Abstract
We previously reported on a 43-year-old patient with Wiskott-Aldrich syndrome (WAS) who experienced progressive clinical improvement and revertant T-cell mosaicism. Deletion of the disease-causing 6-bp insertion was hypothesized to have occurred by DNA polymerase slippage. We now describe 2 additional patients from the same family who also had revertant T lymphocytes that showed selective in vivo advantage. Somatic mosaicism was demonstrated on leukocytes cryopreserved in the first patient when he was 22 years old, 11 years before his death from kidney failure. The second patient is now 16 years old, has a moderate clinical phenotype, and developed revertant cells after the age of 14 years. These results support DNA polymerase slippage as a common underlying mechanism, and they indicate that T-cell mosaicism may have different clinical effects in WAS.
Introduction
The Wiskott-Aldrich syndrome (WAS) is a rare X-linked disorder characterized by thrombocytopenia, eczema, and immunodeficiency and caused by mutations of the WAS protein (WASP) gene.1 Although a phenotype/genotype correlation can usually be verified,2,3 WAS siblings with significantly different phenotypes have also been observed.4 The existence of WASP gene modifiers may explain the latter findings, similar to what is observed for chronic granulomatous disease.5 However, the recent description of a WAS patient with revertant T lymphocytes and spontaneous clinical improvement6 has suggested that somatic mosaicism may also explain the variable phenotypic presentation among WAS siblings and the inconsistent genotype/phenotype correlation in this disease.
WASP expression in peripheral blood mononuclear cells (PBMCs) can be analyzed by fluorescence-activated cell sorting (FACS) that allows easy discrimination of patients with defective WASP expression7-9 from those with somatic mosaicism.6,10,11 Using FACS analysis, we have previously identified a WAS patient with T-cell somatic mosaicism caused by spontaneous in vivo reversion of a 6-bp insertion.6 In the present study, we analyzed samples from 3 additional WAS patients belonging to the same kindred and found that 2 of them also carried WASP-expressing revertant cells.
Study design
Patients
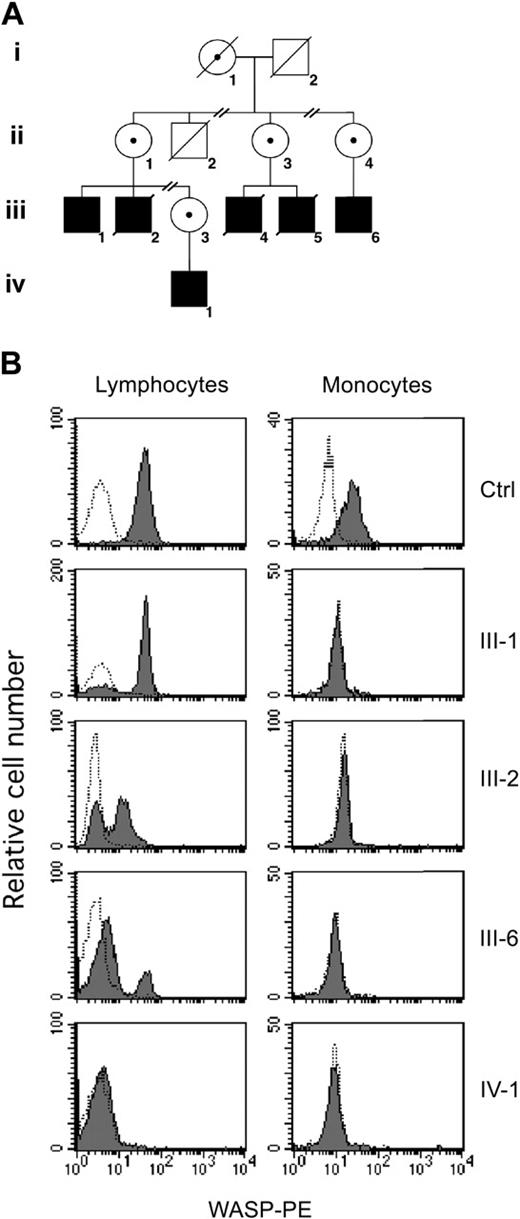
Clinical and immunologic data of patient III-1 (Figure 1A) have already been published.6 Patient III-2, the brother of patient III-1, had petechiae at birth and underwent splenectomy at age 2. He had pneumococcal meningitis at age 7 years and Pneumocystis carinii pneumonia at age 14 years. He also had continuous vasculitis in the lower extremities, esophagus, kidneys, and coronary arteries. At the age of 18 years, he was admitted to the hospital for Escherichia coli sepsis and was noted to have impaired renal function. At age 26 years he had a myocardial infarction, and at age 33 years he died of renal failure. This clinical history is representative of a severe WAS phenotype (clinical score of 5, as per Zhu et al2 ).
Family tree and analysis of WASP expression. (A) Simplified pedigree of the study family. Solid squares represent affected men; diagonal lines indicate deceased patients. Carrier status in female patients is indicated by a central dot. (B) Flow cytometric analysis of WASP. PBMCs were fixed, permeabilized, and stained with anti-WASP monoclonal antibody (mAb) (solid histogram) or a control antibody (open histogram), followed by phycoerythrin (PE)–conjugated goat antimouse antibody. Shown are the results of WASP expression in lymphocytes and monocytes from a healthy control and from patients III-1, III-2, III-6, and IV-1.
Family tree and analysis of WASP expression. (A) Simplified pedigree of the study family. Solid squares represent affected men; diagonal lines indicate deceased patients. Carrier status in female patients is indicated by a central dot. (B) Flow cytometric analysis of WASP. PBMCs were fixed, permeabilized, and stained with anti-WASP monoclonal antibody (mAb) (solid histogram) or a control antibody (open histogram), followed by phycoerythrin (PE)–conjugated goat antimouse antibody. Shown are the results of WASP expression in lymphocytes and monocytes from a healthy control and from patients III-1, III-2, III-6, and IV-1.
Patient III-6 (age 16 years) was given a diagnosis of WAS at birth because of positive family history and platelet counts of 40 to 60 109/L (40 000-60 000/μL). He underwent splenectomy at the age of 1 year and was found at age 3 years to have glomerulitis, mild eczema, and repeated otitis media that resulted in the need for myringotomy when he was 14 years old. Molluscum contagiosum also developed, but it resolved spontaneously. Patient III-6 is now in good health on prophylactic antibiotic treatment (score of 3).
Patient IV-1 (age 1 year) had petechiae and thrombocytopenia (15-20 = 109/L [15 000-20 000/μL]) during the perinatal period. He has no history of infections or other complications of the disease except for moderate eczema. This presentation is compatible with a score of 2, although the clinical picture of the disease might not yet be fully developed in this patient because of his young age.
Analysis of WASP gene mutations and expression
Analysis of the WASP gene mutations and FACS analysis of WASP expression was performed as described.6,11 Detection of WASP exon 4 mutated and revertant sequences from genomic DNA of fresh or cryopreserved PBMCs was performed after polymerase chain reaction (PCR) amplification, as described,6 followed by subcloning of PCR products (Topo TA; Invitrogen, Carlsbad, CA) and sequencing of isolated clones.6,11
Results and discussion
The mutation causing WAS in this family is a 6-bp insertion in exon 4 of WASP, which abrogates protein expression.6,12 We have recently described revertant mosaicism in patient III-1 (Figure 1A).6 Because the ACGAGG insertion followed a tandem repeat of the same 6-bp sequence, we hypothesized that both the original mutation and the reversion were caused by a DNA polymerase slippage facilitated by the specific structural characteristics of the neighboring DNA.6 Accordingly, other affected members from the same family would be expected to be prone to similar slippage events and consequent somatic mosaicism. Therefore, we studied WASP expression in fresh PBMCs from patients III-1, III-6, and IV-1 and frozen PBMCs from patient III-2 at the age of 22 years. As previously observed, mosaic expression of WASP was detected in lymphocytes, but not monocytes, of patient III-1 (Figure 1B). Interestingly, similar results were obtained in samples from patients III-2 and III-6, but no WASP expression could be detected in lymphocytes or monocytes from the youngest patient (patient IV-1).
Analysis of the WASP exon 4 genomic sequence in subcloned PCR products obtained from PBMCs of patients III-2 and III-6 showed the coexistence of the 6-bp insertion and wild-type sequences (data not shown), indicating that the same true back mutation found in patient III-1 was responsible for the occurrence of revertant, WASP-expressing cells in these patients.
As in patient III-1, WASP-expressing cells were clearly detectable only among T lymphocytes of patients III-2 and III-6. CD4+ and CD8+ T-cell subsets contained revertant cells (Table 1),6 whereas no WASP-expressing cells were detectable within B and natural killer (NK) cells. These findings suggest that the revertant event occurred at the level of a T-cell progenitor before CD4/CD8 commitment. Alternatively, the reversion might have occurred in a multipotent hematopoietic progenitor, but it manifested itself only within T cells in which WASP-expressing cells have a selective advantage.6,11,13,14 Evidence of selective advantage of the revertant T lymphocytes was also found in patients III-2 and III-6, whose percentage of WASP-expressing cells among CD45RO+ memory T cells was markedly higher than in CD45RO– naive T lymphocytes (Table 1).
Immunologic features and WASP expression in lymphocyte subsets
. | III-1 . | III-2* . | III-6 . |
|---|---|---|---|
| Absolute blood counts, × 109/L | |||
| White blood cells | 13.6 | 11.2 | 11.1 |
| Lymphocytes | 6.19 | 2.24 | 3.4 |
| CD3+ T cells | 5.8 | 1.65 | 1.29 |
| WASP+ cells in lymphocyte subsets, % | |||
| Lymphocytes | 79.5 | 56.7 | 11.7 |
| CD3+ T cells | 79.6 | 79.5 | 27.6 |
| CD4+ T cells | 85.9 | 60.3 | 1.9 |
| CD8+ T cells | 69.7 | 79.4 | 64.3 |
| CD45RO-CD3+ T cells | 42.0 | 65.4 | 18.9 |
| CD45RO+CD3+ T cells | 86.0 | 87.3 | 43.0 |
| CD20+ B cells | ND | NA | ND |
| CD56+ NK cells | ND | NA | ND |
. | III-1 . | III-2* . | III-6 . |
|---|---|---|---|
| Absolute blood counts, × 109/L | |||
| White blood cells | 13.6 | 11.2 | 11.1 |
| Lymphocytes | 6.19 | 2.24 | 3.4 |
| CD3+ T cells | 5.8 | 1.65 | 1.29 |
| WASP+ cells in lymphocyte subsets, % | |||
| Lymphocytes | 79.5 | 56.7 | 11.7 |
| CD3+ T cells | 79.6 | 79.5 | 27.6 |
| CD4+ T cells | 85.9 | 60.3 | 1.9 |
| CD8+ T cells | 69.7 | 79.4 | 64.3 |
| CD45RO-CD3+ T cells | 42.0 | 65.4 | 18.9 |
| CD45RO+CD3+ T cells | 86.0 | 87.3 | 43.0 |
| CD20+ B cells | ND | NA | ND |
| CD56+ NK cells | ND | NA | ND |
ND indicates not detected; and NA, not available.
Immunophenotyping and flow cytometric analysis of WASP were performed on frozen samples.
Revertant somatic mosaicism has been described in an increasing number of primary immunodeficiencies6,10,11,15-18 and has resulted in progressively improving or mild clinical phenotype in patients with adenosine deaminase deficiency,16 X-linked severe combined immunodeficiency,15 and WAS (patient III-16 ). In the latter, revertant cells comprised approximately 80% of the patient's lymphocytes and CD3+ T cells (Table 1).6 In 3 other WAS patients with levels of revertant lymphocytes ranging between 10% and 15%, clinical improvement could not be demonstrated.10,11 In one of these patients, revertant cells were clearly detected only among CD8+ fresh T cells (although revertant CD4+ T cells could be demonstrated after immortalization), whereas the other 2 patients carried approximately 25% to 37% of revertant CD3+ T cells, with higher proportions of revertant cells in CD4+ cells than in the CD8+ subset. All 3 patients had clinical scores of 5 when revertant cells were described.10,11 Information is limited regarding 5 additional patients with revertant WAS.19,20 Previous studies, therefore, suggested that high levels of revertant T cells may be needed to elicit clinical effects in WAS.
Regarding the patients of our study, the overall moderate phenotype of patient III-6 does not allow us to draw clear conclusions about the effects of revertant cells. In patient III-2, the severe complications of the disease were apparently not mitigated by the presence of approximately 80% WASP-expressing T lymphocytes. One possible explanation is that these cells might have appeared and accumulated in this patient after multiple and irreversible organ damage had developed. Results from previous studies combined with our current observations indicate that despite consistent evidence of an in vivo selective advantage of revertant, WASP-expressing T lymphocytes, the potential clinical consequences of T-cell mosaicism in WAS remain difficult to predict.
Siblings with revertant mosaicism11,19,21 have been reported, but, to our knowledge, 3 patients with revertant disease in a single kindred is unprecedented. The finding of the same true back mutation in all 3 patients with mosaicism strongly suggests a common underlying mechanism that likely involves DNA polymerase slippage.
Finally, in vivo reversion appears to be a phenomenon of relatively high incidence in WAS patients. It seems reasonable to assume that the rate of reversion is similar in WAS as it is in other disorders. If this is so, the high incidence of reversion events in WAS could be attributed to the increased survival of WAS patients, providing a favorable biologic environment to allow revertant cells to accumulate to levels that reach the detection threshold. Alternatively, it is tempting to speculate that increased genomic instability in WAS is responsible for a higher rate of reversion events.
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2004-03-0846.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Robert Childs for his devoted patient care and Jacqueline Keller for her secretarial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal