Abstract
Symptomatic patients with Gaucher disease (GD) (acid β-glucosidase [Gcase] deficiency) are treated with injectable human recombinant GCase. Treatment results in significant decreases in lipid storage in liver, spleen, and bone marrow, but the generalized osteopenia and focal bone lesions present in many adult patients are refractory to treatment. A double-blind, 2-arm, placebo-controlled trial of alendronate (40 mg/d) was performed in adults with GD who had been treated with enzyme for at least 24 months. Primary therapeutic endpoints were improvements in (1) bone mineral density (BMD) and content (BMC) at the lumbar spine, and (2) focal lesions in x-rays of long bones assessed by a blinded reviewer. There were 34 patients with GD type 1 (age range, 18-50 years) receiving enzyme therapy who were randomized for this study. After 18 months, ΔBMD at the lumbar spine was 0.068 ± 0.21 and 0.015 ± 0.034 for alendronate and placebo groups, respectively (P = .001). Long-bone x-rays showed no change in focal lesions or bone deformities in any subject in either arm. Alendronate is a useful adjunctive therapy in combination with enzyme replacement therapy (ERT) for the treatment of GD-related osteopenia in adults, but it cannot be expected to improve focal lesions.
Introduction
Gaucher disease (GD) refers to the heterogeneous clinical signs and symptoms caused by the decreased intracellular hydrolysis of glucosylceramide and other glucosphingolipids, and is primarily due to recessive mutations in the gene encoding the lysosomal hydrolase, acid β-glucosidase (GCase).1 GD has 3 clinical forms based on the absence (type 1) or presence and severity of primary central nervous system (CNS) involvement (type 2, severe; type 3, mild to moderate).2 Massive splenomegaly and large storage cells in the spleen and bone marrow are the hallmarks of the disease,3 but nearly all affected patients also have skeletal involvement.4-8 Episodic, excruciatingly painful infarctive “bone crises” occur in 42% of patients less than 10 years of age and in 25% at more than or equal to 10 years of age.1,9-11 Most patients have focal skeletal abnormalities identified by plain x-ray, the most frequent of which are the “Erlenmeyer flask” deformity of the distal femur and lytic lesions (in descending order of appearance) in the femurs, humeri, vertebral bodies, tibias, ribs, pelvis, bones of the feet, calvarium, and mandible.12,13
In addition to focal abnormalities, generalized osteopenia is nearly universal.5,12,14 Mean bone mineral density (BMD) in adults with GD is substantially less than that for healthy age-matched adults and correlates with the degree of visceral organ involvement.15 Pathologic fracture is widely reported in GD. Femoral fractures are often associated with lytic lesions, but cortical thinning is a contributing cause. Severe osteopenia in the spine is associated with vertebral collapse in both children and adults.
The standard of care for symptomatic patients with GD is periodic infusion of human recombinant GCase. More than 3000 patients worldwide are receiving enzyme therapy.16 Enzyme therapy results in significant decreases in glucosylceramide storage in the liver, spleen, and bone marrow. Subjective improvements in the severity, frequency, and duration of painful crises usually occur within one year of initiating enzyme therapy17-20 and onset of new lytic lesions is usually halted. However, many other bone manifestations, including chronic bone pain, lytic lesions, bone deformities, and osteopenia have either required years of enzyme therapy for improvement or been refractory.20,21 Enzyme therapy's efficacy in reversing the generalized osteopenia likewise appears limited, leaving adult patients who are compliant with enzyme therapy still at risk for pathologic fracture. This delayed and modest skeletal response in some patients receiving enzyme therapy remains the major issue in the overall effectiveness and management of affected patients with GD type 1.
The etiology of Gaucher-related osteopenia is not understood. There is some evidence of elevated bone turnover based on histomorphometric indices.12 Interleukin 6 (IL-6) plasma levels, which have been implicated in localized osteolysis in multiple myeloma, are elevated nearly 3-fold in adults with GD.22 Since anecdotal evidence indicated that bisphosphonates may show some effectiveness in reversing some of the bone manifestations of GD,23-25 a controlled trial of alendronate disodium (ALN) was initiated to test the hypothesis that Gaucher-related bone disease is a high resorption state and to determine whether ALN could improve focal bone lesions and/or BMD to a greater extent than enzyme therapy alone.
Patients, materials, and methods
Study design overview
A 2-arm, double-blind, placebo-controlled design was employed. The 40 mg per day dosage was chosen to increase the chances of reversing focal lesions. Placebo tablets provided by Merck (Whitehouse Station, NJ) were matched in size and shape, and the placebo formulation had been used previously in other industry-sponsored trials by the manufacturer. All study subjects were given identical dosing instructions by the Investigational Pharmacy at Cincinnati Children's Hospital Medical Center. The 2 primary therapeutic endpoints were (1) improvements in lumbar spine BMD and bone mineral content (BMC), measured by dual energy x-ray absorptiometry (DEXA); and (2) improvements of focal lesions as evaluation of plain x-rays by a radiologist blinded to study arm (see protocol summary, Table 1). All study subjects were on enzyme therapy at a dosage selected by their treating physician, which ranged from 15 U/kg to 60 U/kg every 2 weeks. The initial trial design included the use of a second center outside the United States, as a means to test ALN response in patients receiving average lower enzyme (GCase) doses. Because that center was not able to collect key end point data, this report includes only the data collected at the US center.
Overview of study design
Design aspect . | Description . |
|---|---|
| General design | Double-blind, 2-arm; alendronate versus placebo |
| Duration | Up to 24 months |
| Study interval | 6 months |
| Treatment | Alendronate: 40 mg once per day versus placebo |
| Vitamin D: 400 U once per day | |
| Calcium carbonate (Tums): 1.5 g once per day | |
| Key inclusion criteria | Gaucher disease, on ERT more than 24 months |
| Older than 18 years and less than 50 years of age | |
| Lumbar spine Z score less than — 1 | |
| Key exclusion criteria | Pregnancy |
| Prior use of antiresorptive agents | |
| Active gastric or duodenal ulcer disease | |
| Endpoints | |
| Primary | Changes in lumber spine BMD in g/cm2 |
| Reduction in focal changes in long-bone x-rays | |
| Secondary | Reduction of biochemical indices of bone turnover |
Design aspect . | Description . |
|---|---|
| General design | Double-blind, 2-arm; alendronate versus placebo |
| Duration | Up to 24 months |
| Study interval | 6 months |
| Treatment | Alendronate: 40 mg once per day versus placebo |
| Vitamin D: 400 U once per day | |
| Calcium carbonate (Tums): 1.5 g once per day | |
| Key inclusion criteria | Gaucher disease, on ERT more than 24 months |
| Older than 18 years and less than 50 years of age | |
| Lumbar spine Z score less than — 1 | |
| Key exclusion criteria | Pregnancy |
| Prior use of antiresorptive agents | |
| Active gastric or duodenal ulcer disease | |
| Endpoints | |
| Primary | Changes in lumber spine BMD in g/cm2 |
| Reduction in focal changes in long-bone x-rays | |
| Secondary | Reduction of biochemical indices of bone turnover |
Study subjects
All study subjects had enzymatically proven type I GD, were between 18 and 50 years of age, and were on a continuous regimen of enzyme therapy for at least 24 months prior to enrollment. Women who were pregnant, had a positive pregnancy test, or were planning to be pregnant were excluded. Patients with a serum creatinine level of more than 176.8 μM (2.0 mg/dL), and/or who had a recent history of ulcer disease also were excluded. Potential subjects who had elevated lumbar BMD Z scores, or who had diseases likely to affect bone mass, or who had recently taken medications known to have a major effect on skeletal metabolism were also excluded. Subjects who had an initial lumbar BMD Z score of more than –1 were excluded, with the exception of a few individuals judged to be clinically at risk. Subjects traveled to Cincinnati Children's Hospital Medical Center for baseline and follow-up evaluations.
There were 38 patients who were screened, and 36 were enrolled and randomized. Data were collected at baseline and at 6, 12, 18, and 24 months during the course of the study. There were 6 dropouts: 2 cited personal reasons that interfered with travel to the study center; 1 dropped out because of gastrointestinal discomfort (later resolved; subject was receiving placebo); 1 subject wanted to become pregnant; and 2 left the study because of noncompliance with the protocol (no medical reasons cited). There were 2 patients who dropped out in the first 6-month interval; thus, follow-up data were collected on 34 subjects. All subjects were enrolled after Cincinnati Children's Hospital Medical Center institutional review board and Human Subjects Committee approval of the study and written informed consent.
Skeletal imaging
Dual-energy x-ray absorptiometry (DEXA) was performed using Hologic 2000 or 4500 model x-ray absorptiometers (Hologic, Bedford, MA). During the course of the study, the Hologic 2000, supported by the Cincinnati Children's Hospital Medical Center (CCHMC) General Clinical Research Center (GCRC), was replaced by a Hologic 4500. There were 6 subjects who were serially scanned only on the Hologic 2000; there were 24 subjects who were serially scanned only on the Hologic 4500. There were 4 patients who received one or more scans on the 2000 and then were scanned on the 4500 during the remainder of the trial. To control for differences in detection, those individuals were scanned on both machines on the same day and the initial BMD and BMC values (recorded by the Hologic 2000) were adjusted. Plain x-rays were taken of the lateral spine and long bones at baseline and at 12 and 24 months. The x-rays were read at the time of each visit for safety analysis, and then again, in a series, at the end of the study by a radiologist (A.E.O.) who was blinded to study arm. The radiologist specifically commented on the progression or regression of focal lesions, long-bone deformity, or the appearance of periosteal new bone formation.
Clinical biochemistry
Serum levels of osteocalcin, bone-specific alkaline phosphatase, and 25-OH vitamin D were performed using routine methods. Measurement of serum osteocalcin was performed using the IRMA kit from Immunotopics (San Clemente, CA). Bone-specific alkaline phosphatase was measured using the Metra Alkphase-B kit from Quidel (San Diego, CA). Measurement of urinary amino-terminal collagen telopeptides (NTx) was performed using the Osteomark NTx EIA kit from Ostex International (Seattle, WA). Deoxypyridinoline cross-links were measured using the Metra DPD EIA kit from Quidel.
Statistical methods
Means and standard deviations for lumbar spine BMC and BMD and each bone biomarker at baseline and at each of the follow-up examinations were computed for the treatment group and for the placebo group separately. Mean changes from baseline and their standard deviations for BMC and BMD and the bone biomarkers were calculated and plotted at each scheduled follow-up examination. The mean changes from baseline for BMC and BMD and for bone biomarkers were tested for significance by paired t tests. Comparison of changes in BMC and BMD between groups was performed by t tests for 2 independent samples.
The Group Sequential t test26 was used to adjust for the type I error to account for multiple tests in this trial. The null hypothesis was H0, that there were no differences in changes of BMC (or BMD) from baseline between alendronate and placebo group. Two successive interim analyses were performed. The t test statistics for the differences between the alendronate and placebo groups with respect to change of lumbar spine BMC and BMD from baseline were compared with criteria for the group sequential tests. For the interim analysis: if
Longitudinal data for BMC and BMD and the bone biomarkers also were analyzed using random effect models, which summarize the longitudinal data by (1) the general shapes of the curves that individuals display over time (the mean structure) for each group (ie, alendronate or placebo group); (2) how much these curves differ from subject to subject (the between-subject errors); and (3) how closely subjects tend to follow their own predicted curves over time (within-subject errors). In the random effects model, comparisons of changes over time were made between alendronate and placebo groups in BMC, BMD, and the bone biomarkers.
Results
Interim safety data were reviewed periodically throughout the study in a blinded manner. No subject developed radiologic evidence of abnormal bone calcification or woven bone during the study. No individual developed progressively diminishing BMD by DEXA. The study was halted prematurely because of significant and positive differences between alendronate and placebo arms.
To test the randomization of patients at the entry of trial, means and standard deviations were compared for sex, age, lumbar spine BMD, and BMC for each study arm to determine whether there were significant initial differences between the 2 groups (Table 2). The significant group differences in these baseline parameters were mean age (39.5 ± 7.4 years vs 33.0 ± 9.1 years) and enzyme dosage (28.88 ± 8.66 U/kg vs 37.59 ± 15.08 U/kg) for the alendronate and placebo groups, respectively.
Baseline demographics and lumber spine BMC and BMD measurements
. | ALN; N = 17 . | Placebo; N = 17 . | P . |
|---|---|---|---|
| Baseline | |||
| Male sex, % | 29 | 29 | |
| Age, y | 39.50 ± 7.38 | 32.99 ± 9.91 | .0374 |
| Enzyme dose, | |||
| U/kg | 28.88 ± 8.66 | 37.59 ± 15.09 | .049 |
| BMC, g | 59.13 ± 15.60 | 60.62 ± 13.92 | .7707 |
| BMD, g/cm2 | 0.95 ± 0.14 | 0.95 ± 0.14 | .9527 |
. | ALN; N = 17 . | Placebo; N = 17 . | P . |
|---|---|---|---|
| Baseline | |||
| Male sex, % | 29 | 29 | |
| Age, y | 39.50 ± 7.38 | 32.99 ± 9.91 | .0374 |
| Enzyme dose, | |||
| U/kg | 28.88 ± 8.66 | 37.59 ± 15.09 | .049 |
| BMC, g | 59.13 ± 15.60 | 60.62 ± 13.92 | .7707 |
| BMD, g/cm2 | 0.95 ± 0.14 | 0.95 ± 0.14 | .9527 |
Values for ALN and placebo represent the mean ± the standard error of the mean.
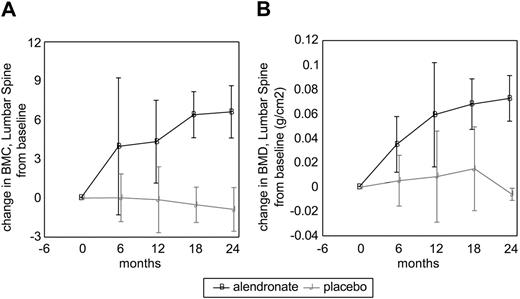
Lumbar spine BMC and BMD progressively increased from baseline in the alendronate group beginning with the first 6-month treatment interval; there was no significant change in the placebo group (Table 3). The extent of improvement in lumbar spine BMD with ALN was not dependent on initial BMD data (not shown). The effect of alendronate was relatively rapid, as over half of the total effect occurred within the first 6 months (Figure 1). Enzyme therapy alone did not produce a significant increase in lumbar spine BMD or BMC in adults who had been on enzyme therapy alone (placebo), in spite of the fact that the placebo group received a higher average enzyme dose that was statistically significant. The whole-body analysis for BMC (corrected to remove cranial mineral content), BMD, total body fat mass, total body lean mass, and percent fat did not show differences between the alendronate and placebo groups (data not shown).
Mean changes from baseline for lumber spine BMC and BMD
Follow-up interval . | ALN . | Placebo . | P . |
|---|---|---|---|
| 6 mo | |||
| ΔBMC, g | 3.97 ± 5.26 | 0.02 ± 1.83 | .0168 |
| ΔBMD, g/cm2 | 0.0349 ± 0.0228 | 0.0052 ± 0.0208 | .0013 |
| 12 mo | |||
| ΔBMC, g | 4.33 ± 3.18 | -0.13 ± 2.53 | .0012 |
| ΔBMD, g/cm2 | 0.0592 ± 0.0427 | 0.0085 ± 0.0373 | .0062 |
| 18 mo | |||
| ΔBMC, g | 6.40 ± 1.77 | -0.52 ± 1.36 | .0004 |
| ΔBMD, g/cm2 | 0.0679 ± 0.0207 | 0.015 ± 0.0343 | .0236 |
| 24 mo | |||
| ΔBMC, g | 6.62 ± 2.01 | -0.87 ± 1.68 | .0110 |
| ΔBMD, g/cm2 | 0.0726 ± 0.0187 | -0.006 ± 0.005 | .0053 |
Follow-up interval . | ALN . | Placebo . | P . |
|---|---|---|---|
| 6 mo | |||
| ΔBMC, g | 3.97 ± 5.26 | 0.02 ± 1.83 | .0168 |
| ΔBMD, g/cm2 | 0.0349 ± 0.0228 | 0.0052 ± 0.0208 | .0013 |
| 12 mo | |||
| ΔBMC, g | 4.33 ± 3.18 | -0.13 ± 2.53 | .0012 |
| ΔBMD, g/cm2 | 0.0592 ± 0.0427 | 0.0085 ± 0.0373 | .0062 |
| 18 mo | |||
| ΔBMC, g | 6.40 ± 1.77 | -0.52 ± 1.36 | .0004 |
| ΔBMD, g/cm2 | 0.0679 ± 0.0207 | 0.015 ± 0.0343 | .0236 |
| 24 mo | |||
| ΔBMC, g | 6.62 ± 2.01 | -0.87 ± 1.68 | .0110 |
| ΔBMD, g/cm2 | 0.0726 ± 0.0187 | -0.006 ± 0.005 | .0053 |
Values for ALN and placebo represent the mean ± the standard error of the mean.
Changes in lumbar spine bone mineral content and bonemineral density in alendronate and placebo groups. (A) Bone mineral content. (B) Bone mineral density. Black line indicates alendronate; and gray line, placebo. Error bars represent standard error of the mean (SEM).
Changes in lumbar spine bone mineral content and bonemineral density in alendronate and placebo groups. (A) Bone mineral content. (B) Bone mineral density. Black line indicates alendronate; and gray line, placebo. Error bars represent standard error of the mean (SEM).
Treatment with alendronate correlated with reduction in 2 serum markers of bone turnover, osteocalcin and bone-specific alkaline phosphatase. Significant group differences between alendronate and placebo groups appeared by 6 months of therapy and were maintained through the first 18 months of treatment. The loss of group differences in these 2 markers at the 24-month treatment interval may be the result of both the small number of subjects treated for the full 24 months prior to early cessation of the trial and the high degree of variability in the laboratory values (Table 4). Urinary markers for bone resorption were inconsistent: NTx were reduced in the alendronate group but pyridinoline cross-links (Pyrilinks-D) were not.
Comparison of the 5 measurements of the bone markers for the ALN group and the placebo group
. | ALN . | Placebo . | P . |
|---|---|---|---|
| Baseline | |||
| n | 17 | 17 | |
| Bone ALP | 16.41 ± 6.33 | 18.65 ± 16.08 | .2937 |
| OC | 4.61 ± 1.38 | 6.01 ± 2.06 | .0233 |
| NTx | 35.69 ± 24.63 | 56.08 ± 46.50 | .1212 |
| D-PYD | 4.81 ± 2.32 | 6.21 ± 2.71 | .1092 |
| Vitamin D-25 | 20.32 ± 8.94 | 21.83 ± 11.00 | .6584 |
| Follow-up at 6 mo | |||
| n | 15 | 15 | |
| Bone ALP | 10.01 ± 4.73 | 17.33 ± 9.21 | .0123 |
| OC | 3.18 ± 1.17 | 5.74 ± 1.49 | < .0001 |
| NTx | 21.77 ± 14.30 | 46.40 ± 28.26 | .0086 |
| D-PYD | 6.01 ± 6.01 | 5.89 ± 3.09 | .9428 |
| Vitamin D-25 | 28.41 ± 11.38 | 25.36 ± 8.97 | .4213 |
| Follow-up at 12 mo | |||
| n | 13 | 12 | |
| Bone ALP | 8.30 ± 3.16 | 17.95 ± 7.60 | .0010 |
| OC | 2.58 ± 0.64 | 5.46 ± 2.26 | .0010 |
| NTx | 25.92 ± 29.61 | 45.91 ± 24.0 | .0886 |
| D-PYD | 3.95 ± 1.91 | 8.00 ± 3.65 | .0034 |
| Vitamin D-25 | 23.65 ± 9.85 | 29.45 ± 8.71 | .1337 |
| Follow-up at 18 mo | |||
| n | 9 | 11 | |
| Bone ALP | 13.90 ± 6.14 | 23.76 ± 11.23 | .0301 |
| OC | 2.88 ± 0.88 | 5.90 ± 1.36 | < .0001 |
| NTx | 13.57 ± 6.27 | 35.03 ± 18.82 | .0058 |
| D-PYD | 3.79 ± 1.23 | 5.59 ± 2.28 | .0505 |
| Vitamin D-25 | 25.26 ± 9.13 | 26.10 ± 6.33 | .8100 |
| Follow-up at 24 mo | |||
| n | 4 | 3 | |
| Bone ALP | 11.58 ± 6.01 | 16.0 ± 9.01 | .4667 |
| OC | 2.25 ± 1.20 | 5.0 ± 1.74 | .0545 |
| NTx | 11.58 ± 2.66 | 43.90 ± 6.54 | .0003 |
| D-PYD | 2.50 ± 0.73 | 5.60 ± 2.57 | .0651 |
| Vitamin D-25 | 19.58 ± 10.22 | 23.67 ± 15.31 | .6861 |
. | ALN . | Placebo . | P . |
|---|---|---|---|
| Baseline | |||
| n | 17 | 17 | |
| Bone ALP | 16.41 ± 6.33 | 18.65 ± 16.08 | .2937 |
| OC | 4.61 ± 1.38 | 6.01 ± 2.06 | .0233 |
| NTx | 35.69 ± 24.63 | 56.08 ± 46.50 | .1212 |
| D-PYD | 4.81 ± 2.32 | 6.21 ± 2.71 | .1092 |
| Vitamin D-25 | 20.32 ± 8.94 | 21.83 ± 11.00 | .6584 |
| Follow-up at 6 mo | |||
| n | 15 | 15 | |
| Bone ALP | 10.01 ± 4.73 | 17.33 ± 9.21 | .0123 |
| OC | 3.18 ± 1.17 | 5.74 ± 1.49 | < .0001 |
| NTx | 21.77 ± 14.30 | 46.40 ± 28.26 | .0086 |
| D-PYD | 6.01 ± 6.01 | 5.89 ± 3.09 | .9428 |
| Vitamin D-25 | 28.41 ± 11.38 | 25.36 ± 8.97 | .4213 |
| Follow-up at 12 mo | |||
| n | 13 | 12 | |
| Bone ALP | 8.30 ± 3.16 | 17.95 ± 7.60 | .0010 |
| OC | 2.58 ± 0.64 | 5.46 ± 2.26 | .0010 |
| NTx | 25.92 ± 29.61 | 45.91 ± 24.0 | .0886 |
| D-PYD | 3.95 ± 1.91 | 8.00 ± 3.65 | .0034 |
| Vitamin D-25 | 23.65 ± 9.85 | 29.45 ± 8.71 | .1337 |
| Follow-up at 18 mo | |||
| n | 9 | 11 | |
| Bone ALP | 13.90 ± 6.14 | 23.76 ± 11.23 | .0301 |
| OC | 2.88 ± 0.88 | 5.90 ± 1.36 | < .0001 |
| NTx | 13.57 ± 6.27 | 35.03 ± 18.82 | .0058 |
| D-PYD | 3.79 ± 1.23 | 5.59 ± 2.28 | .0505 |
| Vitamin D-25 | 25.26 ± 9.13 | 26.10 ± 6.33 | .8100 |
| Follow-up at 24 mo | |||
| n | 4 | 3 | |
| Bone ALP | 11.58 ± 6.01 | 16.0 ± 9.01 | .4667 |
| OC | 2.25 ± 1.20 | 5.0 ± 1.74 | .0545 |
| NTx | 11.58 ± 2.66 | 43.90 ± 6.54 | .0003 |
| D-PYD | 2.50 ± 0.73 | 5.60 ± 2.57 | .0651 |
| Vitamin D-25 | 19.58 ± 10.22 | 23.67 ± 15.31 | .6861 |
ALN and placebo values for bone ALP, OC, NTx, D-PYD, and vitamin D-25 represent the mean ± standard deviation. Bone ALP indicates serum alkaline phosphatase, bone-specific fraction; OC, serum osteocalcin; NTx, urine type I collagen amino-terminal telopeptide; and D-PYD, urine Pyrilinks-D type I collagen cross-linked peptide.
Longitudinal analysis
Repeated measurements for BMC or BMD at the lumbar spine were analyzed using random effects models. In the models, an indicator variable for alendronate treatment and placebo treatment was included for group comparisons. The independent variables are the time intervals from the baseline (ie, alendronate or placebo group) and age at baseline. The interaction between time intervals since the baseline and the group membership was also tested.
The alendronate group had an increase in BMC and BMD after intervention whereas the placebo group had decreased BMC and BMD after baseline. There was a significant difference in the average changes from baseline over the course of the study period between the 2 groups. The rates of changes in the ALN group were greater than those in the placebo group by 6.86 ± 5.82 g for BMC and by 0.02 ± 1.8 g/cm2 for BMD over the 24 months. These group difference changes from baseline BMD varied directly with the duration of treatment within the 24-month trial.
Lack of alendronate effect on focal bone lesions
Blinded review of serial long-bone radiographs failed to discern any group differences with respect to changes in localized skeletal lesions. In fact, the appearance of every localized skeletal abnormality observed in either study group remained unchanged throughout the trial. Long-bone films also were evaluated throughout the trial as a part of the interim safety analysis. No subject developed radiologic evidence of new abnormal bone calcification or woven bone during the study.
The study was halted prematurely because of significant and positive group differences noted in the second scheduled interim analysis. The Group Sequential t test26 was used to adjust for the type I error to account for multiple tests in a trial. There was no significant difference in the first interim analyses for the group difference and the trial continued. At the second interim analysis, there was a significant difference based on the O'Brien and Fleming test. The results presented here are based on the data completed after the second interim analysis.
Discussion
A 2-arm, double-blind, placebo-controlled trial of ALN in adult patients with GD who were on long-term enzyme therapy demonstrated that ALN therapy results in significant improvement in BMD at the lumbar spine and in significant decreases in some biochemical markers of bone turnover. Since the study design excluded individuals with other identifiable causes of osteopenia, including estrogen deficiency, ALN appeared to have an effect on Gaucher-related bone loss. The results from the placebo arm of the trial reinforce previous observations that enzyme therapy alone has a limited ability to reverse Gaucher-related osteopenia in adults. In contrast, alendronate treatment showed no beneficial effect toward improvement of the focal lesions typically seen in patients with GD. There was no improvement in the appearance of lytic lesions and there was no normalization of the “Erlenmeyer flask” deformity in any study patient in either study arm for the duration of the trial.
One of the goals of this trial as it was originally conceived was to indirectly test a hypothesis regarding the pathogenesis of Gaucher bone disease. A dramatic response of either BMD or focal lesions to bisphosphonate therapy could be interpreted as evidence that either or both was due to increased osteoclastic activity. However, the findings from this study do not support a simple osteoclast-mediated mechanism for Gaucher bone manifestations. At initial screening, biochemical markers of bone metabolism were in the normal reference range for all patients, even 9 subjects with more severe osteopenia (T <–2) at the time of screening. Also, the observed improvement in lumbar spine BMD over 24 months is approximately the same response seen in a postmenopausal osteoporosis population,27 which is almost certain to be heterogeneous with respect to both etiology and presence of elevated indices of bone turnover. Thus, the response of BMD to ALN in GD, while almost certainly clinically important, could be viewed as noninstructive with regard to pathogenesis of either focal or generalized bone disease. In addition, the lack of any focal response to alendronate in this trial indicates that lytic bone lesions of GD, unlike those seen in Paget disease, are not characterized by active bone resorption.
The relationship between increased BMD and fracture risk in study subjects is difficult to determine. No fractures were observed in either arm of the study, but the study size was far too small to measure fracture rate as a primary outcome. Pathologic fractures in patients with GD are mechanistically heterogeneous. Generalized osteopenia imposes a discrete fracture risk, particularly for vertebral fractures, but fractures also occur at sites of localized cortical thinning, focal lytic lesions, and focal necrosis, both in the femur and vertebral bodies. Since superimposed generalized osteopenia probably increases fracture risk at these local sites, ALN-related improvement of BMD may reduce risk for fractures at all sites.
ALN may also reduce fracture risk by mechanisms other than improved BMD. Antiresorptive therapy in patients with postmenopausal osteoporosis by means of several classes of drugs including bisphosphonates, nasal calcitonin, or raloxifene reduces fracture risk to a far greater extent (35%-50%) than would be predicted by epidemiologic studies from the rather modest differences in BMD observed (see Watts28 and Riggs and Melton29 for reviews). If protection from fracture above what is predicted by increased BMD is attributable to the above agents' ability to reduce bone turnover, as has been proposed,29,30 then patients with GD treated with alendronate might expect similar protection since a reduction in bone turnover markers was seen in the ALN arm of this study.
The dosage used in this trial represented an attempt to test the effect of ALN both on focal lesions and bone deformity as well as increased BMD. A dosage of 40 mg rather than 20 mg is most effective for reducing focal lesions in Paget disease.31,32 Although the possibility that Gaucher-related osteopenia only responds to the high dosages used in this trial (40 mg/d) cannot be formally excluded from this trial, the optimal dosage is likely to be the 10 mg/d regimen typically used for postmenopausal osteoporosis (or its weekly equivalent). Administration of ALN in dosages of more than 10 mg/d had little additive effect in postmenopausal women.33 Given the lack of response of focal lesions after up to 24 months of therapy, the higher dosage probably is unnecessary and cannot be justified. Nonetheless, this study clearly provides justification for adjunctive ALN therapy for adults with GD who are on enzyme therapy, and who are at risk for osteopenic fracture.
Prepublished online as Blood First Edition Paper, March 9, 2004; DOI 10.1182/blood-2003-11-3854.
Supported by Orphan Products grant FD-R-001537 (R.J.W.) from the US Food and Drug Administration, by grants from Merck and Co and Genzyme Inc, and by US Public Health Service grant M01 RR 08084 (R.J.W.) from the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal