAL [NP-40 detergent], and PBS), mixed by vortex prior to flash freezing in liquid N2 and storage at –80° C. All samples were measured within 1 week of collection. Samples were treated with either sulfanilamide (final concentration 1.5% wt/vol in 2 M HCl, 5 minutes, 25° C) with or without mercuric chloride (50 mM) to measure nitrite and S-nitrosothiols, respectively. Data are calculated by comparison to sodium nitrite and S-nitrosothiol standards, respectively. Sulfanilamide and Hg-resistant NO adducts were also measured and referred to as XNO in the text. Because of the unknown identity of these species and potential differing yields of NO in the I3–-reducing system, data are calculated by comparison to S-nitrosothiol standards. Data are normalized with respect to heme concentrations that were measured by using Drabkins reagent. Membrane and cytosolic compartments of the RBCs were prepared by substituting ultrapure water for PBS in stabilization buffer. Samples were centrifuged at 14 000g supernatant, and pellets were separated and analyzed for NO adducts as described. Molecular weight fractionation of the RBCs was performed by either subjecting RBC lysate to gel filtration chromatography (G-25, 10-kDa cutoff) and collecting fractions eluting with Hb. Alternatively, lysates were fractionated by using centricons (Millipore, Billerica, MA; molecular weight [MWt] cutoff 3 kDa), and the low–molecular weight filtrate was collected.
Nitrosylhemoglobin (HbNO) and methemoglobin (metHb) were determined by EPR spectroscopy. RBCs were collected into an EPR tube and flash frozen in liquid N2. For HbNO electron paramagnetic resonance (EPR), spectra were taken on a Bruker Elexys X-band Spectrometer (Billerica, MA) at 100 K, 1-mW microwave power, 5-G modulation amplitude, 40.96-millisecond time constant and 84-second scan time. Each spectrum was the average of 3 scans. Spectra were baseline corrected and double integrated by using spectrometer software. Quantification was performed by reference to HbNO standards. Spectral deconvolution was performed by multiple linear regression analysis with use of pure basis spectra for the 3 HbNO species and the observed free radical. For metHb measurements EPR spectra were run at 5 K and the metHb signal at g = 6 quantified with reference to standard spectra taken under identical conditions.
For RBC determinations all values are normalized to heme concentration as determined by the Drabkins assay. For plasma determinations, values are expressed by milligram plasma protein (measured by using the Lowry assay). RBC volumes were determined by using a multisizer 3 Coulter counter (Beckman, Fullerton, CA) and found not to differ significantly between control (104.6 ± 0.5 fL) and LPS-treated groups (104.9 ± 0.4 fL).
Synthesis of SNO-RBC
S-nitrosocysteine (10 mM final, 4 hours, 4° C) was added to RBCs (0.5 [50%] hematocrit in PBS + 100 μM DTPA) under light-protected conditions with occasional mixing. RBCs were then washed 5 times in PBS + 100 μM DTPA (4° C).
SNO-RBC stability
RBCs isolated from LPS rats were incubated at 25° C in PBS for 3 hours. At hourly intervals, aliquots were taken for determination of NO adducts and vessel reactivity. Aliquots were also centrifuged to remove RBCs, and S-nitrosothiols were measured in supernatant as an index of S-nitrosothiol export.
Vessel relaxation studies
Isometric tension in isolated rat thoracic aortic ring segments was measured as described previously23 by using phenylephrine and l-nitroarginine methyl ester (L-NAME) contracted vessels. After tension development reached a plateau, RBCs (0-0.02 [0%-2%] hematocrit final concentration) from LPS or control rats were added to the vessel bath (as described in figure legends), and effects on tension were monitored. The n values correspond to individual experiments conducted by using different vessel and RBC preparations. Within a given experiment, each condition was repeated 2 to 4 times. Vessel tension experiments at low oxygen tensions were performed as previously described.23 For these studies high and low oxygen tensions refer to 900 μM and 20 μM (corresponding to 662 mmHg and 15 mmHg O2), respectively. For mechanistic studies, reagents described in the text were added 5 minutes before the addition of RBCs.
Measurement of cGMP
Cyclic guanosine monophosphate (cGMP) was measured in rat thoracic aorta pretreated with 100 μM 3-isobutyl-1-methylxanthine (IBMX) to inhibit phosphodiesterases, indomethacin (15 μM), phenylephrine (100 nM), and NG-monomethyl l-arginine (L-NMMA; 100 μM) exposed to RBCs from LPS or control rats (5 minutes). Vessels were then rinsed, blotted dry, weighed, and flash frozen in liquid N2. cGMP was measured by using a sandwich enzyme-linked immunosorbent assay (ELISA; Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Statistical analysis
All results are reported as the mean ± SEM. Statistical analysis was performed with use of Origin Software (Northampton, MA). Differences between the groups were assessed by Student t test.
Results
Formation of NO adducts in RBCs from LPS-treated rats
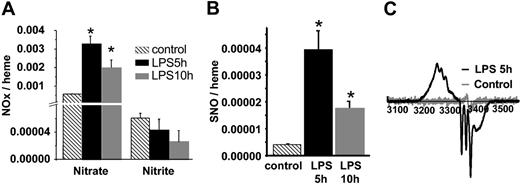
The time-dependent changes of NO metabolites, nitrate, nitrite, and SNO in RBCs isolated from LPS-treated rats are shown in Figure 1A-B. Consistent with an acute inflammatory response and elevated production of NO, nitrate significantly increased (4- to 6-fold) at 5 and 10 hours after LPS administration. In contrast, RBC nitrite concentrations did not change significantly, although they trended toward a decrease. Consistent with previous reports,27,28 RBC S-nitrosothiol concentrations increased significantly (Figure 1B) and were maximal at 5 hours (unless otherwise stated, these conditions were used for the remainder of the studies reported and will be referred to as septic RBCs) and correspond to 600 nM SNO. RBC NO adducts that were resistant to acidified sulfanilamide and Hg were also detected. These have been reported previously and termed XNO. They remain uncharacterized but likely include HbNO, C- or N-nitroso compounds.29 Concentrations of XNO increased significantly from 1.1 × 10–5 to 1.9 × 10–4 and 1.1 × 10–4 moles per heme after 5 hours and 10 hours of LPS treatment, respectively (note these values are based on S-nitrosothiol standards as described in “Materials and methods”). To directly determine the formation of HbNO, RBCs were analyzed by EPR spectroscopy. Figure 1C shows that RBCs isolated from saline-treated rats exhibit a small free radical signal at g = 2.007. After 5 hours of LPS, a spectrum consistent with a mixture of 5- and 6-coordinate α-nitrosyl HbNO, and the g = 2.007 signal was observed. Interestingly, β-nitrosyl Hb was absent, indicating strong chain selectivity for the nitrosylation reaction. The calculated concentration of HbNO (after subtraction of the other species) was 31.7 μM (or 0.0015 HbNO/heme). With the use of EPR, increased formation of metHb was also observed from 15.1 μM to 22.1 μM in control versus septic RBCs, respectively. These data demonstrate that during acute inflammation, RBCs act as targets for the increased NO production in the vascular compartment. Finally, to assess the cellular compartmentalization of RBC S-nitrosothiols, RBC lysates were fractionated by gel-filtration chromatography into a high–molecular weight (> 10 kDa) or low–molecular weight (< 10 kDa) fraction. Table 1 shows in septic RBCs approximately 60% of RBC S-nitrosothiols are in the high–molecular weight fraction. Moreover, determination of S-nitrosothiols in the membrane versus the cytosolic fractions revealed a predominant (> 90%) localization in the cytosol, suggesting the presence of SNOHb. After 10 hours of LPS treatment, although total RBC S-nitrosothiols decreased slightly (Figure 1B), approximately 80% of these were in the high–molecular weight fraction, indicating a dynamic interaction between the high– and low–molecular weight RBC S-nitrosothiol pools during the course of endotoxemia. Interestingly, XNOs were present entirely in the less than 10-kDa fraction, indicating that these cannot be attributed to HbNO or other protein/NO adducts.
NO adducts in RBCs isolated from control and LPS rats. (A) RBC nitrate and nitrite concentrations at 5 and 10 hours following LPS administration compared with control. Data represent mean ± SEM (n = 3). (B) SNO concentrations in LPS RBCs relative to control RBCs. Data represent mean ± SEM (n = 3). (C) Representative EPR spectra of RBCs from saline-injected rats (gray line) versus RBCs isolated from LPS-treated rats (black line). *P ≤ .05 versus control.
NO adducts in RBCs isolated from control and LPS rats. (A) RBC nitrate and nitrite concentrations at 5 and 10 hours following LPS administration compared with control. Data represent mean ± SEM (n = 3). (B) SNO concentrations in LPS RBCs relative to control RBCs. Data represent mean ± SEM (n = 3). (C) Representative EPR spectra of RBCs from saline-injected rats (gray line) versus RBCs isolated from LPS-treated rats (black line). *P ≤ .05 versus control.
Compartmentalization of SNO and XNO in septic RBCs
. | More than 10 kDa . | Less than 10 kDa . |
|---|---|---|
| RBC SNO | ||
| 5 h | 57.8 ± 10 | 42.2 ± 11 |
| 10 h | 79.6 ± 13 | 14.3 ± 1 |
| RBC XNO | ||
| 5 h | 13.1 ± 4 | 86.9 ± 4 |
| 10 h | 14.3 ± 1 | 85.7 ± 0.8 |
. | More than 10 kDa . | Less than 10 kDa . |
|---|---|---|
| RBC SNO | ||
| 5 h | 57.8 ± 10 | 42.2 ± 11 |
| 10 h | 79.6 ± 13 | 14.3 ± 1 |
| RBC XNO | ||
| 5 h | 13.1 ± 4 | 86.9 ± 4 |
| 10 h | 14.3 ± 1 | 85.7 ± 0.8 |
RBCs were fractionated into high- (more than 10 kDa) and low- (less than 10 kDa) molecular weight fractions, and SNO and XNO were measured as described in “Materials and methods.” Values represent the percentage of total within either SNO or XNO pools. Values shown are mean ± SEM (n = 3).
Bioactivity of SNO-RBCs and RBCs isolated from LPS-treated rats
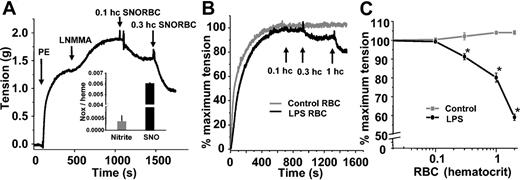
We and others have shown previously that SNOHb is able to stimulate vasodilation.21,23,26 Furthermore, synthesis of S-nitrosothiol–containing RBCs in vitro imparts vasodilatory properties to these cells (Figure 2A). To evaluate the potential biologic effects of increased RBC S-nitrosothiols during endotoxemia, the vasoactive properties of septic RBCs were tested. Figure 2B shows representative tension traces of rat thoracic aortic segments exposed to either RBCs isolated from saline-treated or LPS-treated rats. Whereas control RBCs had no effect, septic RBCs stimulated vessel relaxation. Figure 2C shows this relaxation occurs in a dose-dependent manner. Limitations in the amount of RBCs that can be added to vessel bioassay chambers that cause significant foaming precluded a more extensive dose-response study; however, an EC50 (concentration at which 50% relaxation occurs) between 0.1 and 0.2 (10% and 20%) hematocrit is estimated.
Vasorelaxant effects of RBC S-nitrosothiols. (A) SNO-RBCs were synthesized and contained elevated concentrations of SNO (inset). Addition of these cells to aortic rings precontracted with phenylephrine (PE; 100 nM) and L-NMMA (100 μM) stimulated dilation (indicated by a decrease in tension). (B) Representative vessel tension versus time traces showing that RBCs isolated from LPS-treated rats (black line) stimulate vasorelaxation at 0.003 and 0.01 (0.3% and 1%) hematocrit, whereas control RBCs (gray line) have no effect. (C) Dose-dependent relaxation stimulated by LPS RBCs (black) relative to control RBCs (gray). Data represent mean ± SEM (n = 5). *P ≤ .05 versus control.
Vasorelaxant effects of RBC S-nitrosothiols. (A) SNO-RBCs were synthesized and contained elevated concentrations of SNO (inset). Addition of these cells to aortic rings precontracted with phenylephrine (PE; 100 nM) and L-NMMA (100 μM) stimulated dilation (indicated by a decrease in tension). (B) Representative vessel tension versus time traces showing that RBCs isolated from LPS-treated rats (black line) stimulate vasorelaxation at 0.003 and 0.01 (0.3% and 1%) hematocrit, whereas control RBCs (gray line) have no effect. (C) Dose-dependent relaxation stimulated by LPS RBCs (black) relative to control RBCs (gray). Data represent mean ± SEM (n = 5). *P ≤ .05 versus control.
Role of RBC S-nitrosothiols in vascoactive responses
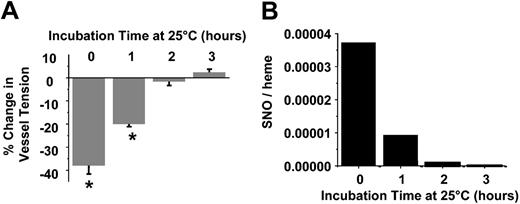
Data shown in Figure 2 suggest that septic RBCs spontaneously stimulate vasodilation and may, therefore, contribute to systemic hypotension observed in the hypodynamic phase of sepsis. To determine whether these responses are mediated by the S-nitrosothiol content, septic RBCs were incubated in PBS at 25° C for 3 hours at ambient O2 tensions. Previous studies have shown that S-nitrosothiols are relatively unstable within the reductive environment of the RBCs and decay readily at room temperature.31 Figure 3 confirms these observations in septic RBCs. S-nitrosothiol concentrations were determined at hourly intervals and completely decayed by 2 hours (Figure 3). HbNO concentrations decreased to 12.5 μM over 3 hours (data not shown). At each time point an aliquot was also taken, and the vessel dilation potential was determined. Figure 3 shows that at time 0, septic RBCs induced 40% dilation, an effect that completely disappeared by 2 hours. Over this time, RBC S-nitrosothiol concentrations also declined with a similar profile. Importantly, XNO concentrations only decreased approximately 25% over 2 hours (not shown).
Role of SNO in septic RBC-mediated vasodilation. Vasorelaxant effects (A) and SNO concentrations (B) of septic RBCs as a function of incubation time in PBS at 25° C. Vasodilatory effects were determined by using 0.02 (2%) hematocrit concentration. A greater negative change in percentage of tension indicates a greater dilation response. Data represent mean ± SEM (n = 3). *P ≤ .05 versus control.
Role of SNO in septic RBC-mediated vasodilation. Vasorelaxant effects (A) and SNO concentrations (B) of septic RBCs as a function of incubation time in PBS at 25° C. Vasodilatory effects were determined by using 0.02 (2%) hematocrit concentration. A greater negative change in percentage of tension indicates a greater dilation response. Data represent mean ± SEM (n = 3). *P ≤ .05 versus control.
Role of oxygen in septic RBC-mediated vasodilation
Oxygen controls the conformational state of Hb and affects vessel responsiveness to nitrosovasodilators. Figure 4 shows that the dilation effects of septic RBCs (0.01 [1%] hematocrit) are approximately 3-fold greater at low oxygen tensions versus high. Importantly, however, septic RBCs stimulated a significant dilation effect at high oxygen concentrations, conditions that would favor the oxygenated conformation state of Hb. No effect on vessel tension of RBCs isolated from saline-injected rats at either high or low oxygen tensions was observed.
Effect of O2 concentrations on vasodilatory effects of septic RBCs. Septic RBCs (0.003 [3%] hematocrit) were added to vessels at either high (900 μM) or low (20 μM) O2 tensions, and changes in vessel tension were measured. RBCs isolated from control animals had no effect on vessel tension at high or low oxygen concentrations. Data represent mean ± SEM (n = 3). *P ≤ .05 versus control. #P ≤ .05 versus LPS RBCs at high O2.
Effect of O2 concentrations on vasodilatory effects of septic RBCs. Septic RBCs (0.003 [3%] hematocrit) were added to vessels at either high (900 μM) or low (20 μM) O2 tensions, and changes in vessel tension were measured. RBCs isolated from control animals had no effect on vessel tension at high or low oxygen concentrations. Data represent mean ± SEM (n = 3). *P ≤ .05 versus control. #P ≤ .05 versus LPS RBCs at high O2.
Mechanism of septic RBC-mediated vasodilation
To gain insights into potential mechanisms by which septic RBCs stimulate vasodilation we first tested for a role of soluble guanylate cyclase (sGC) activation. Figure 5A shows that cGMP (product of sGC activation) increased significantly in vessels exposed to septic RBCs but not those treated with control RBCs. This finding suggests that at some point NO is produced that in turn activates sGC. To test whether NO is involved, we performed vasodilation experiments with either intact septic RBCs or cells that had been lysed by repeated (30 times) shearing through a 27-gauge needle. This resulted in 75.5 ± 8 μM cell-free Hb for a 0.01 (1%) hematocrit concentration of RBCs, which is equivalent to 40% lysis. Cell-free Hb rapidly inhibits relaxation induced by NO. Figure 5B shows dose-response curves for intact and lysed septic RBCs and demonstrates that relaxation was significantly inhibited in the presence of cell-free Hb. At a concentration equivalent to 0.01 (1%) hematocrit, approximately 50% inhibition was observed (Figure 5C). Interestingly, however, addition of C-PTIO, a specific NO (nitrogen monoxide radical) scavenger, had no effect on relaxation responses (Figure 5C). Previous studies have shown a critical role for thiols in mediating SNOHb-dependent vasodilation. In contrast to this effect, addition of glutathione (GSH) inhibited septic RBC-dependent relaxation, whereas addition of ascorbate, a reductant that reduces S-nitrosothiols in a metal-dependent manner, had no effect. These findings suggest that S-nitrosothiols are not released from septic RBCs as occurs with synthesized S-nitrosated RBCs. Consistent with these conclusions, no S-nitrosothiols were observed in the extracellular media of septic RBCs incubated for 0 to 3 hours in PBS ± GSH (100 μM) at 25° C (data not shown). Finally, to test a role for adenosine 5′-triphosphate (ATP) release (which has been shown to occur from deoxygenated RBCs and mediate NO formation from endothelial cells by way of activation of purinergic receptors), relaxation studies were performed in the presence of apyrase (grade VII 5 U/mL), an enzyme cocktail that degrades ATP and adenosine 5′-diphosphate (ADP). No effect of apyrase was observed (Figure 5C), suggesting that release of ATP from septic RBCs was not playing a role in the observed relaxation responses.
Mechanism of septic RBC-mediated vasodilation. (A) Vessel cGMP content in rat thoracic aorta segments increased following incubation with septic RBCs (0.01 [1%] hematocrit). (B) Relaxation effects of septic RBCs were inhibited on cell lysis. (C) Effects of lysis, GSH (100 μM), C-PTIO (100 μM), ascorbate (100 μM), or apyrase (5 U/mL) on septic RBC– (0.01 [1%] hematocrit) mediated relaxation. For RBC lysis, amounts of RBC lysates equivalent to 0.01 (1%) hematocrit intact RBCs were added. Data represent mean ± SEM (n = 3). *P ≤ .05 versus LPS RBCs.
Mechanism of septic RBC-mediated vasodilation. (A) Vessel cGMP content in rat thoracic aorta segments increased following incubation with septic RBCs (0.01 [1%] hematocrit). (B) Relaxation effects of septic RBCs were inhibited on cell lysis. (C) Effects of lysis, GSH (100 μM), C-PTIO (100 μM), ascorbate (100 μM), or apyrase (5 U/mL) on septic RBC– (0.01 [1%] hematocrit) mediated relaxation. For RBC lysis, amounts of RBC lysates equivalent to 0.01 (1%) hematocrit intact RBCs were added. Data represent mean ± SEM (n = 3). *P ≤ .05 versus LPS RBCs.
Role of iNOS in formation of RBC S-nitrosothiols
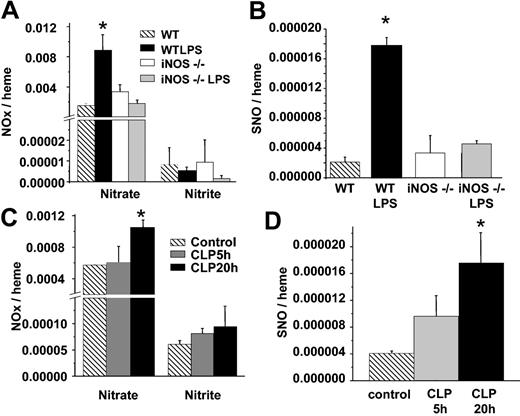
Conditions associated with iNOS induction are conducive to nitrosative chemistry and may occur through a variety of mechanisms. Moreover, incubation of RBCs with relatively high concentrations of NO or iNOS leads to SNOHb formation.40 We, therefore, determined formation of S-nitrosothiols in RBCs isolated from wild-type (WT) or iNOS-deficient mice treated with saline or LPS. Figure 6 shows that RBC S-nitrosothiol concentrations increased 8-fold in WT mice treated with LPS but did not increase in iNOS–/– mice. Interestingly, RBC nitrite concentrations did not change significantly in any treatment group.
Formation of RBC S-nitrosothiols in iNOS–/– mice and a surgical model of sepsis. (A) RBC nitrate and nitrite in WT or iNOS–/– mice treated with saline or LPS. (B) Corresponding RBC S-nitrosothiol concentrations. (C-D) Changes in RBC nitrate, nitrite, and S-nitrosothiols measured at 5 or 20 hours (▦ and ▪, respectively) after cecal ligation and puncture. ▧ represents control RBCs. Data represent mean ± SEM (n = 3-5). *P ≤ .05 versus control.
Formation of RBC S-nitrosothiols in iNOS–/– mice and a surgical model of sepsis. (A) RBC nitrate and nitrite in WT or iNOS–/– mice treated with saline or LPS. (B) Corresponding RBC S-nitrosothiol concentrations. (C-D) Changes in RBC nitrate, nitrite, and S-nitrosothiols measured at 5 or 20 hours (▦ and ▪, respectively) after cecal ligation and puncture. ▧ represents control RBCs. Data represent mean ± SEM (n = 3-5). *P ≤ .05 versus control.
Formation of RBC S-nitrosothiols in cecal ligation puncture model of sepsis
LPS administration simulates endotoxic shock and the hypodynamic phase of sepsis. CLP is a variable but more relevant model for sepsis. Many of the features of clinical sepsis have been reproduced with use of CLP. Furthermore, iNOS induction and NO production (measured as nitrite and nitrate) increases in a time-dependent manner after CLP, with little induction after 5 hours, but maximal formation at 20 hours.35 We, therefore, measured RBC NO adducts in CLP rats. Figure 6C-D shows that significant increases in RBC nitrate and S-nitrosothiols occur after 20 hours. Similar to LPS treatment, no changes in RBC nitrite were observed, however. Moreover, fractionation studies showed that all RBC S-nitrosothiols isolated from 20-hour CLP rats were present in the high (> 10 kDa) fraction (data not shown).
Role of plasma nitrite in RBC S-nitrosothiol formation
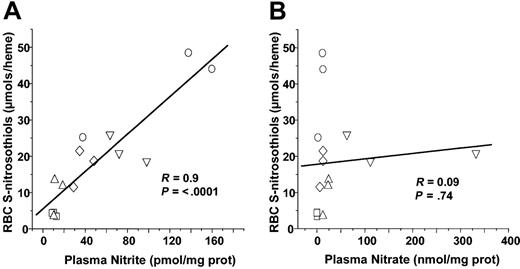
Recent studies have shown that nitrite addition to deoxygenated red cells of Hb results in SNOHb formation.41,42 RBC nitrite concentrations did not significantly change in either LPS or CLP models or sepsis (Figures 1 and 6). However, plasma nitrite did increase significantly from 1.1 × 10–11 to 1.12 × 10–10 and 7.8 × 10–11 mol/mg plasma protein after 5 hours of LPS or 20 hours of CLP, respectively. To test whether plasma nitrite is a potential source of SNOHb, therefore, correlations were performed between either RBC or plasma nitrite and RBC S-nitrosothiols. Figure 7A shows that plasma nitrite measured in either control, LPS 5 hours, LPS 10 hours, CLP 5 hours, or CLP 10 hours correlated significantly with RBC S-nitrosothiol concentrations. No significant correlation between plasma nitrate and RBC S-nitrosothiols was observed, however, (Figure 7B) nor was any correlation between RBC nitrite and RBC S-nitrosothiols observed (not shown).
Correlation analysis of plasma nitrite or nitrate with RBC S-nitrosothiols. Each data point represents measurements of plasma nitrite (A) or nitrate (B) made from samples collected from an individual animal. □ indicates control; ○, LPS for 5 hours; ⋄, LPS for 10 hours; ▵, CLP for 5 hours; and ▿, CLP for 20 hours. Line shown is best fit line determined by linear regression. Correlation coefficients (R) and statistical P values are shown in respective figures.
Correlation analysis of plasma nitrite or nitrate with RBC S-nitrosothiols. Each data point represents measurements of plasma nitrite (A) or nitrate (B) made from samples collected from an individual animal. □ indicates control; ○, LPS for 5 hours; ⋄, LPS for 10 hours; ▵, CLP for 5 hours; and ▿, CLP for 20 hours. Line shown is best fit line determined by linear regression. Correlation coefficients (R) and statistical P values are shown in respective figures.
Discussion
Sepsis is a disease of high mortality with an increasing prevalence. The extreme complexity of the pathology of systemic inflammation in sepsis is emphasized by the multiple failed therapeutic interventions targeted on a single inflammatory mediator or pathway. To this end, the role of NO in sepsis has been widely studied, and inhibition of its production by way of iNOS or NO scavenging has not led to success at the clinical level despite numerous trials.13 These studies underscore the beneficial effects of NO (eg, maintaining organ perfusion and inhibiting leukocyte-endothelial interactions43,44 ) and imply that targeting all NO production during sepsis is unlikely to be a viable therapy. The lack of success of trials targeted at NO also highlights the deficiencies in our understanding of mechanisms through which NO modulates vascular function during the course of sepsis. Herein, we show that NO production from iNOS results in nitrosative reactions within the RBCs and increases circulating concentrations of S-nitrosothiols, potential storage forms of NO bioactivity.
Previously, the role of the RBC in sepsis has been limited to discussion of effects of its O2 transport functions and dysfunctional rheologic properties that may contribute to vascular plugging.2 The current study demonstrates the potential role of RBCs in mechanisms that control NO and its vascular functions during sepsis and forwards the novel concept that RBCs actively contribute to systemic hypotensive responses by storing and transporting NO bioactivity in the form of S-nitrosothiols.
With use of animal models of endotoxemia (LPS) and sepsis (CLP), S-nitrosothiols, HbNO, and NO metabolites were found to increase in RBCs. In the latter case this was restricted to nitrate, which is likely to be formed from oxyhemoglobin-dependent oxidation of nitrite and/or NO. Maximal concentrations of RBC S-nitrosothiols were 4 × 10–5 SNO per heme (Figure 1B). Fractionation studies indicated that a significant fraction (> 60%-80%) of RBC S-nitrosothiols were high molecular weight (> 10 kDa) and cytosolic. Moreover, RBC S-nitrosothiols were detected in a clinically relevant (cecal ligation puncture) model of sepsis and consistent with a recent study documenting increased SNOHb concentrations in patients with gram-negative sepsis.32 Given that Hb comprises more than 90% of the dry weight of the total RBC protein, it is likely that the predominant high MWt RBC S-nitrosothiol was SNOHb.
SNOHb has been discussed as a potential regulator of physiologic blood flow, but this contention has been disputed by a number of recent studies.19,20,24,30 Consistent with a lack of any effect of RBCs, Figure 4 shows that RBCs isolated from control rats do not dilate rat thoracic aorta at either 662 mmHg or 15 mmHg oxygen, tensions that, respectively, favor oxygenation and deoxygenation of erythrocytic Hb. The fact that SNOHb stimulates vasodilation under certain conditions is universally accepted, however. We and others have characterized the vasodilatory properties of SNOHb and have shown an absolute dependence of reduced thiols.21,23,26 The exact mechanisms for this effect are not known but are regulated by heme redox state and may involve formation of redox derivatives of NO, including nitroxyl (HNO).23 We hypothesized that SNOHb production during sepsis may contribute to hypotension observed in this disease through similar mechanisms. Consistent with this hypothesis, septic RBCs, or RBCs containing S-nitrosothiols synthesized in vitro, but not control RBCs, stimulated vasodilation spontaneously on addition to vessels. Interestingly, no exogenous thiols were required for this relaxation response. Moreover, the degree of relaxation correlated with the concentrations of RBC S-nitrosothiols (Figures 2, 3). Taken together, these data indicate that SNOHb may play a role in the pathogenesis of vascular dysfunction associated with sepsis.
Interestingly, NO adducts in the RBCs that were not S-nitrosothiols were also detected. Previous studies have referred to these as XNO and may represent HbNO and/or N-nitroso compounds.29,45 However, in septic RBCs the majority (> 80%) of XNOs were found in the less than 10-kDa fraction, suggesting HbNO constitutes only a small fraction of this species. HbNO was detected by EPR at concentrations of 0.0015 NO/heme, approximately 50-fold higher than the concentration of SNOHb, again, indicating that the observed XNO does not derive from HbNO. HbNO formation is consistent with previous studies and may arise from a number of different mechanisms, including reactions of deoxyhemoglobin with NO, s-nitrosothiols, or nitrite.42,46,47 Gradients in HbNO between the arterial and venous circulation after NO inhalation suggest a role in mediating blood flow.48 However, ferrous Hb has an extremely high affinity for NO. Moreover, HbNO was still present at approximately 12 μM in septic RBCs, which no longer stimulated vasodilation, and taken together the data suggest that HbNO is unlikely to be the vasodilatory agent. N-nitrosamines also stimulate vasodilation.49 However, a role for these is unlikely since, under conditions in which septic RBCs did not stimulate vasodilation (Figure 3; 2 hours), significant XNO concentrations (> 75% of maximum) were still measured.
To evaluate mechanisms through which septic RBCs stimulate vasodilation, first a role for the classic NO-dependent signaling pathway involving activation of sGC was tested. An increase in vessel cGMP concentrations indicates the release of a nitrosovasodilator from septic RBCs. The most logical candidate for mediating this effect is NO. However, a role for NO is difficult to rationalize because NO produced in the RBC is unlikely to escape as a result of rapid scavenging by Hb. However, if NO is produced outside the RBC, the presence of diffusion barriers or unstirred boundary layers that significantly decrease the rate of NO scavenging by RBC Hb may allow for NO-dependent vasodilation.50-52 To test a role for NO directly, therefore, vasorelaxation responses of septic RBCs were tested either in the presence of C-PTIO or after lysis. Interestingly, lysed septic RBCs in which diffusion/unstirred boundary layers are no longer a factor and Hb-dependent scavenging of NO is rapid, inhibited relaxation responses. However, C-PTIO had no effect. These data imply that either NO is not released by septic RBCs or an intact RBC is absolutely required to stimulate vasodilation. A role for released S-nitrosothiols was excluded on the basis that none were detected and neither GSH nor ascorbate potentiated the dilation response. Moreover, GSH inhibited the relaxation response which is in contrast to its effects on cell-free SNOHb-dependent dilation. An intriguing possibility that may reconcile these observations is the role of HNO, which has been discussed previously in the context of SNOHb biochemistry and vasodilation effects.23,53,54 Interestingly, C-PTIO does not affect HNO-mediated responses, whereas cell-free Hb and GSH are potent scavengers.55 Insights into the physiologic effects of HNO suggest that this molecule may exert effects on the vasculature by way of a mechanism unique from NO and involving release of CGRP.56 It is tempting to speculate that our findings suggest a link between NO overproduction and increased circulating CGRP seen in sepsis, by ways of formation of SNOHb and HNO release. The mechanism through which septic RBCs stimulate dilation remains to be determined.
RBCs are integrated into physiologic mechanisms that control blood flow. Proposed mechanisms include nitrite reactions with deoxygenated RBCs42 and/or release of ATP from hypoxic RBCs. In the latter case, ATP binds to endothelial purinergic receptors to stimulate NO formation and vasodilation.57 Importantly, these are controlled processes that increase blood flow in response to hypoxia. The finding in the present study differs in that septic RBCs stimulated vasodilation at both high and low oxygen tensions. At high oxygen tensions, oxygenated Hb will be prevalent, precluding any role for nitrite-dependent effects under these conditions. Relaxation was more efficient at low oxygen tensions, and this may be due to initiation of nitrite-dependent reactions and/or due to the enhanced responsiveness of vessels to nitrosovasodilators at low oxygen.23,26 It is interesting to note that the oxygen dependence of cell-free SNOHb-mediated vasodilation is similar to that observed here with septic RBCs.
Data shown in Figures 1 and 3 also illustrate the dynamic nature of S-nitrosothiols in septic RBCs, which will be determined by rates of formation and decomposition. In the context of decomposition, previous studies have shown that, within the reductive environment of the RBC,31 SNOHb is relatively unstable. In septic RBCs significant oxidation of Hb to metHb was observed, indicating an increased oxidative environment and/or decreased reductive potential within the RBC contributes to SNOHb formation. iNOS plays an absolute role in formation as illustrated by the lack of S-nitrosothiols in RBCs isolated from LPS-treated iNOS–/– mice. Many cellular sources of iNOS have been described during inflammation. Which ones are responsible for S-nitrosothiol formation in the red blood cell is not known but are likely to include cells that either come into close contact with red blood cells and/or can increase plasma nitrite (described later). Logical candidates include cells in the vessel wall (eg, smooth muscle cells) and circulating white blood cells that have been shown to express iNOS during inflammation.8
How iNOS-derived NO leads to S-nitrosothiol formation is not known, but recent studies have also documented that nitrosative reactions in the vascular compartment occur during acute inflammation.29 An intriguing and potential mechanism is the role of plasma nitrite. In both experimental models of acute inflammation studied here, plasma nitrite, but not RBC nitrite, increased. Interestingly, plasma nitrite, but not nitrate, correlated significantly with RBC S-nitrosothiols, suggesting that nitrite outside the RBC is regulating S-nitrosothiol formation inside the RBC. Coupled with altered blood flow and vascular remodeling that characterizes sepsis and development of hypoxia, we propose that plasma nitrite drives RBC S-nitrosothiol formation. It is important to note that other potential mechanisms of S-nitrosation are not excluded and include peroxynitrite formation, autoxidation of NO to form nitrosating intermediates particularly in hydrophobic regions of RBC membranes.16,58-61 Moreover, recent studies indicate that HbNO formation accelerates NO uptake by RBC51 and could represent a feed-forward mechanism that further “focuses” NO to the RBCs. Our studies show that during acute inflammation, RBCs are foci for nitrosative reactants, resulting in the formation of S-nitrosothiols. Through these products, RBCs may redirect nitrosative reactivity to the modulation of systemic vascular functions of NO, including stimulating blood flow and platelet function.
In summary, we have characterized formation of NO adducts and metabolites in RBCs during sepsis and show significant formation of S-nitrosothiols in an iNOS-dependent manner. This results in RBCs that are able to spontaneously stimulate vasodilation. Physiologically, RBCs can affect blood flow through mechanisms that are controlled by O2. Septic RBCs, however, dilate vessels at high O2 tensions, indicating that endogenous control mechanisms are no longer functional. In this model, therefore, we propose that septic RBCs contribute to hypotensive responses by a continuous stimulation of NO-dependent pathways. In addition, SNO within the RBCs may serve as a marker of disease progression in sepsis and also presents a therapeutic target distinct from enzymatic NO production.
Prepublished online as Blood First Edition Paper, May 18, 2004; DOI 10.1182/blood-2004-03-0880.
Supported by National Institutes of Health grants HL70146 (R.P.P.), GM55792 and EB001980 (N.H.), and MSTP T32 GM08361 (J.H.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Torry Tucker and Dr Eric Schwiebert for help with RBC volume determinations.

![Figure 4. Effect of O2 concentrations on vasodilatory effects of septic RBCs. Septic RBCs (0.003 [3%] hematocrit) were added to vessels at either high (900 μM) or low (20 μM) O2 tensions, and changes in vessel tension were measured. RBCs isolated from control animals had no effect on vessel tension at high or low oxygen concentrations. Data represent mean ± SEM (n = 3). *P ≤ .05 versus control. #P ≤ .05 versus LPS RBCs at high O2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-0880/5/m_zh80170466140004.jpeg?Expires=1765926032&Signature=nlVERmzdsJJgy1tZwH~1hxJoM9HHb-CBc4knvyepeQmxTuwajIMR9srE0dc83mLdICXcnmNJac4fWi7rcZhhLdmyyzJdNBiSqP0RX9lzol~vj4dnTHvjbjqda6~FmBjykwvhxuYjcvGGOowXyckh09MCoSE3ZZg2oKl4b~tRIOgkwnAlm6YPGHB57ZGEG4yrySWt1cc3OwDrENhvShGulV5-WgLYVACI802cQVdvKzz4ykkPHPtq0EfdtMhcPWz2wk3BmG7Bi-zcFiFH4djPsto7XC38cC1gd-RHIH1U8WIZ4soFftA9E-ya~iAgOcioRY5sXGAXQm9mi~51s-sS9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Mechanism of septic RBC-mediated vasodilation. (A) Vessel cGMP content in rat thoracic aorta segments increased following incubation with septic RBCs (0.01 [1%] hematocrit). (B) Relaxation effects of septic RBCs were inhibited on cell lysis. (C) Effects of lysis, GSH (100 μM), C-PTIO (100 μM), ascorbate (100 μM), or apyrase (5 U/mL) on septic RBC– (0.01 [1%] hematocrit) mediated relaxation. For RBC lysis, amounts of RBC lysates equivalent to 0.01 (1%) hematocrit intact RBCs were added. Data represent mean ± SEM (n = 3). *P ≤ .05 versus LPS RBCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-0880/5/m_zh80170466140005.jpeg?Expires=1765926032&Signature=JJU7Za4ZlXfG1pShrbK4bCNKHuzcJy3IQm652n6K0CTinpN6DS8-GyXOaIT79rr9OK7BgorQUCvNBDuuwzzsD7Z29CL6ocl2Tm-nV9UDyVhSsbBC6NZ1Y~1Vq3TMHcI3uBzADL0C31RLP1Ioabf6rqC6LaPY5vLAEI5ZRmxu4wUjAE9RlxwidOREclv~WLOIhSdHxKWgQ21HLufII6t9KKMubFaFgd2oajgJ1lmH1srKxK2IhNYigeKK83HFpsFqHgt3A5bHSMLraPK70XqhPgf5~L5bY7Oy5eM7YehWB6O462YsaXlHWdFczLLe6Qsxhbk5vBot4xjKDlPsAGKs1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal