Abstract
Deficiencies of coagulation factors other than factor VIII and factor IX that cause bleeding disorders are inherited as autosomal recessive traits and are rare, with prevalences in the general population varying between 1 in 500 000 and 1 in 2 million for the homozygous forms. As a consequence of the rarity of these deficiencies, the type and severity of bleeding symptoms, the underlying molecular defects, and the actual management of bleeding episodes are not as well established as for hemophilia A and B. We investigated more than 1000 patients with recessively inherited coagulation disorders from Italy and Iran, a country with a high rate of recessive diseases due to the custom of consanguineous marriages. Based upon this experience, this article reviews the genetic basis, prevalent clinical manifestations, and management of these disorders. The steps and actions necessary to improve the condition of these often neglected patients are outlined.
Introduction
Hemophilia A and B are the most frequent inherited bleeding disorders. Together with von Willebrand disease, a defect of primary hemostasis associated with a secondary defect in coagulation factor VIII (FVIII), these X-linked disorders include 95% to 97% of all the inherited deficiencies of coagulation factors.1,2 The remaining defects, generally transmitted as autosomal recessive traits in both sexes, are rare, with prevalences of the presumably homozygous forms in the general population ranging from approximately 1 in 2 million for factor II (prothrombin) and factor XIII (FXIII) deficiency to 1 in 500 000 for factor VII (FVII) deficiency (Table 1).3,4 Exceptions to these low prevalences are countries with large Jewish communities (where FXI deficiency is much more prevalent), Middle Eastern countries, and Southern India. In the last 2 regions, consanguineous marriages are relatively common, so that autosomal recessive traits occur more frequently in homozygosity.
General characteristics of recessively inherited coagulation disorders
Deficient factor and OMIM no.† . | Prevalence of homozygous forms . | Gene (size in kb) . | Chromosome location . |
|---|---|---|---|
| Fibrinogen #202400 | 1 in 1 000 000 | ||
| +134820 | FGA (7.6) | 4q31.3 | |
| *134830 | FGB (8.1) | 4q31.3 | |
| *134850 | FGG (8.5) | 4q32.1 | |
| Prothrombin | 1 in 2 000 000 | ||
| *176930 | F2 (20.3) | 11p11.2 | |
| V | 1 in 1 000 000 | ||
| *227400 | F5 (72.3) | 1q24.2 | |
| VII | 1 in 500 000 | ||
| *227500 | F7 (14.2) | 13q34 | |
| X | 1 in 1 000 000 | ||
| *227600 | F10 (26.7) | 13q34 | |
| XI | 1 in 1 000 000‡ | ||
| *264900 | F11 (22.6) | 4q35.2 | |
| XIII | 1 in 2 000 000 | ||
| +134570 | F13A (176.6) | 6p25.1 | |
| +13580 | F13B (28.0) | 1q31.3 | |
| V + VIII #227300 | 1 in 2 000 000 | ||
| *601567 | LMAN1 (29.4) | 18q21.32 | |
| *607788 | MCFD2 (13.9) | 2p21 | |
| Vitamin K—dependent | 1 in 2 000 000 | ||
| type I #277450 | |||
| *137167 | GGCX (12.4) | 2p11.2 | |
| type II #607473 | |||
| *608547 | VKORC1 (5.1) | 16p11.2 |
Deficient factor and OMIM no.† . | Prevalence of homozygous forms . | Gene (size in kb) . | Chromosome location . |
|---|---|---|---|
| Fibrinogen #202400 | 1 in 1 000 000 | ||
| +134820 | FGA (7.6) | 4q31.3 | |
| *134830 | FGB (8.1) | 4q31.3 | |
| *134850 | FGG (8.5) | 4q32.1 | |
| Prothrombin | 1 in 2 000 000 | ||
| *176930 | F2 (20.3) | 11p11.2 | |
| V | 1 in 1 000 000 | ||
| *227400 | F5 (72.3) | 1q24.2 | |
| VII | 1 in 500 000 | ||
| *227500 | F7 (14.2) | 13q34 | |
| X | 1 in 1 000 000 | ||
| *227600 | F10 (26.7) | 13q34 | |
| XI | 1 in 1 000 000‡ | ||
| *264900 | F11 (22.6) | 4q35.2 | |
| XIII | 1 in 2 000 000 | ||
| +134570 | F13A (176.6) | 6p25.1 | |
| +13580 | F13B (28.0) | 1q31.3 | |
| V + VIII #227300 | 1 in 2 000 000 | ||
| *601567 | LMAN1 (29.4) | 18q21.32 | |
| *607788 | MCFD2 (13.9) | 2p21 | |
| Vitamin K—dependent | 1 in 2 000 000 | ||
| type I #277450 | |||
| *137167 | GGCX (12.4) | 2p11.2 | |
| type II #607473 | |||
| *608547 | VKORC1 (5.1) | 16p11.2 |
Gene sizes and chromosomal locations were obtained from the UCSC Genome Browser (http://genome.ucsc.edu/).
FGA indicates fibrinogen Aα-chain gene; FGB, fibrinogen Bβ-chain gene; FGG, fibrinogen γ-chain gene; LMAN1, lectin mannose-binding 1; MCFD2, multiple coagulation factor deficiency 2; GGCX, γ-glutamyl carboxylase; and VKORC1, vitamin K epoxide reductase complex subunit 1. # indicates a descriptive locus that does not represent a unique locus; +, an entry that contains the description of a gene of known sequence and aphenotype; and *, a gene of known sequence.
Online Mendelian Inheritance in Man is the largest registry of human genetic diseases (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM).
Much more prevalent in countries with large Jewish communities.
The natural history and spectrum of clinical manifestations of recessively inherited coagulation disorders (RICDs) are not well established, because very few clinical centers have the opportunity to see a significant number of these rare patients. Even though much progress was recently done to unravel the molecular lesions underlying RICDs,3,4 this knowledge is not widely exploited to control them through genetic counseling and prenatal diagnosis. Most importantly, there was little progress in treatment. Only one product produced by recombinant DNA technology is licensed for FVII deficiency (recombinant activated FVII). A few plasma-derived single-factor concentrates (fibrinogen, FVII, FXI, FXIII) are available or licensed in some European countries but not in the United States. FV deficiency and the combined deficiency of FV and FVIII can only be treated with fresh-frozen plasma; prothrombin and FX deficiencies are often treated with prothrombin complex concentrates (PCCs) containing vitamin K–dependent factors other than those actually deficient.
We focused on the molecular, clinical, and therapeutic aspects of RICDs on the basis of the experience gained on large series of patients from Italy (n = 353) and Iran (n = 750) (Table 2). Inherited deficiencies of FXII, prekallikrein, and high molecular weight kininogen are not featured because they are not associated with a bleeding tendency.
Number of patients and relative frequency of recessively inherited coagulation disorders in Iran and Italy
Deficiency . | Iran, n (%) . | Italy, n (%) . |
|---|---|---|
| Fibrinogen | 80 (11) | 30 (8) |
| Prothrombin | 15 (2) | 18 (5) |
| V | 50 (7) | 35 (10) |
| VII | 300 (39) | 90 (25) |
| X | 75 (10) | 28 (8) |
| XI | 50 (7) | 80 (24) |
| XIII | 100 (13) | 40 (11) |
| V + VIII | 80 (11) | 32 (9) |
| Total | 750 | 353 |
Deficiency . | Iran, n (%) . | Italy, n (%) . |
|---|---|---|
| Fibrinogen | 80 (11) | 30 (8) |
| Prothrombin | 15 (2) | 18 (5) |
| V | 50 (7) | 35 (10) |
| VII | 300 (39) | 90 (25) |
| X | 75 (10) | 28 (8) |
| XI | 50 (7) | 80 (24) |
| XIII | 100 (13) | 40 (11) |
| V + VIII | 80 (11) | 32 (9) |
| Total | 750 | 353 |
The general population of Iran is 65 million; Italy, 55 million. Data on RICD are obtained from the most recent adjournments (2004) of the registries kept in Iran (courtesy of Dr. M. Lak, Iman Khomeini Hospital, Tehran) and in Italy (Associazione Italiana Centri Emofilia).
Molecular basis
General features
Patients with RICDs and clinically significant manifestations are usually homozygous or compound heterozygous. Heterozygotes (parents and children of the probands) have approximately half-normal levels of coagulation factors and are usually asymptomatic, although a recent North American survey found a high rate of bleeding symptoms5 (see “Clinical manifestations”). Pseudominant transmission from an affected homozygous parent to a child may sometimes be observed due to heterozygosity of the unaffected parent.6 Most RICDs are expressed phenotypically by a parallel reduction of plasma factors measured by functional assays and immunoassays (so-called type I deficiencies). Qualitative defects, characterized by normal, slightly reduced, or increased levels of factor antigen contrasting with much lower or undetectable functional activity (type II), are less frequent.3,4
Gene defects
RICDs are usually due to DNA defects in the genes that encode the corresponding coagulation factors (Table 1). Exceptions are the combined deficiency of FV and FVIII, characterized by defects in genes encoding proteins involved in the intracellular transport of these factors,7,8 and the combined deficiency of vitamin K–dependent FII, FVII, FIX, and FX, characterized by defects in the genes encoding enzymes involved in posttranslational modifications of these factors and in vitamin K metabolism9,10 (Table 1). In contrast with hemophilia A, due in approximately half of the patients to an inversion mutation involving introns 1 or 22 of the FVIII gene,1 RICDs are often due to mutations unique for each kindred and scattered throughout the genes.3,4 In approximately 10% to 20% of patients, no putative mutation is found. These cases may be due to defects in noncoding regions or in genes coding for regulators of intracellular transport and posttranslational modifications of coagulation factors.
Genotype-phenotype relationships
In severe type I deficiencies, functional levels of the deficient coagulation factor are frequently below the detection limit of the currently available assays. In most cases, however, severely reduced but still measurable antigen levels can be detected by sensitive immunoassays. The complete absence of a coagulation factor probably occurs only with large gene deletions.11 “Null” mutations predicting the production of truncated proteins or of unstable mRNAs (partial deletions, out-of-frame insertions, splicing abnormalities, nonsense mutations) are usually associated with very low or undetectable plasma factors and severe clinical manifestations.4 The effect of missense mutations is less homogenous: While in some instances they lead to severe factor deficiency, in others they are associated with partial deficiencies and milder clinical manifestations.4 Some missense mutations cause the production of mutant proteins with heightened procoagulant properties associated with thrombotic phenotypes, usually transmitted as autosomal dominant traits.12
Expression studies
Expression of a number of mutations in cultured mammalian cells and characterization of the trafficking and secretion of the corresponding recombinant proteins has helped to explain how they lead to factor deficiency. In some instances—as, for example, due to missense mutations in the genes of fibrinogen,13 FVII,14 FX,15 and FXI16 —mutant proteins are produced normally but ultimately not secreted because impaired folding and/or conformational changes cause their retention by the quality control system of the secretory pathway, eventually leading to intracellular degradation or accumulation.13-15 In others, the mutant recombinant protein is fully secreted, so that it is possible to compare in vitro the abnormal functional properties with those of the wild-type protein and to understand the nature of the defect and sometimes the mechanism of the corresponding bleeding tendency.17-19
A previous review article listed all the gene mutations identified for each RICD until 2002.4 A large collection of mutations can also be obtained from the Human Gene Mutation Database maintained at the Institute of Medical Genetics in Cardiff.20 We provide below only general or very recent new information for each RICD, summarizing in Table 3 the total number of each type of mutation reported in full so far (March 2004).
Mutations in recessively inherited coagulation disorders
. | Mutation type . | . | . | . | . | Total no. of mutations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deficient factor and gene . | Missense . | Nonsense . | Insertion/deletion . | Splicing . | Gross deletions . | . | ||||
| Fibrinogen* | ||||||||||
| FGA | 0 | 7 | 7 | 3 | 3 | 20 | ||||
| FGB | 4 | 2 | 0 | 2 | 0 | 8 | ||||
| FGG | 0 | 2 | 1 | 3 | 0 | 6 | ||||
| Prothrombin | ||||||||||
| F2 | 27 | 2 | 4 | 1 | 0 | 34 | ||||
| V | ||||||||||
| F5 | 9 | 6 | 9 | 2 | 0 | 26 | ||||
| VII | ||||||||||
| F7 | 84 | 6 | 8 | 17 | 0 | 124† | ||||
| X | ||||||||||
| F10 | 55 | 0 | 4 | 3 | 3 | 65 | ||||
| XI | ||||||||||
| F11 | 25 | 11 | 7 | 7 | 0 | 50 | ||||
| XII | ||||||||||
| F13A | 26 | 6 | 10 | 8 | 1 | 51 | ||||
| F13B | 1 | 0 | 2 | 0 | 0 | 3 | ||||
| V + VIII | ||||||||||
| LMAN1 | 1 | 3 | 10 | 4 | 0 | 18 | ||||
| MCDD2 | 2 | 0 | 3 | 2 | 0 | 7 | ||||
| Vitamin K dependent | ||||||||||
| GGCX | 2 | 0 | 0 | 0 | 0 | 2 | ||||
| VKORC1 | 1 | 0 | 0 | 0 | 0 | 1 | ||||
. | Mutation type . | . | . | . | . | Total no. of mutations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deficient factor and gene . | Missense . | Nonsense . | Insertion/deletion . | Splicing . | Gross deletions . | . | ||||
| Fibrinogen* | ||||||||||
| FGA | 0 | 7 | 7 | 3 | 3 | 20 | ||||
| FGB | 4 | 2 | 0 | 2 | 0 | 8 | ||||
| FGG | 0 | 2 | 1 | 3 | 0 | 6 | ||||
| Prothrombin | ||||||||||
| F2 | 27 | 2 | 4 | 1 | 0 | 34 | ||||
| V | ||||||||||
| F5 | 9 | 6 | 9 | 2 | 0 | 26 | ||||
| VII | ||||||||||
| F7 | 84 | 6 | 8 | 17 | 0 | 124† | ||||
| X | ||||||||||
| F10 | 55 | 0 | 4 | 3 | 3 | 65 | ||||
| XI | ||||||||||
| F11 | 25 | 11 | 7 | 7 | 0 | 50 | ||||
| XII | ||||||||||
| F13A | 26 | 6 | 10 | 8 | 1 | 51 | ||||
| F13B | 1 | 0 | 2 | 0 | 0 | 3 | ||||
| V + VIII | ||||||||||
| LMAN1 | 1 | 3 | 10 | 4 | 0 | 18 | ||||
| MCDD2 | 2 | 0 | 3 | 2 | 0 | 7 | ||||
| Vitamin K dependent | ||||||||||
| GGCX | 2 | 0 | 0 | 0 | 0 | 2 | ||||
| VKORC1 | 1 | 0 | 0 | 0 | 0 | 1 | ||||
The list is updated to March 2004; only fully published mutations have been counted.
Only mutations identified in afibrinogenemic or severe hypofibrinogenemic patients were considered.
The total number of FVII mutations includes also 6 additional mutations located in the 5′UTR region of the FVII gene.
Fibrinogen deficiency
Fibrinogen deficiency presents phenotypically as afibrinogenemia, hypofibrinogenemia, and dysfibrinogenemia and is due to mutations in any of the 3 genes that encode the Aα,Bβ, and γ chains of fibrinogen (Table 1). In afibrinogenemia and severe hypofibrinogenemia, null mutations leading to no production or severe truncation of either chain are frequent (deletions, nonsense and splicing mutations),11,21-23 but missense mutations leading to the deficient secretion of fibrinogen are also described and characterized.13,24,25 In at least 2 cases of hypofibrinogenemia, heterozygous missense mutations in the γ chain were associated with endoplasmic reticulum storage within hepatocytes of the mutant fibrinogens.26,27 The first case of complete isodisomy of chromosome 4 was recently identified as a cause of afibrinogenemia.28 Dysfibrinogenemia is an exception to the general paradigm of RICDs as recessive disorders, because it is usually transmitted as an autosomal dominant trait. Most patients are clinically asymptomatic,17 some present with a bleeding diathesis, others with thrombophilia,29 and occasionally with both.29 A list of mutations in the fibrinogen genes can be found in the Fibrinogen Database (http://www.geht.org/databaseang/fibrinogen).30
Prothrombin (FII) deficiency
Hypoprothrombinemia is characterized by concomitantly low activity and antigen levels in plasma.31 To our knowledge, no case of aprothrombinemia is reported, suggesting that this deficiency is incompatible with life. Missense mutations in the prothrombin gene are the most frequent defects in the relatively few patients with hypoprothrombinemia genotyped so far.4,31 Several cases of dysprothrombinemia, characterized by normal levels of a dysfunctional protein, were reported and the relevant mutant proteins expressed and characterized.18,32
FV deficiency
FV deficiency is almost invariably expressed phenotypically as type I deficiency.3 Only one genetic defect (FV New Brunswick, Ala221Val) causing type II deficiency was recently demonstrated to interfere with the stability of activated FV.19 So far, mainly null mutations were identified in severe or moderately severe FV deficiency.33,34 Approximately half of them are in the large exon 13 encoding the B domain that, like the corresponding domain of the highly homologous FVIII, is disposed during the enzymatic activation of FV.33-36 A few missense mutations, distributed among the A2, A3, and C2 domains, were found to lead to secretion defects by expression studies.33,37 The in-trans association of the FV Leiden mutation12 with FV gene mutations causing type I deficiency leads to the so-called “pseudohomozygous” activated protein C resistance phenotype, characterized by reduced FV antigen levels, no bleeding symptom, but a thrombotic tendency similar to that of FV Leiden homozygotes.38
FVII deficiency
The FVII gene maps on chromosome 13 close to that encoding FX (Table 1). The availability of an Internet database allows access to a large number of mutations (http://193.60.222.13/index.htm).39 More than two thirds of them are missense mutations; the remaining ones are null mutations that decrease or abolish the expression of FVII (Table 3).4,40-42 In general, patients with mild to moderate clinical phenotypes are homozygous or compound heterozygous for missense mutations, whereas more severe phenotypes are associated with deletions, insertions, splicing, and promoter mutations. However, some missense mutations are also associated with a severe phenotype.39
FX deficiency
Most patients (approximately three fourths) have missense mutations (Table 3).4,15,43-47 Phenotypically, most affected individuals have low but measurable levels of FX activity,44 suggesting that the complete absence of FX in plasma may be incompatible with adult life. Another feature of FX deficiency is the complete absence of reported nonsense mutations,4 whereas a small number of deletions leading to stop codons were identified (Table 3).
FXI deficiency
This RICD—FXI deficiency—is peculiar because a founder effect appears to underlie its frequency among some communities. The so-called type II mutation, particularly frequent in Ashkenazi and Iraqi Jews, is a nonsense mutation in exon 5 (Glu117Stop), causing very low or undetectable plasma levels of FXI in homozygotes.48 Another mutation (type III), frequent in Ashkenazi Jews, is a missense mutation in exon 9 (Phe283Leu) that leads to a defective secretion, with plasma levels of FXI low but detectable at approximately 10%.49,50 Compound heterozygosity for both mutations is the most common cause of the deficiency among Jews, characterized by detectable plasma levels of FXI.48 Haplotype analysis indicates that a founder effect is also implicated in the ancient missense mutations found in non-Jewish communities from France16 and the United Kingdom.51 A founder effect was not established for another recently described ancient French mutation.52 Some missense mutations were shown to exert a dominant negative effect through heterodimer formation between the mutant and wild-type polypeptides, resulting in a pattern of dominant transmission.53
FXIII deficiency
Most cases of FXIII deficiency are associated with alterations in the gene that encodes the catalytic A subunit of this transglutaminase that cross-links the α and γ chains of fibrin monomers to yield stable clots (Tables 1 and 3).54 A minority of cases are due to defects of the carrier B subunit.54 Mutations, with a prevalence of missense mutations, are located throughout the coding region of the FXIII-A gene, with little evidence of recurring mutations.4,54-56
Combined deficiency of FV and FVIII
Patients with combined deficiency of FV and FVIII have concomitantly low but detectable levels of coagulant activity and antigen of both factors (usually between 5% and 20%). In approximately two thirds of cases, mutations are located in a gene on chromosome 18 that encodes a lectin mannose-binding protein (LMAN1, also called ERGIC-53).7,57,58 LMAN1 binds both FV and FVIII in the endoplasmic reticulum/Golgi intermediate compartment (ERGIC) and functions as chaperone in their intracellular transport. LMAN1 mutations are mainly null mutations.7,57,58 In other kindreds the deficiency is associated with mutations in a gene on chromosome 2 called multiple coagulation factor deficiency 2 gene (MCFD2).8 MCFD2 encodes a protein that forms a Ca2+-dependent stoichiometric complex with LMAN1 and acts as a cofactor in the intracellular trafficking of FV and FVIII.8
Multiple deficiency of vitamin K–dependent coagulation factors
Plasma defects are not limited to the procoagulants FII, FVII, FIX, and FX but also involve such naturally occurring anticoagulants as protein C and protein S and vitamin K–dependent bone proteins.9,59,60 Factors range from less than 1% to 30% in plasma; clinical manifestations occur early in life and are usually severe, such as umbilical cord and central nervous system bleeding.9,59 The multiple deficiency may be due to defects in enzymes involved in γ-glutamyl carboxylation, the posttranslational modification of vitamin K–dependent proteins necessary for calcium binding, and attachment to membrane phospholipids. The molecular basis of the multiple deficiency are missense mutations in the γ-glutamyl carboxylase gene (GGCX) located on chromosome 2, which lead to the production of a dysfunctional enzyme.9 The multiple deficiency can also be associated with missense mutations in the gene vitamin K epoxide reductase complex subunit 1 (VKORC1), which encodes a small transmembrane protein of the endoplasmic reticulum necessary for the full function of vitamin K in the carboxylase reaction.61
Clinical manifestations
Knowledge on the spectrum of clinical manifestations of RICDs is much greater after the recent description of registries from Iran, Italy, and North America including a large number of unselected patients.
Registries of RICDs
In Iran, a country where the custom of marriages among first cousins makes RICDs 3 to 5 times more frequent than in Western countries,4 a registry of bleeding disorders was kept since the early 1970s. Starting in 1996 we established a collaboration based upon visits, workshops, and exchange of technology and samples. A form for the uniform collection of clinical history, with special emphasis on the onset and frequency of objectively documented symptoms and long-term consequences of RICDs, has been developed in collaboration with the hemophilia treatment centers of the 2 main Iranian cities (Teheran in the north and Shiraz in the south). Urban patients and those living in rural areas are periodically summoned for clinical and laboratory evaluation. A program for genotyping is initiated, with the goal to offer prenatal diagnosis to families that had already witnessed the birth of severely affected children. Even though ascertainment of RICDs is not complete in Iran, the actual cohort of 750 patients (Table 1), initially retrospective but now prospectively followed, is likely to be the largest investigated so far.4,62-68
In Italy, where a registry of inherited coagulation disorders was kept since 1980, 353 presumably homozygous patients with RICD have been diagnosed. The third large set of data stems from the North American registry, which includes patients with afibrinogenemia, FII, FVII, FX, FV, and FXIII deficiencies on the basis of a questionnaire delivered to 225 hemophilia treatment centers in the United States and Canada.5 Approximately one fourth of the centers provided information on 294 individuals pertaining to disease prevalence, clinical events that led to diagnosis, type and frequency of symptoms, treatment strategies, and disease- and treatment-related complications. At variance with the Iranian registry that gathered information only on patients presumably homozygous with factor levels below 10% (less than 10 mg/dL for fibrinogen), the North American registry also gathered information on individuals presumably heterozygous, with factor levels of 20% or more (about half of the entire cohort).5 An unexpected finding, peculiar of the North American registry, is that approximately 40% of the heterozygotes were symptomatic.5 Questionnaire surveys have inherent limitations in terms of accurate collection of bleeding symptoms, which are reported even in up to half of healthy persons.69,70
Main clinical features
The most typical symptom, common to all RICDs, is the occurrence of excessive bleeding at the time of invasive procedures such as circumcision and dental extractions. Bleeding in mucosal tracts (particularly epistaxis and menorrhagia) is also a frequent feature, and impaired wound healing is rather typical of FXIII deficiency. Such life-endangering symptoms as umbilical cord and recurrent hemoperitoneum during ovulation, as well as limb-endangering hemarthroses and soft tissue hematomas, occur with higher frequency in patients with prothrombin, FX, and FXIII deficiency than in other RICDs (Table 4). Afibrinogenemia is sometimes associated with thrombotic episodes, thought to be triggered by thrombin-induced platelet aggregation in vivo due to the absence of the thrombin-neutralizing properties of fibrin.62 FVII deficiency presents with a wide spectrum of symptom severity that sometimes correlates poorly with FVII levels, a number of patients with undetectable FVII being totally asymptomatic.5,64 Intracranial bleeding, reported to be frequent and severe after birth in a series of FVII-deficient patients,71 was rare in the Iranian, Italian, and American cohorts.5,64 This severe clinical manifestation is usually rare in patients with RICDs, except in approximately one fourth of patients with FXIII deficiency.68 There have been reports of thrombotic symptoms in FVII-deficient patients.72 A recent survey by Mariani et al73 provides little evidence that thrombosis is especially prevalent in these patients but indicates that the deficiency does not protect from thrombosis. Menorrhagia is frequent in women with RICDs and may cause iron deficiency anemia.5,62-68 Recurrent miscarriages, frequently described in afibrinogenemic and FXIII-deficiency women,62,68 are not prominent in women with other RICDs. Postpartum bleeding often occurs if replacement therapy is not administered for 2 to 3 days after delivery.62-68
Clinical features of recessively inherited coagulation disorders
Deficient factor . | Main clinical symptoms* . | Hemostatic levels . | Plasma half-life . |
|---|---|---|---|
| Fibrinogen | Umbilical cord, joint, and mucosal tract bleeding; recurrent miscarriages, rarely thrombosis | 50 mg/dL | 2-4 d |
| Prothrombin | Umbilical cord, joint, and mucosal tract bleeding | 20%-30% | 3-4 d |
| V | Mucosal tract bleeding | 15%-20% | 36 h |
| VII | Mucosal tract, joint, and muscle bleeding | 15%-20% | 4-6 h |
| X | Umbilical cord, joint, and muscle bleeding | 15%-20% | 40-60 h |
| XI | Posttraumatic bleeding | 15%-20% | 40-70 h |
| XIII | Umbilical cord, intracranial, and joint bleeding; recurrent miscarriages, impaired wound healing | 2%-5% | 11-14 d |
| V + VIII | Mucosal tract bleeding | 15%-20% | 36 h for factor V and 10-14 h for factor VIII |
| Vitamin K—dependent, multiple deficiency | Umbilical cord and intracranial bleeding | 15%-20% | See corresponding factors |
Deficient factor . | Main clinical symptoms* . | Hemostatic levels . | Plasma half-life . |
|---|---|---|---|
| Fibrinogen | Umbilical cord, joint, and mucosal tract bleeding; recurrent miscarriages, rarely thrombosis | 50 mg/dL | 2-4 d |
| Prothrombin | Umbilical cord, joint, and mucosal tract bleeding | 20%-30% | 3-4 d |
| V | Mucosal tract bleeding | 15%-20% | 36 h |
| VII | Mucosal tract, joint, and muscle bleeding | 15%-20% | 4-6 h |
| X | Umbilical cord, joint, and muscle bleeding | 15%-20% | 40-60 h |
| XI | Posttraumatic bleeding | 15%-20% | 40-70 h |
| XIII | Umbilical cord, intracranial, and joint bleeding; recurrent miscarriages, impaired wound healing | 2%-5% | 11-14 d |
| V + VIII | Mucosal tract bleeding | 15%-20% | 36 h for factor V and 10-14 h for factor VIII |
| Vitamin K—dependent, multiple deficiency | Umbilical cord and intracranial bleeding | 15%-20% | See corresponding factors |
Excessive bleeding after invasive procedures carried out without replacement therapy is a symptom common to all RICDs.
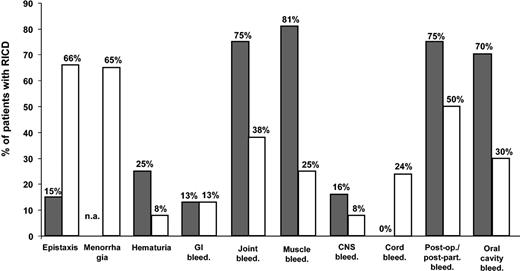
From the data of the 3 registries, as well as from other relatively large cohorts,74,75 a few general considerations can be drawn on the spectrum of clinical manifestations in RICDs (Table 4). Bleeding symptoms that endanger life or cause long-term musculoskeletal handicaps appear to be less frequent in patients with RICDs as a whole than in hemophiliacs of comparable degree of severity (Figure 1). The measurement of plasma levels of factor activity usually helps to predict the severity and frequency of clinical manifestations, but the relationship between factor levels and bleeding tendency is sometimes poor, particularly for FVII and FXI deficiencies.5,64,67,75 The minimal plasma levels of each factor compatible with normal hemostasis, as obtained from the evaluation of the natural history of each RICD in Iranian and Italian patients, are given in Table 4. It must be emphasized that more clinical experience on even larger series of patients is necessary to confirm these hemostatic levels for each RICD.
Bleeding symptoms in RICD patients versus hemophiliacs. Percentage of Iranian patients (n = 750) presumably homozygous for recessively inherited coagulation disorders who had a given bleeding symptom at least once (□), compared with hemophilia A Iranian patients with comparable factor VIII deficiency (less than 10% in plasma) (▪). n.a. denotes not applicable.
Bleeding symptoms in RICD patients versus hemophiliacs. Percentage of Iranian patients (n = 750) presumably homozygous for recessively inherited coagulation disorders who had a given bleeding symptom at least once (□), compared with hemophilia A Iranian patients with comparable factor VIII deficiency (less than 10% in plasma) (▪). n.a. denotes not applicable.
Management
Diagnosis
The combined performance of the global coagulation tests prothrombin time (PT) and activated partial thromboplastin time (APTT) is usually apt to identify RICDs of clinically significant severity but not FXIII deficiency. A prolonged APTT contrasting with a normal PT is indicative of FXI deficiency, provided hemophilia A and B and the asymptomatic defects of the contact phase are ruled out. The specular pattern (normal APTT and prolonged PT) is typical of FVII deficiency, whereas the prolongation of both tests directs further analysis on the possible deficiencies of FX, FV, prothrombin, or fibrinogen. This paradigm is not valid for RICDs due to combined deficiencies, which prolong both the PT and the APTT. Specific assays of factor coagulant activity are necessary when the degree of prolongation of the global tests suggests the presence of severe, clinically significant deficiencies. These assays are routinely available in the average coagulation laboratory in Europe and North America but are seldom carried out, so that proficiency and standardization may be limited. Factor antigen assays are not strictly necessary for diagnosis and treatment but are necessary to distinguish type I from type II deficiencies.
The main application of genotyping is the control of RICDs through prenatal diagnosis on DNA samples obtained by chorionic villus sampling or amniocentesis.76 Genotyping of RICDs is not extensive so far: For instance, only 5.4% of affected individuals were genotyped in the North American5 and 12.7% in the Iranian cohort (F. P., unpublished data, January 2004). Genotyping is complicated by the overall paucity of repetitive mutations (except for FXI deficiency in some populations), which usually makes it necessary to sequence the whole coding region of the gene and its boundaries.
Treatment
As for the hemophilias, replacement of the deficient coagulation factor is the mainstay of treatment for RICDs, but safe and efficacious products are fewer and experiences on their optimal use much more limited. The recommendations given herewith are mainly based on the clinical experience gained with the Iranian and Italian series of patients62-68 and on those from the United States.5,77
Replacement materials
Plasma concentrates of single coagulation factors are available for replacement therapy, licensed or on an investigational basis, in a few European countries but not in the United States (Table 5). Their main advantages are the small volume of infusion, fewer allergic reactions, and the adoption of virus-inactivation procedures during manufacturing. PCCs, licensed for the treatment of FIX deficiency but containing also large amounts of FII, FVII, and FX, can also be used to treat these deficiencies, even though not all the available products are labeled in terms of coagulation factors other than FIX.
Coagulation factor concentrates for use in recessively inherited coagulation disorders
Coagulation factor (brand) . | Manufacturer . | Fractionation . | Viral inactivation . | Comments . |
|---|---|---|---|---|
| Fibrinogen (Haemocomplettan HS) | ZLB Behring, Marburg, Germany | Multiple precipitation | Pasteurization at 60°C, 20 h | Albumin added |
| Fibrinogen (Clottagen) | LFB, Les Ulis, France | Cryoprecipitation, adsorption on aluminum hydroxide gel, anion exchange chromatography | TNBP/polysorbate 80 | — |
| Fibrinogen | SNBTS, Edinburgh, Scotland | Multiple precipitation, ion exchange chromatography | TNBP/polysorbate 80; dry heat, 80°C, 72 h | — |
| Fibrinogen (Fibroraas) | RAAS, Shanghai, China | Multiple fractionation | TNBP/polysorbate 80 | — |
| Factor VII | Bio Products Laboratory, Elstree, United Kingdom | Ion exchange chromatography | Dry heat, 80°C, 72 h | — |
| Factor VII (Facteur VII) | LFB | DEAE adsorption, anion exchange chromatography | TNBP/polysorbate 80 | — |
| Factor VII (Provertin) | Baxter, Vienna, Austria | Ion exchange chromatography | Vapor heat, 60°C, 10 h, at 190 mbar + 80°C, 1 h, at 37/5 mbar | — |
| Recombinant activated factor VII (NovoSeven) | NovoNordisk, Bagsvaerd, Denmark | Recombinant | Yes, not disclosed | Primarily licensed for hemostasis in presence of inhibitors |
| Factor XI | Bio Products Laboratory | Affinity heparin Sepharose chromatography | Dry heat, 80°C, 72 h | Heparin and antithrombin added |
| Factor XI (Hemoleven) | LFB | Dialysis, cation exchange chromatography | TNBP/polysorbate, 15 nm nanofiltration | Heparin, antithrombin, and C-1 esterase inhibitor added |
| Factor XIII (Fibrogammin HS) | ZLB Behring | Multiple precipitation | Pasteurization at 60°C, 10 h | Albumin added |
Coagulation factor (brand) . | Manufacturer . | Fractionation . | Viral inactivation . | Comments . |
|---|---|---|---|---|
| Fibrinogen (Haemocomplettan HS) | ZLB Behring, Marburg, Germany | Multiple precipitation | Pasteurization at 60°C, 20 h | Albumin added |
| Fibrinogen (Clottagen) | LFB, Les Ulis, France | Cryoprecipitation, adsorption on aluminum hydroxide gel, anion exchange chromatography | TNBP/polysorbate 80 | — |
| Fibrinogen | SNBTS, Edinburgh, Scotland | Multiple precipitation, ion exchange chromatography | TNBP/polysorbate 80; dry heat, 80°C, 72 h | — |
| Fibrinogen (Fibroraas) | RAAS, Shanghai, China | Multiple fractionation | TNBP/polysorbate 80 | — |
| Factor VII | Bio Products Laboratory, Elstree, United Kingdom | Ion exchange chromatography | Dry heat, 80°C, 72 h | — |
| Factor VII (Facteur VII) | LFB | DEAE adsorption, anion exchange chromatography | TNBP/polysorbate 80 | — |
| Factor VII (Provertin) | Baxter, Vienna, Austria | Ion exchange chromatography | Vapor heat, 60°C, 10 h, at 190 mbar + 80°C, 1 h, at 37/5 mbar | — |
| Recombinant activated factor VII (NovoSeven) | NovoNordisk, Bagsvaerd, Denmark | Recombinant | Yes, not disclosed | Primarily licensed for hemostasis in presence of inhibitors |
| Factor XI | Bio Products Laboratory | Affinity heparin Sepharose chromatography | Dry heat, 80°C, 72 h | Heparin and antithrombin added |
| Factor XI (Hemoleven) | LFB | Dialysis, cation exchange chromatography | TNBP/polysorbate, 15 nm nanofiltration | Heparin, antithrombin, and C-1 esterase inhibitor added |
| Factor XIII (Fibrogammin HS) | ZLB Behring | Multiple precipitation | Pasteurization at 60°C, 10 h | Albumin added |
Information on these products was obtained from the January 2004 update of Registry of Clotting Factor Concentrates (http://www.wfh.org/showdoc.asp?Rubrique=31&Document=121). DEAE indicates diethylaminoethyl; and TNBP, tri-n-butyl phosphate.
The mainstay of RICD treatment, single-donor fresh-frozen plasma (FFP) (that contains all coagulation factors), is relatively inexpensive and widely available. However, the risk of volume overload is real when repeated infusions are administered to raise and keep the deficient factor at hemostatic levels. Hence, concentrates should be preferred for major surgical procedures or when the severity of the clinical manifestations predicts a long-lasting treatment. Most importantly, infectious complications with such bloodborne viruses as the hepatitis viruses or human immunodeficiency virus (HIV) are still perceived as a threat of FFP. Therefore, even though improvements in donor selection and screening, including nucleic acid testing, is likely to have minimized the actual absolute risk of bloodborne infections (probably less than 1 in 100 000 for the hepatitis viruses and HIV taken together),78 virus-inactivated FFP is preferable to plain FFP. The most widely used method of virus inactivation is based upon the addition to pooled FFP of a solvent-detergent mixture that quenches the infectivity of enveloped viruses but preserves the activity of coagulation factors.79 Another virucidal method such as photoinactivation in the presence of methylene blue80 preserves the activity of coagulation factors, but clinical experience is limited. These products are available in several European countries but not in the United States. None of the aforementioned virucidal methods affects the infectivity of abnormal prions, possibly transmitted by blood transfusion in at least one case.81
Nontransfusional therapies
Antifibrinolytic amino acids may be useful, alone or in combination with replacement therapy, in the management of the less severe forms of mucosal tract hemorrhages. Epsilon aminocaproic acid (50-60 mg/kg every 4 to 6 hours) and tranexamic acid (20-25 mg/kg every 8 to 12 hours) can be administered orally or intravenously. The continued use of estrogen-progestogen preparations helps to reduce menstrual blood loss in women with iron deficiency anemia due to menorrhagia. Because levels of the deficient coagulation factors are not significantly modified, efficacy of this treatment is likely to be due to the changes induced on the endometrium, which bleeds less at the time of menstruation.
Specific recommendations
Specific recommendations are based on the hemostatic levels of each factor, on plasma half-life of the infused factors (which governs the frequency of dose administration) and, most importantly, on safety. The strength of these recommendations (Table 6) is limited by the fact that the size of the series of patients on which they are based is not as large as those of hemophilia A and B 1,2 and that accurate pharmacokinetic studies are lacking.
Recommended schedules of treatment of different clinical situations in patients with recessively inherited coagulation disorders
Factor deficient . | Major surgery . | Minor surgery . | Spontaneous bleeding . | Comments . |
|---|---|---|---|---|
| Fibrinogen | A. Concentrate (20-30 mg/kg) | FFP (15-20 mL/kg) | FFP (15-20 mL/kg) | Prophylaxis with weekly concentrate doses (20-30 mg/kg) if spontaneous bleeding is frequent and severe |
| B. FFP (15-20 mL/kg) | Target: > 50 mg/dL for 2-3 d | Target: > 50 mg/dL until bleeding stops | ||
| C. Cryoprecipitate (1 bag per 10 kg) | ||||
| Target: > 50 mg/dL until healing is complete | ||||
| Prothrombin | A. PCC (20-30 U/kg) | FFP (15-20 mL/kg) | FFP (15-20 mL/kg) | After PCC, FVII, FIX, and FX should not exceed 150% |
| B. FFP (15-20 mL/kg) | Target: > 30% for 2-3 d | Target: > 30% until bleeding stops | ||
| Target: > 30% until healing is complete | ||||
| V | FFP (15-20 mL/kg) | As for major surgery | As for major surgery | No available concentrate |
| Target: > 20% until healing is complete | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| VII | A. rFVIIa (15-30 μg/kg at 12-h intervals) | As for major surgery | As for major surgery | Monitor with factor VII activity assays |
| B. Concentrate (30-40 U/kg at 12-h intervals) | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| Target: > 20% until healing is complete | ||||
| X | A. PCC (20-30 U/kg) | FFP (15-20 mL/kg) | As for major surgery | After PCC, FII, FVII, and FIX should not exceed 150% |
| B. FFP (15-20 mL/kg) | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| Target: > 20% until healing is complete | ||||
| XI | FFP (15-20 mL/kg) | As for major surgery | As for major surgery | Target levels of FXI can usually be achieved also with infusions at alternate days |
| Target: > 20% until healing is complete | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| XIII | A. Concentrate (10-20 U/kg) | As for major surgery | As for major surgery | Prophylaxis in all patients. The replacement material can be infused every 20-30 d |
| B. FFP (15-20 mL/kg) | Target: > 5% for 2-3 d | Target: > 5% until bleeding stops | ||
| C. Cryoprecipitate (1 bag per 10 kg) | ||||
| Target: > 5% until healing is complete |
Factor deficient . | Major surgery . | Minor surgery . | Spontaneous bleeding . | Comments . |
|---|---|---|---|---|
| Fibrinogen | A. Concentrate (20-30 mg/kg) | FFP (15-20 mL/kg) | FFP (15-20 mL/kg) | Prophylaxis with weekly concentrate doses (20-30 mg/kg) if spontaneous bleeding is frequent and severe |
| B. FFP (15-20 mL/kg) | Target: > 50 mg/dL for 2-3 d | Target: > 50 mg/dL until bleeding stops | ||
| C. Cryoprecipitate (1 bag per 10 kg) | ||||
| Target: > 50 mg/dL until healing is complete | ||||
| Prothrombin | A. PCC (20-30 U/kg) | FFP (15-20 mL/kg) | FFP (15-20 mL/kg) | After PCC, FVII, FIX, and FX should not exceed 150% |
| B. FFP (15-20 mL/kg) | Target: > 30% for 2-3 d | Target: > 30% until bleeding stops | ||
| Target: > 30% until healing is complete | ||||
| V | FFP (15-20 mL/kg) | As for major surgery | As for major surgery | No available concentrate |
| Target: > 20% until healing is complete | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| VII | A. rFVIIa (15-30 μg/kg at 12-h intervals) | As for major surgery | As for major surgery | Monitor with factor VII activity assays |
| B. Concentrate (30-40 U/kg at 12-h intervals) | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| Target: > 20% until healing is complete | ||||
| X | A. PCC (20-30 U/kg) | FFP (15-20 mL/kg) | As for major surgery | After PCC, FII, FVII, and FIX should not exceed 150% |
| B. FFP (15-20 mL/kg) | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| Target: > 20% until healing is complete | ||||
| XI | FFP (15-20 mL/kg) | As for major surgery | As for major surgery | Target levels of FXI can usually be achieved also with infusions at alternate days |
| Target: > 20% until healing is complete | Target: > 20% for 2-3 d | Target: > 20% until bleeding stops | ||
| XIII | A. Concentrate (10-20 U/kg) | As for major surgery | As for major surgery | Prophylaxis in all patients. The replacement material can be infused every 20-30 d |
| B. FFP (15-20 mL/kg) | Target: > 5% for 2-3 d | Target: > 5% until bleeding stops | ||
| C. Cryoprecipitate (1 bag per 10 kg) | ||||
| Target: > 5% until healing is complete |
Clinical situations and treatment options in order of priority. FFP denotes fresh-frozen plasma, preferably virus-inactivated; PCC, prothrombin complex concentrate; and rFVIIa, recombinant FVIIa.
Fibrinogen deficiency. Four products are available for replacement: FFP and cryoprecipitate (not virus inactivated), virus-inactivated FFP, and fibrinogen concentrates. Safety commands avoidance of the first 2, if possible. Virus-inactivated FFP is in most instances the first choice to stop or prevent spontaneous bleeding and minor surgery, but concentrates are preferable to avoid volume overload during regular prophylaxis or when bleeding episodes and surgical procedures must be handled. The plasma half-life of fibrinogen is relatively long (Table 4), making regular prophylaxis with weekly infusions possible. The latter mode of treatment delivery, however, is only recommended for patients who bleed frequently and severely in such critical sites as the muscles, joints, gastrointestinal tract, and central nervous system.
Prothrombin deficiency. According to our experience in Iranian and Italian patients, the minimal levels of prothrombin necessary for hemostasis are somewhat higher than for most RICDs (25%-30% instead of 15%-20%) (Table 4). Products for replacement are FFP and PCCs. The latter are virus-inactivated but raise FVII, FIX, and FX to very high plasma levels, particularly when repeated infusions are administered. These high levels of vitamin K–dependent factors may increase the risk of thrombosis, so that laboratory monitoring is advisable to avoid levels in excess of 150% when prolonged treatments are predicted.
FV deficiency. No FV concentrate is available; the main replacement material is FFP, preferably virus-inactivated. The recommended starting dosage is 15 to 20 mL/kg, to be repeated daily to keep FV at hemostatic levels when a prolonged treatment is needed (Table 6). In some instances this schedule may cause volume overload, so that surveillance is mandatory and diuretics are sometimes needed. FFP is also recommended to treat combined FV and FVIII deficiency, with the advantage that in these patients baseline FV levels are usually higher than in isolated FV deficiency. On the other hand, the half-life of FVIII is approximately one third of that of FV (10 to 14 versus 36 hours), so that relatively frequent doses of FFP must be given if FVIII levels are to be kept at the same levels as those of FV. We and others82,83 have sometimes successfully used desmopressin to further raise FVIII when the post-FFP trough levels of this factor were thought to be inadequate for hemostasis.
FVII deficiency. The very short half-life of FVII makes it difficult to use FFP without causing volume overload. Recombinant activated FVII, originally used to bypass the hemostatic defect of hemophilia complicated by inhibitory anti-FVIII alloantibodies, is licensed for FVII deficiency in Europe84,85 (Table 5). The recommended dosage of 15 to 30 μg/kg, repeated according to the given clinical situation, is able to keep FVII levels above 15% to 20% (Table 5). Two commercial manufacturers produce virus-inactivated FVII concentrates, used successfully in small series of patients.86,87 The recommended starting dose is 30 to 40 U/kg, repeated every 12 hours as necessary (Table 6).
FX deficiency. This deficiency can be treated in a way similar to prothrombin deficiency (see “Prothrombin deficiency”), cognizant that the plasma half-life of FX is much shorter than that of prothrombin (40 to 60 hours versus 3 to 4 days). Daily infusions of 20 to 30 U/kg PCCs are thus necessary when a relatively long-lasting treatment is needed, but patients should be closely monitored to avoid that FII, FVII, and FIX levels rise in excess of 150% (Table 6).
FXI deficiency. Although these patients seldom bleed spontaneously, replacement is necessary at the time of major invasive procedures. FXI has a relatively long half-life (40-70 hours), so that the infusion of 15 to 20 mL/kg virus-inactivated FFP at alternate days should be sufficient to keep FIX at trough hemostatic levels of 15% to 20% (Table 6). Plasma concentrates of FXI are manufactured in France and in the United Kingdom,88 but thrombotic complications due to hypercoagulability were observed.89-91 Manufacturers have tried to circumvent this problem by adding heparin and/or protease inhibitors to concentrates (Table 5).
FXIII deficiency. The clinical severity of this deficiency prompts regular prophylaxis.5,68 This mode of treatment is facilitated by hemostatic levels of FXIII as low as 2% to 5% and the very long plasma half-life of infused FXIII (11-14 days), so that replacement material can be infused at large intervals (20-30 days). There are 3 types of FXIII-containing products: FFP (preferably virus-inactivated), cryoprecipitate, and a pasteurized plasma concentrate92-94 (Table 5). A preparation of recombinant FXIII has undergone a phase 1 study in healthy persons.95 The pasteurized concentrate and virus-inactivated FFP are to be preferred to cryoprecipitate. The recommended dosages are 15 to 20 mL/kg for FFP and 10 to 20 U/kg for concentrate (Table 6).
Complications of treatment
There is little information on the incidence and prevalence of alloantibodies inactivating coagulation factors in patients with RICDs. According to the North American registry, 3% of patients with FV and FXIII deficiency developed alloantibodies following treatment with FFP and FXIII concentrates, respectively.5 A few cases of anti-FXIII inhibitors are also mentioned in a European questionnaire survey.96 In a recent study by Salomon et al97 on 64 FXI-deficient Israeli patients treated with FFP, inhibitors developed in 7 of 21 patients (33%) homozygous for the Glu117Stop nonsense mutation but in none of 43 patients with less severe mutations (combined heterozygosity for type II and III, homozygosity for type III, and others).97 The main clinical message that stems from this study is that FXI-containing plasma products should be used sparingly in patients with the type II nonsense mutation. For minor surgical procedures, delivery, and dental extractions, the use of antifibrinolytic amino acids can often substitute for replacement therapy. If replacement therapy cannot be avoided during major surgery or severe bleeding episodes, the development of inhibitors should be monitored in the posttreatment period.
Bloodborne viral infections are another potential complication of treatment in RICDs, but there are few published data. According to the North American registry, seropositivity was 15.6% for hepatitis B, 25% for hepatitis C, and a reassuring 1% for HIV.5 Among Iranian patients, data are currently available only for FXI and FXIII deficiency: The prevalence of hepatitis C infection was 50% for both, with no patient HIV positive.67,68 Hence, bloodborne infections appear to be less frequent in RICDs than in patients with hemophilia, 90% of whom were infected in the past with the hepatitis C virus. This lower frequency is perhaps due to the fact that the need for treatment due to bleeding is generally less frequent in RICDs and that, until recently, nonvirus inactivated, large plasma pool concentrates, the main source of bloodborne infections in hemophiliacs, were used less frequently in RICDs.
General conclusions and recommendations
RICDs are typically orphan diseases, relatively neglected by health care providers, advocacy organizations, and drug manufacturers. Accordingly, patients and their families usually have less extensive information on their ailments than patients with hemophilia A and B. Control of RICDs must rely upon 2 strategies: genetic counseling in the frame of marriages between consanguineous couples and prenatal diagnosis in kindreds at risk for having members with severe disease. Both are not of simple realization in practice. The cultural, religious, and economic roots of the custom of consanguineous marriages are still deep among some communities, even though they are becoming less frequent in large cities and among younger generations. The implementation of prenatal diagnosis in kindreds at risk is hindered not only by cultural and religious reasons but also by the technically demanding, time-consuming, and expensive need to sequence the whole coding region of the gene to find the causative mutation in most kindreds with RICDs.
Amidst these shadows, there are some lights. The International Society for Thrombosis and Haemostasis (ISTH) has established a working group on RICDs with the goals to develop a registry of mutations and establish more precisely genotype-phenotype correlations, to standardize laboratory methods for phenotypic diagnosis, and foster the investigation and licensing of recombinant and plasma-derived products, particularly for those deficiencies (typically, FV deficiency) with no available therapeutic concentrate. Accurate pharmacokinetic and clinical studies, similar to those carried out in patients with hemophilia A and B, should be carried out in patients with RICDs to establish with more precision the plasma half-life of some coagulation factors and the trough hemostatic levels. The medical advisory board of the National Hemophilia Foundation is pursuing the same goals in the United States in collaboration with the ISTH working group. It is hoped that an organization with such a large expertise in standardization and quality control of coagulation tests as the United Kingdom National External Quality Assessment Scheme (UK NEQAS) will soon tackle the issue of laboratory methods for the diagnosis of RICDs. The World Federation of Hemophilia is investing considerable efforts and financial resources on RICDs, particularly in those developing countries where they are more prevalent.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2004-02-0595.
Supported by grants from the Italian Ministry of Education, Telethon-Italy (grant no. GGP030261), World Federation of Hemophilia, and Fondazione Italo Monzino. F.P. was supported by a 2003 Bayer Hemophilia Award (Early Career Investigator).
We thank Drs M. Lak, R. Sharifian, and M. Karimi and all the staff of the Teheran and Shiraz hemophilia centers for their help in supplying some of the information contained in this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal