Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated transcription factor important in lipid metabolism, diabetes, and inflammation. We evaluated whether human platelets and megakaryocytes express PPARγ and whether PPARγ agonists influence platelet release of bioactive mediators. Although PPARγ is mainly considered a nuclear receptor, we show that enucleate platelets highly express PPARγ protein as shown by Western blotting, flow cytometry, and immunocytochemistry. Meg-01 megakaryocyte cells and human bone marrow megakaryocytes also express PPARγ. Platelet and Meg-01 PPARγ bound the PPARγ DNA consensus sequence, and this was enhanced by PPARγ agonists. Platelets are essential not only for clotting, but have an emerging role in inflammation in part due to their release or production of the proinflammatory and proatherogenic mediators CD40 ligand (CD40L) and thromboxanes (TXs). Platelet incubation with a natural PPARγ agonist, 15d-PGJ2, or with a potent synthetic PPARγ ligand, rosiglitazone, prevented thrombin-induced CD40L surface expression and release of CD40L and thromboxane B2 (TXB2). 15d-PGJ2 also inhibited platelet aggregation and adenosine triphosphate (ATP) release. Our results show that human platelets express PPARγ and that PPARγ agonists such as the thiazolidinedione class of antidiabetic drugs have a new target cell, the platelet. This may represent a novel mechanism for treatment of inflammation, thrombosis, and vascular disease in high-risk patients.
Introduction
Peroxisome proliferator-activated receptors (PPARs) are members of a nuclear hormone receptor superfamily of ligand-activated transcription factors. There are 3 PPAR subtypes: PPARα, PPARβ/δ, and PPARγ. The genes encoding the PPAR subtypes each reside on different chromosomes and have distinct tissue expression patterns.1 While many reports focus on PPAR expression in the nucleus, PPARγ, in particular, is also found in the cytoplasm.2,3
PPARγ is highly expressed in white adipose tissue and was initially described as important for regulating gene expression in metabolism, insulin responsiveness, and adipocyte differentiation.4,5 While PPARγ was originally thought to be found mainly in fat tissue, it is in fact widely expressed by many types of cells including macrophages, B and T lymphocytes, epithelial, endothelial, smooth muscle, and fibroblastic cells.2,6-11 PPARγ has also come to prominence as PPARγ agonists play an important role in immune function by dampening inflammation, attenuating macrophage/monocyte synthesis of proinflammatory cytokines, and inducing apoptosis in B lymphocytes.2,6,12,13 PPARγ has also emerged as a key target for malignant cells as PPARγ agonists have shown therapeutic potential for B lymphoma and various epithelial-derived cancers.2,14,15
Megakaryocytes are the biggest cell of the bone marrow and the parent cell of platelets. Platelets are derived from the cytoplasm of megakaryocytes and are released to the bloodstream under the effects of cytokines such as interleukin-6 (IL-6) and IL-11.16,17 Platelets are enuclear cells that have a plasma membrane, surface-connected canalicular and tubular system, mitochondria, granules, lysosomes, and peroxisomes.18 Recent studies demonstrate that platelets and many of their products are important not only in hemostasis, but have now emerged as important in immunoregulation and inflammation. For example, platelets produce key inflammatory mediators such as transforming growth factor-β (TGF-β), thromboxane A2 (TXA2), and prostaglandin E2 (PGE2).19-21 The recent key demonstration that activated human platelets express and expel CD40 ligand (CD40L, formally known as CD154) provides a mechanism of interaction with CD40 expressing cells that include macrophages and vascular structural cells.22-25 These cells when activated through CD40 express cyclooxygenase-2 (Cox-2) and prostaglandins, adhesion molecules, and cytokines such as IL-6 and tissue factor.26,27 Many new studies now demonstrate that elevated CD40L levels in blood are associated with acute coronary syndromes and stroke.28 Interestingly, elevated serum levels of CD40L predict an increased cardiovascular risk in a healthy population.29
The enucleate platelet is not usually thought of as a cell containing transcription factors. Nonetheless, we investigated whether the human megakaryoblast cell line (Meg-01), human bone marrow megakaryocytes, and human platelets express PPARγ protein and whether platelets themselves might be targets of selected PPARγ agonists. Herein, we report the surprising findings that human megakaryocytes and platelets do express PPARγ and are susceptible to PPARγ agonists that dampen platelet release of the key proinflammatory and proatherogenic mediators CD40L and thromboxane B2 (TXB2). These novel findings support a role for the PPARγ system in modulating platelet function.
Materials and methods
Cell line and culture conditions
Meg-01 cells were purchased from the American Type Culture Collection (Rockville, MD) and are widely used as a model of human megakaryocytes.30 Meg-01 cells were cultured in RPMI-1640 tissue culture medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma, St Louis, MO), 2 mM l-glutamine (Invitrogen), 4.5 g/L glucose (Invitrogen), and 50 μg/mL gentamicin (Invitrogen).
Preparation of platelets
Blood samples (500 mL) were collected from healthy volunteers by venipuncture into a CPDA-1 blood collection bag (Baxter Healthcare, Deerfield, IL). The platelet-rich plasma (PRP) was obtained by centrifugation at 1800g for 8 minutes and extracted into the transfer bag (Charter Medical, Winston-Salem, NC) at room temperature. The Pall Biomedical Purecell LRF high-efficiency leukoreduction filter (East Hills, NY) was used to reduce leukocytes, microaggregates, and anaphylatoxin C3a. Leukocytes were removed by adherence in the filter. Platelets were washed with 0.9% saline using a COBE 2991 Blood Cell Processor (Lakewood, CO). Cell counts were performed on an Abbott Cell-Dyn 1700 (Abbott Park, IL), and the final platelet count was 5.5 × 1010/unit. The maximum numbers of contaminant nonplatelet cells were 1 × 105 white blood cells and 1 × 108 red blood cells, the percentages being 0.0001% and 0.1818% of platelets, respectively. Pooled PRP was prepared by the same procedure from 2 to 5 donors and combined into a pool bag (Charter Medical). The platelets were isolated by an additional centrifugation step at 1200g of the PRP for 4 minutes, and the pellet was washed twice with 1 × phosphate-buffered saline (PBS).
Western blot for PPARγ
Meg-01 and platelet total protein was isolated using nonidet P-40 lysis buffer containing a protease inhibitor cocktail (4-(2-aminoethyl)-benzenesulfonyl fluoride, pepstatin A, transepoxysuccinyl-l-leucylamido (4-guanidino) butane, bestatin, leupeptin, and aprotinin; Sigma). Total protein was quantified with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). A total of 15 μg protein was electrophoresed on 10% denaturing polyacrylamide-stacking gels and transferred to nitrocellulose membrane (Amersham, Piscataway, NJ) at 4° C. After blocking with 10% Blotto (PBS/0.1% Tween 20 and 10% milk) for 2 hours at room temperature, membranes were then incubated with a mouse monoclonal anti-PPARγ antibody from Santa Cruz Biotechnology (1:1000; Santa Cruz, CA) or with a rabbit polyclonal anti-PPARγ antibody from Calbiochem (1:5000; San Diego, CA) diluted in 2.5% Blotto for 1 hour. They were then washed in PBS/0.1% Tween 20 and incubated with a goat anti–rabbit horseradish peroxidase (Santa Cruz Biotechnology) secondary antibody at 1:2000 dilution for 1 hour. The membranes were washed in PBS/0.1% Tween 20 and bands were visualized using a Western Lightning chemiluminescence kit according to the manufacturer's instructions (Perkin Elmer Life Sciences, Boston, MA). The platelet PPARγ band detected by Western blot was identified as PPARγ by MALDI-TOF mass spectroscopy (MS) peptide mapping analysis at the University of Rochester MicroChemical Protein/Peptide Core Facility (data not shown).
Meg-01 and human platelet immunocytochemistry for PPARγ
Meg-01 cells (1 × 105) and platelets (1 × 107) were cytospun on slides and fixed with 1% paraformaldehyde and stained with a rabbit polyclonal anti-PPARγ antibody (Santa Cruz Biotechnology) or with an immunoglobulin G (IgG) isotype control (both at 4 μg/mL) (Santa Cruz Biotechnology) as described.7 Slides were developed with aminoethyl carbazole (AEC) reagent (Zymed Laboratories, San Francisco, CA) and visualized with an Olympus BX51 microscope (Melville, NY). Photographs were taken using a SPOT camera with SPOT RT software (New Hyde Park, NY). The objectives used were a 60 × Olympus UPlan F1 with a 1.25 numerical aperture and a 100 × Olympus UPlan F1 with a 1.3 numerical aperture.
Preparation of human bone marrow smears and immunocytochemistry for PPARγ
Human bone marrow aspiration material was obtained from the hip bone of anemia patients. A drop of material about 2 mm in diameter was put onto slides and immediately spread over by coverslip and air dried for 24 hours. Smears were fixed with acetone-methanol solutions. Except for the fixation step, immunocytochemistry was performed as described.7 Slides were stained with a mouse monoclonal anti-PPARγ antibody (Santa Cruz Biotechnology) or with IgG1 isotype control (both at 4 μg/mL) (Santa Cruz Biotechnology), and biotin-labeled horse anti–mouse IgG (Vector Laboratories, Burlingame, CA) was used as secondary antibody. After staining for PPARγ, counterstaining with hematoxylin was performed. One slide from the same patient was stained with a Diff-Quik stain set (Dade Behring, Newark, DE).
cDNA synthesis and RT-PCR assay
Total RNA was extracted with Tri-Reagent from platelets and Meg-01 according to the supplier's protocol (MRC, Cincinnati, OH). A total of 2 μg RNA was used for the reverse-transcription (RT) reaction, and polymerase chain reaction (PCR) for PPARγ and β-actin was performed as described.7 A reaction was performed without reverse transcriptase for each cDNA synthesis and used as a negative control in the PCR. cDNA (10 μL) was used in the PCR reaction. The RT-PCR products were separated by gel electrophoresis on 1% agarose gels and stained with ethidium bromide. Adipose tissue and THP1 human monocyte cells were used as positive controls.
Flow cytometric analysis
The washed platelets were resuspended and incubated in 1 mL fluorescence-activated cell-sorter (FACS) lysis solution (FLS; BD Biosciences, Immunocytometry Systems, San Jose, CA) at a concentration of 1 × 107/mL in 1 × FLS for 10 minutes in the dark at room temperature. After centrifugation at 500g for 5 minutes, the cells were permeabilized with 1 × FLS + 0.2% saponin (Sigma) for 10 minutes. Samples then were incubated with 8 μg/mL monoclonal fluorescein isothiocyanate (FITC)–labeled anti-PPARγ antibody (BD Biosciences, San Diego, CA) or FITC-labeled IgG1 isotype control (BD Biosciences) for 30 minutes in the dark at room temperature. Cells were washed with 1 × PBS containing 1% bovine serum albumin (BSA) and 0.1% sodium azide (NaN3). Samples were resuspended in 1% paraformaldehyde and analyzed on a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA).
For CD40L surface staining, washed platelets were pretreated with PPARγ agonists for 15 minutes and then exposed to 0.8 U/mL thrombin for 60 minutes at 37° C in the presence of 200 μM fibrinogen receptor antagonist (Bachem, King of Prussia, PA) and 5 mM EDTA(ethylenediaminetetraacetic acid; Sigma) to prevent clotting. The platelets were then stained for CD40L using a mouse IgG1 anti–human CD40L biotinylated monoclonal antibody (Ancell, Bayport, MN), or a mouse IgG1 isotype control antibody (Caltag, Burlingame, CA) followed by streptavidin conjugated to allophycocyanin (Caltag).
PPARγ activity assay
Concentrated platelets were washed twice and treated with 20 μM 15d-PGJ2 (Biomol, Plymouth Meeting, PA), rosiglitazone (Cayman Chemical, Ann Arbor, MI), ciglitazone (Biomol), or dimethyl sulfoxide (DMSO, vehicle control) for 2 hours at 37° C. Platelets were lysed with hypotonic buffer (10 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], 0.5% nonidet P-40, and 0.2 mM phenylmethylsulfonyl fluoride [PMSF]), 10 μg of cell extract was incubated in each well of TransAM PPARγ assay kit (Active Motif, Carlsbad, CA), and PPARγ DNA binding was determined as per the manufacturer's protocol.
Electrophoretic mobility shift assay (EMSA) for PPARγ
Nuclear extracts of Meg-01 cells were prepared as described previously.31 Cells were treated with 5 μM 15d-PGJ2, 10 μM ciglitazone, or DMSO (vehicle control) for 4 hours. The cells were washed in cold PBS and then incubated on ice in hypotonic buffer (10 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5% nonidet P-40, and 0.2 mM PMSF) for 10 minutes. The lysates were vortexed for 10 seconds and centrifuged for 15 seconds. The pellet was isolated carefully and resuspended in 80 μL hypertonic buffer (20 mM HEPES-KOH [pH 7.9], 1.5 mM MgCl2, 25% glycerol, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, and 0.2 mM PMSF). After incubation on ice for 20 minutes, lysates were centrifuged for 20 seconds and the supernatant containing the nuclear protein was transferred to new tubes. Protein quantification was performed using a BCA assay kit. Platelet protein isolation was done as described for the PPARγ activity assay. For the gel shift assay of Meg-01 and platelets, the consensus sequence for PPARγ (5′-CAAAACTAGGTCAAAGGTCA-3′) was labeled with [γ-32P] adenosine triphosphate (ATP) using T4 Polynucleotide Kinase (Life Technologies, Bethesda, MD). Micro Bio-Spin P-30 Tris Chromatography Columns were used to remove the unbound nucleotides (Bio-Rad, Hercules, CA). Meg-01 or platelet protein extracts were incubated with binding buffer (10 mM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 7.5], 50 mM NaCl, 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, and 0.05 mg/mL poly (dI-dC)) and 50 000 counts of labeled oligonucleotide or cold oligonucleotide for 15 minutes at room temperature. Supershift experiments were completed by adding 2 μgofthe anti-PPARγ antibody (Calbiochem) to the binding reaction. The samples were then run on a 4% nondenaturing polyacrylamide gel. The gel was dried on a Savant SGD 2000 gel dryer (Savant, Farmingdale, NY) for 1 hour at 50° C and exposed to film overnight.
Measurement of CD40L and TXB2
Platelets were isolated as described in “Preparation of platelets” and cultured with buffer or with 15d-PGJ2 or rosiglitazone (both at 20 μM) for 15 minutes at 37° C. Platelets were then activated with 0.8 U/mL thrombin or buffer, and samples were taken at the 5-, 10-, 15-, 30-, and 60-minute time points to measure human soluble CD40L and PGE2. CD40L assays were performed with a commercially available enzyme-linked immunosorbent assay (ELISA) specific for CD40L (Bender Biomedical Systems, San Bruno, CA). Virtually identical results were obtained using an ELISA for CD40L developed in our lab (data not shown). The stable end product of platelet TXA2 synthesis, namely TXB2, was measured using a highly specific enzyme immunoassay from Cayman Chemical as per the manufacturer's directions.
Platelet aggregation and ATP release
Platelet aggregation was performed using the turbidimetric method of Born32 with simultaneous measurement of ATP release using a Chrono-log Lumi-aggregometer with AGGRO/LINK for Windows Software version 5.1.6 (Chrono-log, Havertown, PA). Blood was collected by clean venipuncture from healthy donors who abstained from drugs known to affect platelet aggregation into 0.105 M/sodium citrate. PRP was prepared by centrifugation at 150g for 10 minutes at 20° C, and the platelet count adjusted to 250 × 109/L by mixing PRP and platelet-poor plasma from the same donor. All experiments were performed within 3 hours of blood collection. Aggregation was performed with adenosine diphosphate (ADP), and the slope of aggregation and amplitude was computed using accompanying software. The effects of the PPARγ agonist 15d-PGJ2 were tested by addition of varying concentrations to PRP for 15 minutes before aggregation. The 15d-PGJ2 was dissolved in DMSO, with a final concentration of DMSO in the samples of approximately 0.1%. Control experiments showed no effect of this concentration of DMSO on platelet aggregation or release.
Statistics
Statistical analysis of time-dependent changes in supernatant levels of soluble CD40L (sCD40L) and TXB2 used the log-rank test performed using Statview (SAS Institute, Cary, NC). P values of less than .05 were considered significant.
Results
Meg-01 megakaryocytes and human blood platelets express PPARγ protein
Meg-01 cells have been extensively used as a model of human megakaryocytes.30 To determine whether megakaryocytes and platelets express PPARγ protein we first tested Meg-01 cells and human platelets by Western blot for PPARγ. Meg-01 cells and platelets were lysed and the protein was analyzed for PPARγ by Western blot using commercially available and widely used anti-PPARγ antibodies. Meg-01 cells express PPARγ protein that co-migrated with human fat tissue PPARγ, used as a known positive control (Figure 1A). We next evaluated highly purified human platelets for PPARγ expression. There were 3 different single donor platelets and 3 multiple donor pooled platelet samples tested for PPARγ using 2 different anti-PPARγ antibodies (Figure 1B-C). Human platelets express a PPARγ band, which migrated similarly to the adipose tissue PPARγ band. While the platelet preparations were highly purified (> 99.99% platelets), they did contain the rare white blood cell. To determine how many white blood cells were needed to generate a PPARγ band on a Western blot, experiments were completed with different numbers of white blood cells. At least 1 × 106 white blood cells were needed to show a PPARγ band on Western blots (data not shown). Therefore contamination with white blood cells in purified platelets could not account for the Western blot signal. Western blot experiments of red blood cells were also were completed for PPARγ and red blood cells do not express PPARγ (Figure 1B). Additionally, PPARγ of platelet origin was confirmed by MALDI-TOF mass spectroscopy peptide mapping (data not shown).
PPARγ protein is expressed in the human megakaryoblast cell line, Meg-01, and by human platelets. (A) Western blot of Meg-01 cell line (15 μg) using a polyclonal anti-PPARγ antibody (Calbiochem). PPARγ bands co-migrated with the human adipose tissue protein extract (15 μg) used as positive control. (B-C) Platelet cell lysates (15 μg) from rigorously purified single donor or pooled platelets were analyzed by Western blot for PPARγ using 2 different anti-PPARγ antibodies (panel B is monoclonal anti-PPARγ [Santa Cruz Biotechnology]; panel C is polyclonal anti-PPARγ [Calbiochem]). Human adipose tissue protein extract (5 μg) was used as positive control (left lane). The PPARγ protein was shown for 3 different single donor and pooled platelet samples. Purified human red blood cells (30 μg) are negative for PPARγ (B). Data are representative of more than 5 experiments.
PPARγ protein is expressed in the human megakaryoblast cell line, Meg-01, and by human platelets. (A) Western blot of Meg-01 cell line (15 μg) using a polyclonal anti-PPARγ antibody (Calbiochem). PPARγ bands co-migrated with the human adipose tissue protein extract (15 μg) used as positive control. (B-C) Platelet cell lysates (15 μg) from rigorously purified single donor or pooled platelets were analyzed by Western blot for PPARγ using 2 different anti-PPARγ antibodies (panel B is monoclonal anti-PPARγ [Santa Cruz Biotechnology]; panel C is polyclonal anti-PPARγ [Calbiochem]). Human adipose tissue protein extract (5 μg) was used as positive control (left lane). The PPARγ protein was shown for 3 different single donor and pooled platelet samples. Purified human red blood cells (30 μg) are negative for PPARγ (B). Data are representative of more than 5 experiments.
The presence of PPARγ in Meg-01 cells and human platelets was further examined by immunocytochemistry. Meg-01 cells (Figure 2A) and platelets (Figure 2B) contain PPARγ protein, confirming the Western blot data. The PPARγ staining pattern of Meg-01 is cytoplasmic, as well as nuclear. In platelets, the staining pattern for PPARγ appeared throughout the cell, with apparent denser staining in platelet granules.
Immunocytochemistry demonstrating PPARγ expression in human megakaryoblast cells and platelets. Immunocytochemistry was performed with a rabbit polyclonal anti-PPARγ antibody as described in “Materials and methods.” Nonspecific staining was assessed using a rabbit IgG isotype control. (A) Nucleated cells and enucleated plateletlike cells of the Meg-01 cell line were stained for PPARγ. Meg-01 cells stain in the nucleus and the cytoplasm. Results were repeated 4 times with separate preparations of Meg-01 cells. Original magnification is × 600. (B) Human platelets express PPARγ. The staining pattern for PPARγ is throughout the platelets. Data are representative of 4 different donor platelet experiments with similar results. Original magnification is × 1000. The inset represents 1 platelet with a final magnification of × 2000. (C) Flow cytometric analysis for intracellular expression of PPARγ in human platelets. Purified platelets were washed and stained with a monoclonal FITC-labeled anti-PPARγ antibody (open histogram) or FITC-labeled IgG1 isotype control (shaded histogram) as described in “Materials and methods.” Forward- and side-scatter gates were set to analyze only platelets. This experiment was repeated 3 times with similar results. (D) Immunocytochemistry of human bone marrow megakaryocyte for PPARγ. (Left) Diff-Quik staining of a human bone marrow megakaryocyte. Immunohistochemistry was performed with a mouse monoclonal PPARγ antibody as described in “Materials and methods.” (Right) PPARγ expression. (Middle) Mouse IgG1 isotype control was also used to show nonspecific staining. In addition to PPARγ immunostaining, light counterstaining was performed with hematoxylin to visualize the cells. The arrows are pointing at human megakaryocytes. Original magnification is × 600. Data are representative of 4 experiments from 4 patients with similar results.
Immunocytochemistry demonstrating PPARγ expression in human megakaryoblast cells and platelets. Immunocytochemistry was performed with a rabbit polyclonal anti-PPARγ antibody as described in “Materials and methods.” Nonspecific staining was assessed using a rabbit IgG isotype control. (A) Nucleated cells and enucleated plateletlike cells of the Meg-01 cell line were stained for PPARγ. Meg-01 cells stain in the nucleus and the cytoplasm. Results were repeated 4 times with separate preparations of Meg-01 cells. Original magnification is × 600. (B) Human platelets express PPARγ. The staining pattern for PPARγ is throughout the platelets. Data are representative of 4 different donor platelet experiments with similar results. Original magnification is × 1000. The inset represents 1 platelet with a final magnification of × 2000. (C) Flow cytometric analysis for intracellular expression of PPARγ in human platelets. Purified platelets were washed and stained with a monoclonal FITC-labeled anti-PPARγ antibody (open histogram) or FITC-labeled IgG1 isotype control (shaded histogram) as described in “Materials and methods.” Forward- and side-scatter gates were set to analyze only platelets. This experiment was repeated 3 times with similar results. (D) Immunocytochemistry of human bone marrow megakaryocyte for PPARγ. (Left) Diff-Quik staining of a human bone marrow megakaryocyte. Immunohistochemistry was performed with a mouse monoclonal PPARγ antibody as described in “Materials and methods.” (Right) PPARγ expression. (Middle) Mouse IgG1 isotype control was also used to show nonspecific staining. In addition to PPARγ immunostaining, light counterstaining was performed with hematoxylin to visualize the cells. The arrows are pointing at human megakaryocytes. Original magnification is × 600. Data are representative of 4 experiments from 4 patients with similar results.
To further demonstrate expression of PPARγ protein in human platelets, flow cytometry experiments were performed. Concentrated and washed human platelets were incubated with monoclonal FITC-labeled anti-PPARγ antibody or FITC-labeled IgG1 isotype for 30 minutes and analyzed on a Becton Dickinson FACS Caliber flow cytometer. Platelets, being very small enucleate cells, have a low forward- and side-scatter profile compared with white blood cells. The flow cytometry results showed that PPARγ protein was expressed in more than 85% of platelets (Figure 2C).
Human bone marrow megakaryocytes express PPARγ protein
Based on the fact that platelets and the Meg-01 cells expressed PPARγ protein, we hypothesized that human megakaryocytes would also express PPARγ protein. Expression of PPARγ in human bone marrow megakaryocytes was detected by immunocytochemistry using a monoclonal anti-PPARγ antibody. Human bone marrow was stained with Diff-Quik to identify human megakaryocytes (Figure 2D). The megakaryocyte is the largest cell of bone marrow with multilobated nuclei and abundant granular cytoplasm. Bone marrow smears were also prepared for immunocytochemistry to stain for PPARγ. The right-hand panel of Figure 2D shows staining of human megakaryocytes for PPARγ. The middle panel shows no staining with an isotype control antibody (smear is lightly counterstained with hematoxylin).
PPARγ mRNA is expressed in the Meg-01 cell line but not in platelets
Expression of PPARγ mRNA in Meg-01 and platelets was examined by RT-PCR. Platelets, while enucleate, do express a range of mRNA species.33 Total RNA was isolated from Meg-01 cells and single donor or pooled platelets and reverse transcribed as described in “Materials and methods.” Then cDNA was run in PCR reactions with control β-actin primers or primers specific for human PPARγ. RNA from human adipose tissue and THP1 human monocyte cells was used as positive control for PPARγ. The results revealed a single RT-PCR product of the expected size of 360 bp for PPARγ in adipose tissue (Figure 3, lane 2). Meg-01 cells and the THP1 monocytic cells express PPARγ mRNA (lanes 6 and 7, respectively). PPARγ mRNA was not present in platelet samples (lanes 3-5). All samples did express β-actin mRNA, consistent with reports that platelets express mRNA encoding β-actin.34
The human megakaryocyte cell line, Meg-01, but not human platelets express PPARγ mRNA. Total RNA was isolated from Meg-01 cells (lane 6) and human platelets (lanes 3-5) and reverse transcribed into cDNA. The cDNA was amplified with primers specific for β-actin (539-bp product, as a control) or PPARγ (360-bp product). A 100-bp ladder was loaded in lane 1. Human adipose tissue (lane 2) and the human monocyte cell line (THP1; lane 7) were used as positive controls. Platelet samples were from a single donor (lane 3) or pooled from several donors (lanes 4-5). Reverse-transcriptase (–) controls were negative in all cases (data not shown).
The human megakaryocyte cell line, Meg-01, but not human platelets express PPARγ mRNA. Total RNA was isolated from Meg-01 cells (lane 6) and human platelets (lanes 3-5) and reverse transcribed into cDNA. The cDNA was amplified with primers specific for β-actin (539-bp product, as a control) or PPARγ (360-bp product). A 100-bp ladder was loaded in lane 1. Human adipose tissue (lane 2) and the human monocyte cell line (THP1; lane 7) were used as positive controls. Platelet samples were from a single donor (lane 3) or pooled from several donors (lanes 4-5). Reverse-transcriptase (–) controls were negative in all cases (data not shown).
Meg-01 PPARγ has DNA binding ability that is enhanced by treatment with PPARγ ligands
To determine if the PPARγ protein in Meg-01 cells can bind DNA, gel shift assays were performed. In many systems enhanced DNA binding is observed if PPARγ-expressing cells are first exposed to a PPARγ agonist.35 Meg-01 cells were treated with the PPARγ agonists 15d-PGJ2 (5 μM) or ciglitazone (10 μM) or vehicle (DMSO) for 4 hours in culture. Nuclear protein was then incubated with a radiolabeled probe containing the consensus DNA binding sequence for PPARγ (Figure 4A). Figure 4 shows that Meg-01 cells have a constitutive level of active PPARγ (lane 2), which was increased by exposure to the natural PPARγ agonist 15d-PGJ2 (lane 3) and to the synthetic PPARγ agonist ciglitazone (lane 4). A supershift using an anti-PPARγ antibody further supported PPARγ expression in Meg-01 cells (lane 6). We conclude that 15d-PGJ2 and ciglitazone increase the activation of PPARγ in Meg-01 cells.
Meg-01 cells and human platelets contain PPARγ that binds the PPARγ DNA consensus sequence. (A) 15d-PGJ2 and ciglitazone induce DNA binding of PPARγ protein in MEG-01 cells. After treatment with 15d-PGJ2 (lane 3) or ciglitazone (lane 4) or DMSO (vehicle control, lane 2), EMSA was performed. Lane 1 was loaded with free probe (no lysate), and lane 5 is nuclear extract from 15d-PGJ2–treated cells incubated with unlabeled probe (cold competitor) as a control for binding specificity. Lane 6 shows the locations of shifted and supershifted PPARγ (supershift with an anti-PPARγ antibody). Shift assays were repeated 3 times with similar results. (B) EMSA shows that platelets have PPARγ DNA binding activity. Platelet extracts were prepared without any treatment from 3 different pooled platelets as described in “Materials and methods.” Lane 1 shows radioactive-labeled probe. Cell extracts (50 μg) were incubated with 32P-labeled PPARγ oligonucleotides (lanes 2-4) or cold competitor (unlabeled probe) (lanes 5-7) and run on a 4% nondenaturing gel. Lanes 8 to 10 indicate the locations of supershifted bands with anti-PPARγ antibody. (C) TransAMTM solid-phase PPARγ DNA binding activity measurements show that platelets have some active DNA binding PPARγ without treatment with PPARγ agonist. However, exposure to PPARγ agonist (20 μM 15d-PGJ2, ciglitazone, rosiglitazone) significantly enhances binding to the PPARγ DNA response element. Assay background in this experiment was 0.02 optical density (OD).
Meg-01 cells and human platelets contain PPARγ that binds the PPARγ DNA consensus sequence. (A) 15d-PGJ2 and ciglitazone induce DNA binding of PPARγ protein in MEG-01 cells. After treatment with 15d-PGJ2 (lane 3) or ciglitazone (lane 4) or DMSO (vehicle control, lane 2), EMSA was performed. Lane 1 was loaded with free probe (no lysate), and lane 5 is nuclear extract from 15d-PGJ2–treated cells incubated with unlabeled probe (cold competitor) as a control for binding specificity. Lane 6 shows the locations of shifted and supershifted PPARγ (supershift with an anti-PPARγ antibody). Shift assays were repeated 3 times with similar results. (B) EMSA shows that platelets have PPARγ DNA binding activity. Platelet extracts were prepared without any treatment from 3 different pooled platelets as described in “Materials and methods.” Lane 1 shows radioactive-labeled probe. Cell extracts (50 μg) were incubated with 32P-labeled PPARγ oligonucleotides (lanes 2-4) or cold competitor (unlabeled probe) (lanes 5-7) and run on a 4% nondenaturing gel. Lanes 8 to 10 indicate the locations of supershifted bands with anti-PPARγ antibody. (C) TransAMTM solid-phase PPARγ DNA binding activity measurements show that platelets have some active DNA binding PPARγ without treatment with PPARγ agonist. However, exposure to PPARγ agonist (20 μM 15d-PGJ2, ciglitazone, rosiglitazone) significantly enhances binding to the PPARγ DNA response element. Assay background in this experiment was 0.02 optical density (OD).
Platelets have constitutively active PPARγ protein that has DNA binding ability
EMSA was next performed to determine if platelet PPARγ protein can bind to the DNA PPAR response element. Lysates from 3 different rigorously purified platelet samples were incubated with a radioactive probe (PPARγ consensus DNA binding sequence) or cold probe (Figure 4B). A discrete DNA binding band appears in the 3 different platelet samples (lanes 2-4). The band disappears when extracts were incubated with excess cold probe (lanes 5-7). A supershift assay using a specific anti-PPARγ antibody was also performed and the bands shifted to a higher mass consistent with PPARγ (lanes 8-10). We also measured the ability of platelet-derived PPARγ to bind its DNA consensus sequence using the TransAMTM PPARγ assay kit (Active Motif). In this method the consensus DNA sequence for PPARγ binding (or as a control, mutated oligonucleotides) is plate bound. A cell lysate is then added to the well, washed, and next incubated with an enzyme-conjugated anti-PPARγ antibody that recognizes only DNA-bound PPARγ. Following substrate addition, a colored product is formed. Platelets were exposed to buffer, 15d-PGJ2, ciglitazone, or rosiglitazone (20 μM for all) for 2 hours at 37° C and then protein was extracted. The measurements demonstrate that platelet PPARγ binds DNA even without treatment with PPARγ agonists, but binds 3- to 4-fold more strongly in the presence of PPARγ agonists (Figure 4C). The ability of platelet PPARγ to bind DNA in the absence of deliberate addition of PPARγ ligand suggests that platelets do contain an endogenous ligand. One possible ligand is lysophosphatidic acid, which platelets are known to produce.36 Overall, these results further support that platelets express PPARγ and that platelet PPARγ retains its DNA binding ability.
PPARγ agonists prevent activated platelet release of CD40L, TXB2, and ATP and inhibit platelet aggregation
We speculated that platelet PPARγ played a role in attenuating platelet activation. In order to begin to test the theory, we isolated human platelets and exposed them to the PPARγ ligands, 15d-PGJ2 or rosiglitazone, for 15 minutes at 37° C. Platelets were then incubated with buffer or with thrombin, a powerful platelet activator. Upon platelet activation, the cells expel key bioactive mediators important for thrombosis, inflammation, and vascular disease including CD40L and TXB2.23,37 As shown in Figure 5, the release of CD40L and TXB2 was largely prevented in platelets exposed to a naturally occurring PPARγ agonist, 15d-PGJ2, as well as to rosiglitazone, a synthetic PPARγ agonist. The thrombin-induced increase in platelet surface CD40L was also prevented by the PPARγ agonists as measured by flow cytometry (Figure 6).
PPARγ agonists block platelet release of CD40L and TXB2. Purified human platelets were exposed to buffer or with 20 μM 15d-PGJ2 or rosiglitazone for 15 minutes. The platelets were then activated with 0.8 U/mL thrombin and the supernatants collected at the times shown. Specific ELISA and enzyme immunoassays for CD40L (A) and TXB2 (B) levels were performed. (A) The increase in supernatant CD40L over time was statistically significant after thrombin activation compared with untreated or PPARγ agonist pretreated samples (P = .0006 by the log-rank test). There were no significant differences in CD40L release when comparing untreated samples with those treated with PPARγ agonist and thrombin. Mean ± SD values are shown. (B) The increase in supernatant TXB2 over time was statistically significant after thrombin activation compared with untreated or PPARγ agonist and thrombin-treated platelets (P = .0004 by the log-rank test). These data are representative of 3 separate experiments. *Significantly different from samples treated with 15d-PGJ2 or rosiglitazone.
PPARγ agonists block platelet release of CD40L and TXB2. Purified human platelets were exposed to buffer or with 20 μM 15d-PGJ2 or rosiglitazone for 15 minutes. The platelets were then activated with 0.8 U/mL thrombin and the supernatants collected at the times shown. Specific ELISA and enzyme immunoassays for CD40L (A) and TXB2 (B) levels were performed. (A) The increase in supernatant CD40L over time was statistically significant after thrombin activation compared with untreated or PPARγ agonist pretreated samples (P = .0006 by the log-rank test). There were no significant differences in CD40L release when comparing untreated samples with those treated with PPARγ agonist and thrombin. Mean ± SD values are shown. (B) The increase in supernatant TXB2 over time was statistically significant after thrombin activation compared with untreated or PPARγ agonist and thrombin-treated platelets (P = .0004 by the log-rank test). These data are representative of 3 separate experiments. *Significantly different from samples treated with 15d-PGJ2 or rosiglitazone.
PPARγ agonists block the thrombin-induced increase in platelet surface CD40L expression. Purified human platelets were exposed to 20 μM 15d-PGJ2 or rosiglitazone for 15 minutes and were then stimulated with 0.8 U/mL thrombin for 60 minutes. The platelets were then stained and prepared for flow cytometry with a monoclonal antihuman CD40L antibody or with control isotype antibody. The graph shows a representative experiment with the results presented as the percent of surface CD40L+ platelets.
PPARγ agonists block the thrombin-induced increase in platelet surface CD40L expression. Purified human platelets were exposed to 20 μM 15d-PGJ2 or rosiglitazone for 15 minutes and were then stimulated with 0.8 U/mL thrombin for 60 minutes. The platelets were then stained and prepared for flow cytometry with a monoclonal antihuman CD40L antibody or with control isotype antibody. The graph shows a representative experiment with the results presented as the percent of surface CD40L+ platelets.
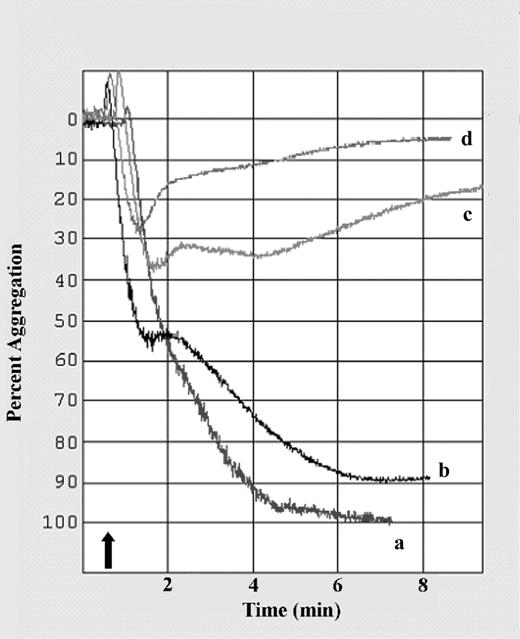
To determine if a PPARγ agonist would inhibit platelet aggregation, the natural PPARγ agonist 15d-PGJ2 was added to PRP and aggregation and ATP release were stimulated with ADP. As shown in Figure 7, there was a concentration-dependent inhibition of platelet aggregation as shown by the results of a representative experiment. The initial slope of platelet aggregation, measured within the first 16 seconds after ADP addition, and the amplitude were significantly inhibited with 20 μM 15d-PGJ2. The slope was 83 ± 5% (mean ± SEM) of the normal (untreated) and the amplitude of aggregation was 64 ± 11% of normal platelets (n = 7, P = .02 for both). ATP release was also significantly inhibited by 20 μM 15d-PGJ2 with a slope of 15 ± 5% of normal and an amplitude of 22 ± 10% of normal platelets (n = 7, P < .0008 for both). These findings support a role for PPARγ in down-modulating platelet activation.
The PPARγ agonist 15d-PGJ2 inhibits platelet aggregation induced by ADP. Platelet aggregation was stimulated by addition of 5 μM ADP in the absence (a) or presence of 5 μM (b), 10 μM (c), or 20 μM (d) 15d-PGJ2. Addition of ADP is indicated by the arrow. 15d-PGJ2 dose dependently inhibited platelet aggregation. Shown is 1 representative experiment of 7.
The PPARγ agonist 15d-PGJ2 inhibits platelet aggregation induced by ADP. Platelet aggregation was stimulated by addition of 5 μM ADP in the absence (a) or presence of 5 μM (b), 10 μM (c), or 20 μM (d) 15d-PGJ2. Addition of ADP is indicated by the arrow. 15d-PGJ2 dose dependently inhibited platelet aggregation. Shown is 1 representative experiment of 7.
Discussion
PPARγ is believed to be expressed only by nucleated cells since it is known as a transcription factor mainly located in the nucleus.38 However, recent studies have showed that PPARγ is not restricted to the nucleus, but is also expressed in the cytoplasm.2,3 Moreover, based on the emerging concept that platelets and their products enhance inflammation and atherogenesis, we hypothesized that human megakaryocytes and their cytoplasmic fragments, namely platelets, express PPARγ.
Our results provide the first evidence that the Meg-01 cell line, human bone marrow megakaryocytes, and human platelets express PPARγ. The presence of PPARγ protein was demonstrated by Western blotting with several different anti-PPARγ antibodies, immunocytochemistry, flow cytometry, and by peptide mapping analysis. As shown by EMSA and gel shift assay, the Meg-01 cell line and human platelets have active PPARγ protein with the ability to bind DNA. This was also shown by the TransAM PPARγ DNA binding assay. Megakaryocytes, the precursor cell of platelets, express a wide range of mRNA encoding for a variety of bioactive mediators.39 The Meg-01 cell line was used to test for the presence of PPARγ mRNA, and these cells do express PPARγ mRNA. Interestingly, the enucleate platelet does express some mRNAs.33 However, while we found β-actin mRNA in platelets, no PPARγ mRNA was detected. This finding supports the concept that platelets have preformed PPARγ protein.
Our findings that platelets contain the transcription factor PPARγ and that PPARγ agonists blunt platelet activation suggest a novel nontranscriptional function for PPARγ. The exact location of PPARγ in the platelet is unknown, but based on immunohistochemical staining of platelets (Figure 2B), it may be contained in granules with the bulk of the PPARγ being distributed throughout the platelet. Since there is abundant PPARγ permeating the platelet, it will likely have a pivotal role in regulating multiple platelet functions. Clearly, platelet PPARγ retains its DNA binding ability, which would appear to be unneeded in platelets; we therefore suggest that PPARγ must also possess other functions, which may include interactions with intracellular platelet proteins. There are several steps during platelet exocytosis wherein PPARγ could interfere, including calcium or protein kinase C signaling pathways, rearrangement of the cytoskeleton during platelet activation, or docking and fusion of granules with the plasma membrane. Further studies to determine the novel PPARγ targets in platelets will be necessary to thoroughly define the mechanism of platelet inhibition by PPARγ agonists.
Little is known about the in vivo ligands for PPARγ. One possibility in the bone marrow is that megakaryocytes generate 15d-PGJ2, as they are known to produce its precursor PGD2.40 This could modulate PPARγ activity in the bone marrow. PPARγ may be involved in the differentiation and proliferation of bone marrow cells and may have additional immunologically relevant effects in erythroid, myeloid, monocytic, megakaryocytic, T- and B-lymphocytic, stromal, and endothelial cell function. In the study described herein, we demonstrate that 15d-PGJ2 and the thiazolidinedione class of antidiabetic drugs, ciglitazone and rosiglitazone, play an important role in attenuating platelet activation. This was demonstrated by the ability of PPARγ agonists to block thrombin-induced platelet release of TXB2, CD40L, and surface-associated CD40L. In addition, the PPARγ agonist 15d-PGJ2 blunted ADP-induced platelet aggregation and ATP release. Platelets, the most numerous, enucleate, and tiny blood cells, are not only essential for clotting, but are broadly involved in inflammation and pathogenesis. Platelets contain proinflammatory and bioactive mediators that include transforming growth factor-β, prostaglandins, thromboxanes, and CD40L. TXA2 potentiates platelet aggregation at concentrations produced by activated platelets and mediates fever and inflammation by induction of the Cox-2 enzyme.41,42 Platelets have the highest expression of CD40L of any human cell. Platelet-released CD40L, as well as CD40L expressed on the platelet surface, could activate nearby CD40-expressing cells. Recent studies show that platelets contribute to mucosal inflammation and the atherosclerosis process by expressing and releasing CD40L.24,28 CD40L is now also considered a primary platelet agonist.43 Since platelets are activated by their own released CD40L through B3 integrin binding, a decrease in CD40L by PPARγ ligands could reduce platelet activation, including thrombosis.43 Patients with unstable angina have higher blood concentrations of CD40L than healthy people, perhaps due to release from activated platelets.44 Platelet surface expression of CD40L and evidence for high CD40L levels in atheromatous plaques have served to focus attention on platelets in atherosclerosis. CD40-CD40L interaction promotes proinflammatory and proatherogenic effects in vitro and in vivo.45 It has been shown that the binding of CD40L to its corresponding cellular receptors stimulates production of other proinflammatory cytokines, such as tumor necrosis factor-alpha and IL-1 by leukocytes and vascular endothelium.22
The pathogenesis of type 1 and type 2 diabetes involves inflammation with elevated blood levels of CD40L as in atherosclerosis.46 PPARγ-activating thiazolidinediones, novel insulin-sensitizing antidiabetic agents, have been shown to exhibit anti-inflammatory effects.6,12 Interestingly, it was recently shown that treatment of diabetic patients with a thiazolidinedione-type drug decreased circulating CD40L blood levels.46,47 Based on our findings, we speculate that the reduced blood levels of CD40L in that study could have been due to inhibition of platelet release of CD40L by the dampening effects of the PPARγ agonist drug. Furthermore, our findings that the PPARγ agonist 15d-PGJ2 inhibited platelet aggregation and ATP release support a potential therapeutic approach to inhibit platelet function in diabetics and other patients. Obviously, further clinical study is required to fully evaluate the effects of natural and synthetic PPARγ agonists on platelets in human beings.
The foundation studies we report demonstrating platelet PPARγ expression and its role in tempering platelet activation have revealed a novel target for PPARγ agonists. The emerging role of platelets as mediators of inflammation suggests that some of the anti-inflammatory effects of PPARγ may be mediated through dampening platelet activation, especially CD40L release. Our findings support continued evaluation of natural and synthetic PPARγ agonists as regulators of thrombosis and anti-inflammatory agents.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2004-03-0926.
Supported by TUBITAK (The Scientific and Technical Research Council of Turkey)/NATO-A2, National Institutes of Health (NIH) Training Program in Oral Infectious Diseases (T32-DE07165, NIH DE011390, and ES001247), a Cancer Center Discovery Award (HL-30616), and RR14682 from the National Center for Research Resources of the NIH.
F.A. and D.M.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rosemary Ziemba-Ball for providing human bone marrow smears, and Chantal Turner and Kathryn Seweryniak for performing some of the ELISAs. We also thank Brian H. Smith and Stephen Pollock for providing technical assistance.

![Figure 1. PPARγ protein is expressed in the human megakaryoblast cell line, Meg-01, and by human platelets. (A) Western blot of Meg-01 cell line (15 μg) using a polyclonal anti-PPARγ antibody (Calbiochem). PPARγ bands co-migrated with the human adipose tissue protein extract (15 μg) used as positive control. (B-C) Platelet cell lysates (15 μg) from rigorously purified single donor or pooled platelets were analyzed by Western blot for PPARγ using 2 different anti-PPARγ antibodies (panel B is monoclonal anti-PPARγ [Santa Cruz Biotechnology]; panel C is polyclonal anti-PPARγ [Calbiochem]). Human adipose tissue protein extract (5 μg) was used as positive control (left lane). The PPARγ protein was shown for 3 different single donor and pooled platelet samples. Purified human red blood cells (30 μg) are negative for PPARγ (B). Data are representative of more than 5 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-0926/5/m_zh80170465850001.jpeg?Expires=1769097158&Signature=QON0CTJsgvptRUVvGALmnj9EX-BjSuh8t0sUS8c40BSPahe1bv3WovEx9gedFZB7cGuiDijtl7xm4F6brDUCuK0KlfTXO8z4fy6yhxAWHQVvqrF~ZGKSO6L2vHuWyBOYhaMbaUM8hhOsaPCK3gb~oDgIDRlthzxejmZ25TyHvi5biQGIDsawYaxdQhP8ztt1rolXCDl-BYeODoiUZeK884Ii3paNZZG7G3OPH125zNlIZgrfG5aOQo0aIwMH~f8kh751EYWlR-gasYGhoMC2HfyA~QxTh4XBYBSA6Ujc~zSFRivpA8X8AT2QQPhXtYYAX1rDwrzbTXKpHGrgnb6MCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal