Abstract
Neutrophil degranulation is important in many inflammatory disorders, although the intracellular mechanisms underlying this process remain poorly understood. The Rho GTPase, Rac2, has been implicated in control of degranulation in earlier studies. We hypothesized that Rac2 selectively regulates neutrophil primary granule release. Using bone marrow and peritoneal exudate neutrophils from rac2-/- mice in comparison with similar cells from wild-type C57Bl/6 mice, we found that primary granule myeloperoxidase and elastase release was absent in Rac2-/- neutrophils in response to chemoattractant stimulation, cytochalasin B/f-Met-Leu-Phe (CB/fMLP), and CB/leukotriene B4. Rac2-/- neutrophils also failed to exhibit mobilization of the primary granule marker CD63+ during CB/fMLP stimulation as determined by confocal microscopy. Priming of Rac2-/- neutrophils with tumor necrosis factor (TNF) or by peritoneal elicitation did not rescue the defect in primary granule release. However, phosphorylation of p38 mitogen-activated protein (MAP) kinase in Rac2-/- neutrophils was evident in response to CB/fMLP and/or TNF. Primary granule density and morphology were normal in Rac2-/- neutrophils. Secondary specific and tertiary granule release, measured by lactoferrin immunoassay and zymography, was normal in response to CB/fMLP and adhesion to fibronectin. These findings suggest an obligatory role for Rac2 in regulation of primary granule release by neutrophils.
Introduction
Neutrophils constitute 40% to 80% of circulating white blood cells and are a critical component in the maintenance of innate immunity. Overexuberant activation of neutrophils can lead to extensive degranulation, which may be fatal in septic shock, acute lung injury, and other serious inflammatory disorders. Neutrophils contain 4 different granule populations: primary azurophilic, secondary specific, tertiary granules, and secretory vesicles. These granule types exhibit a hierarchy of release in response to intracellular Ca2+ spikes,1 suggesting that granule-specific pathways exist to regulate their secretion. Earlier studies using permeabilized or patch-clamped neutrophils have shown that the final step of granule fusion with membrane, leading to release of granule contents, is dependent on guanosine triphosphate (GTP) and Ca2+.2,3 Determining the identity of GTPase(s) involved in this step has been elusive. Recently, Rho GTPases Rac1, Rac2, and Cdc42 have been suggested as possible exocytotic GTPases.4
Rho GTPases, an 18-member subfamily of ras-related GTPases, have been shown to be important in regulating a diverse array of cell activation events downstream of receptor activation.5 Their principle functions are associated with regulation of cytoskeletal reorganization, formation of lamellipodia, chemotaxis, transcriptional events, and cell growth and differentiation.6 Rac1 and Rac2 exhibit distinct tissue distribution patterns; whereas Rac1 is ubiquitously expressed, Rac2 is limited to hematopoietic cells.7 Human neutrophils primarily express Rac2 rather than Rac1,8 whereas murine neutrophils express equivalent levels of Rac1 and Rac2.9 Although Rho GTPases are involved in regulating cell functions at different levels, each isoform demonstrates substantial cross-reactivity with each other. Rac1 and Rac2 exhibit considerable homology at the amino acid level (92% identity)10 and are functionally interchangeable in their ability to activate cytoskeletal remodeling in chemotaxis, as well as superoxide generation through the respiratory burst nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex.11 Such functional overlap may be partially due to the ability of Rac1 and Rac2 to activate common downstream effectors, such as those containing Cdc42/Rac interactive binding (CRIB) motifs (p21-activating kinases 1-6, among others), and in cascades associated with c-Jun kinase and p38 mitogen-activated protein (MAP) kinase.12,13 Inhibition of p38 MAP kinase with SB203580 has been demonstrated to partially block f-Met-Leu-Phe (fMLP)–induced primary and secondary granule release in neutrophils, suggesting an important role for p38 MAP kinase in activating receptor-mediated degranulation.14
Rac2 and Cdc42 have been implicated in the regulation of degranulation in mast cells.4,15,16 Addition of active recombinant isoforms of Rac2 and Cdc42 delayed the loss of sensitivity to GTPγS and Ca2+ during rundown of exocytotic responses in permeabilized rat mast cells,4 while dominant-negative forms of Cdc42 and Rac1 introduced by vaccinia virus vectors into rat basophilic leukemia (RBL-2H3) cells inhibited antigen-induced secretion.16 In addition, Clostridium difficile toxin B, an inhibitor of Rac1, Rac2, and Cdc42 but not RhoA, blocked FcϵRI-mediated degranulation from RBL cells.17,18 However, these studies could not discriminate between pathways selectively regulated by distinct GTPase isoforms. Thus, the use of gene deletion models is essential for defining the role of each specific Rho GTPase isoform in degranulation.
Gene deletion of Rac2 in mice was shown to lead to a profound loss of chemotactic ability in peripheral blood and bone marrow neutrophils, as well as decreased superoxide production in response to fMLP, tumor necrosis factor (TNF), or phorbol myristate acetate (PMA).19 Phosphorylation of p38 MAP kinase was slightly decreased in stimulated Rac2-/- neutrophils,20 whereas that of ERK1/2 was more significantly diminished.19
Recently, a male infant was reported expressing a dominant-negative form of Rac2 (Rac2D57N),21,22 which led to multiple life-threatening infections. Peripheral blood neutrophils from this child exhibited marked deficiencies in adhesion and respiratory burst, while degranulation assays suggested a defect in primary granule release. However, degranulation responses have not been studied in detail in Rac2-deficient neutrophils.
In this study, we hypothesized that Rac2 is a crucial regulator of neutrophil primary granule release. Peritoneal exudate and bone marrow neutrophils from rac2-/- mice were harvested to investigate degranulation responses to chemoattractant stimulation. We show an absence of primary granule release, with intact secondary and tertiary granule exocytosis, in Rac2-/- neutrophils.
Materials and methods
Materials
Chemicals were purchased from Sigma-Aldrich (Oakville, ON) unless otherwise noted. Ca2+, Mg2+, and phenol red–free Hanks balanced salt solution (HBSS) and phosphate-buffered saline (PBS; pH 7.2) were obtained from Life Technologies (Grand Island, NY). HBSS (pH 7.4) was supplemented with bovine serum albumin (BSA; 0.1%) and glucose (1%). Phenol red–free RPMI-1640 was purchased from Life Technologies. Recombinant murine TNF was from BD Pharmingen (San Diego, CA). Antibodies to p38 MAP kinase and phospho-p38 were obtained from BD Biosciences (Mississauga, ON).
Animals
The rac2-/- mice used in this study were previously generated by targeted disruption of the rac2 gene,19 which had been backcrossed into C57Bl/6 mice for more than 11 generations. Wild-type C57Bl/6 control mice were from Charles River Canada (Saint-Constant, PQ, Canada). Animals were housed under specific pathogen-free conditions and fed autoclaved food and water as needed. Mice used in these experiments were between 8 and 20 weeks of age.
Preparation of bone marrow and peritoneal neutrophils
Bone marrow neutrophils (BMNs) were obtained by density gradient centrifugation using a modification of previously described techniques.20,23 Briefly, BMNs were isolated from femurs and tibias flushed with 3 mL HBSS supplemented with BSA and glucose (HBSS-BG) using a 22-gauge needle (Becton Dickinson, San Jose, CA). Cells were pelleted by centrifugation at 300g for 10 minutes at 4°C before mixing with 45% Percoll (Pharmacia, Uppsala, Sweden) and layering onto gradients consisting of 3 mL 81%, 2 mL 62%, 2 mL 55%, and 2 mL 50% Percoll in HBSS-BG. After centrifugation of gradients at 600g for 30 minutes at 10°C, BMNs were removed from the interface between the 62% and 81% layers and washed twice with HBSS-BG. To remove contaminating red blood cells, BMNs were centrifuged on Histopaque 1119 at 600g for 30 minutes at 10°C, and washed twice in HBSS-BG. We obtained 66% to 70% neutrophils for wild-type (WT) and Rac2-/- bone marrow preparations as determined by Diff-Quik (Fisher Scientific, Nepean, ON, Canada) staining, which is similar to previously published values.20 Neutrophil viability was more than 90% as determined by trypan blue exclusion.
Peritoneal exudate neutrophils (PENs) were isolated by intraperitoneal injection of mice with 2 mL 0.05% sodium caseinate in sterile saline 4 hours prior to sacrifice and peritoneal lavage with 6 mL to 8 mL HBSS-BG supplemented with 2 U/mL heparin.24 Cells were washed with phenol red–free RPMI-1640 and stained with Kimura stain for differential counts. The purity averaged between 88% and 94% for neutrophils from 9 animals, with contaminating mast cells. Injection of animals with sodium caseinate was essential for eliciting PENs, since no PENs could be obtained from uninjected animals.
Incubation of cells for degranulation assays
PENs and BMNs were washed and resuspended at 5 × 106 cells/mL to 10 × 106 cells/mL in phenol red–free RPMI-1640 for all degranulation assays, which were carried out on cells in suspension. Aliquots (50 μL) of cells were preincubated with 5 μg/mL cytochalasin B (CB) for 5 minutes at 37°C before adding increasing doses of fMLP (0 μM-40 μM) or leukotriene B4 (LTB4; 0 μM-1 μM). Cells were incubated for an additional 15 minutes or 1 hour before terminating by centrifugation at 4°C for 5 minutes at 300g. Supernatants were removed for assay of released granule proteins, whereas unstimulated cells were lysed by addition of an equivalent volume of RPMI-1640 containing 0.5% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate). For fibronectin adhesion assays, BMNs were incubated on recombinant human fibronectin-coated plates (coated with RetroNectin from PanVera, Fisher Scientific) for 1 hour at 37°C in a humidified incubator as previously described,25,26 and in some experiments, stimulated for an additional 15 minutes with 5 μM fMLP.
Measurement of myeloperoxidase, elastase, and lactoferrin
Myeloperoxidase (MPO), a marker for primary granules, was assayed by using tetramethylbenzidine (TMB) based on a previously established technique.27 Briefly, 150 μL TMB substrate solution was added to 50 μLof sample and incubated (ambient temperature, 30 minutes) prior to termination with 50 μL 1 M H2SO4. Plates were read spectrophotometrically at 450 nm (Molecular Devices, Sunnyvale, CA). Elastase activity was measured by an EnzCheck Elastase assay kit according to the manufacturer's instructions (Molecular Probes, Eugene, OR). Briefly, 100 μL fresh sample was incubated with 100 μL substrate solution (DQ elastin labeled with BODIPY FL dye; Molecular Probes) for 24 hours at room temperature in the dark (shorter incubations generated less fluorescence) before reading the resulting fluorescence at 505 nm for excitation and 515 nm for emission. Absorbance values for released MPO and elastase were divided into the average of values from 0.5% CHAPS-lysed cells to give percentage of total cellular mediator released.
Lactoferrin (LTF) was assayed by enzyme-linked immunosorbent assay (ELISA) based on a previously published observation demonstrating cross-reactivity of human anti-LTF (Sigma-Aldrich) for murine LTF.28 Values of release were plotted as optical density, since this assay technique produced low values for total cellular LTF.
Zymography
Supernatants of resting and stimulated cells were subjected to gel electrophoresis and analyzed for gelatinase activity as previously described.29 Briefly, 4× zymography loading buffer (40% glycerol, 8% sodium dodecyl sulfate [SDS], 20 μg/mL bromophenol blue in 0.25 M Tris, pH 6.8) was added to cell supernatants and the mixture was loaded on 10% acrylamide ready-made zymogram gels (BioRad Laboratories, Hercules, CA). Electrophoresis was carried out at 160 mV for 2 hours and gels were washed 3 times for 20 minutes with 2.5% Triton X-100 at room temperature with shaking. Gels were incubated with zymography buffer (0.9% NaCl, 5 μM CaCl2, 0.000 25% NaN3 in 50 mM Tris) for up to 1 week at 37°C. Gel staining was carried out using 0.5% Coomassie blue G-250 in 25% methanol, 10% acetic acid for 1 hour with shaking at room temperature, followed by destaining overnight with 4% methanol and 8% acetic acid.
Confocal microscopy
Confocal analysis of neutrophil cytospins was carried out as previously reported30 using 10 μg/mL mouse monoclonal antibody to CD63 (Serotec, Raleigh, NC). Binding of CD63 was detected with 2 μg/mL goat antimouse immunoglobulin G (IgG) secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR). Confocal images were acquired on a Zeiss LSM510 confocal laser scanning system (Carl Zeiss Imaging, Thornwood, NY) using a 40× (1.3 numerical aperture) Plan-Neofluor oil immersion objective.
Electron microscopic (EM) analysis of BMNs
BMNs were pelleted from WT and rac2-/- mice and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer before treatment with diaminobenzidine (DAB) solution for 1 hour (0.01% H2O2, 0.05 M Tris-HCl, pH 6.8, and 5 mg/mL DAB), using a modification of a previously described technique,31 to enhance staining of peroxidase-containing granules. Samples were submitted to conventional fixation procedure with 1% osmium tetroxide and sequential dehydration steps before addition of Epon resin and sectioning onto uncoated grids. Sections were subjected to a postfix staining step with lead citrate and uranyl acetate, then analyzed (Hitachi model H7000 transmission electron microscope).
Data presentation
Release of MPO and elastase was calculated as a percentage of total cellular mediator activity by dividing the corrected absorbance of supernatants into the sum of supernatants and average corrected values for lysed pellets. Data were analyzed by 2-tailed Student t test or one-way analysis of variance (ANOVA) analysis followed by Tukey column comparison, and depicted in figures as mean plus or minus the standard error of the mean (SEM). All figures shown represent averages of at least 3 separate experiments.
Results
Rac2 is essential for primary granule release in response to chemoattractants
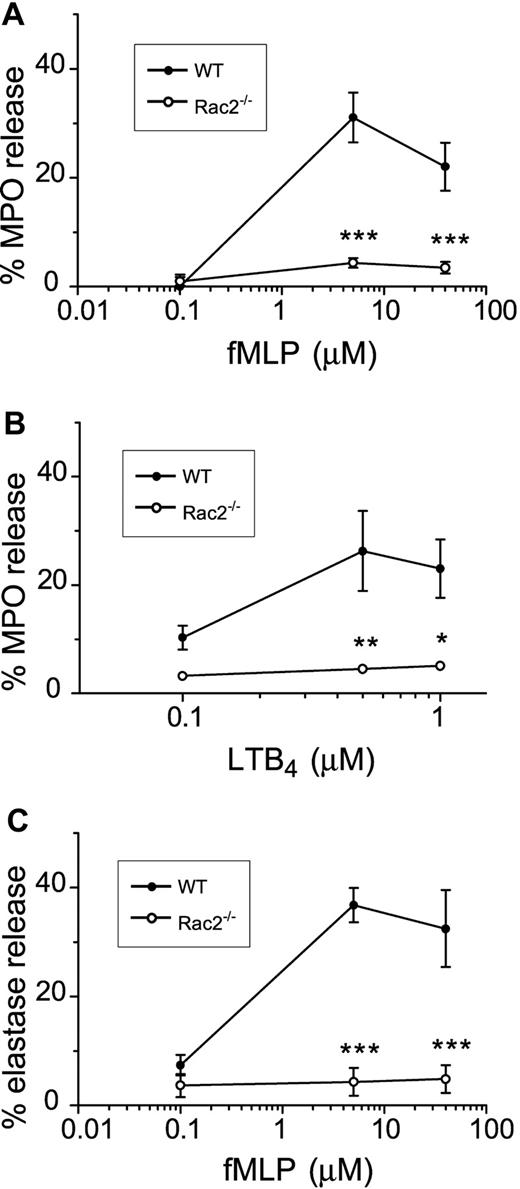
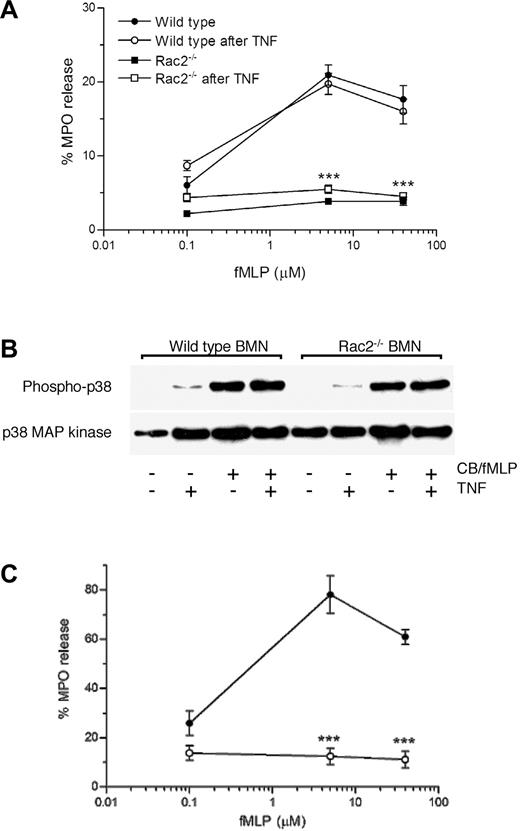
Stimulation of neutrophils in vitro with cytochalasin B in combination with chemoattractants results in release of primary granule contents, which include mediators that are crucial for microbial killing.32 We tested the effect of CB/fMLP on primary granule release from freshly prepared WT and Rac2-/- BMNs by measuring the release of MPO. As shown in Figure 1A, MPO release from WT neutrophils was detected in response to 5 μM to 40 μM fMLP stimulation, with maximal release at 5 μM fMLP (31% ± 2%). In contrast, Rac2-/- BMNs were defective in their ability to release primary granule MPO during CB/fMLP stimulation. Maximal MPO release from Rac2-/- neutrophils was 4.4% ± 0.5% (5 μM fMLP), which did not significantly differ from baseline values obtained in unstimulated Rac2-/- BMNs. WT and Rac2-/- BMNs treated with CB alone did not release significant levels of MPO (2.6% ± 1.9% and 0.8% ± 0.8%, respectively). The MPO content in lysed unstimulated cells was not significantly different between WT and Rac2-/- BMNs (cell pellets averaged 0.34 ± 0.04 versus 0.46 ± 0.23 optical density [OD], respectively), suggesting that MPO was synthesized and stored at similar levels in Rac2-/- BMNs as those of WT BMNs. Similar release profiles were obtained with BMNs stimulated by dihydrocytochalasin B (5 μg/mL) and 0.1 μM to 40 μM fMLP (data not shown), suggesting that preincubation with CB did not adversely affect secretion because of interference with glucose transport. Similarly, increasing doses of another chemoattractant, LTB4, induced the release of MPO from WT BMNs but not Rac2-/- BMNs (Figure 1B). Addition of 50 U/mL superoxide dismutase did not enhance CB/fMLP-induced secretion of MPO, suggesting that released MPO was not significantly inactivated by MPO-catalyzed oxidation.33 These findings suggest that Rac2-/- neutrophils exhibit a deficiency in MPO release in response to stimulation by 2 different agonists.
Absence of primary granule release in chemoattractant-stimulated Rac2-/- neutrophils. Freshly prepared BMNs from WT and rac2-/- neutrophils were examined for their ability to release the primary granule marker MPO in response to increasing doses of fMLP (A) for 15 minutes following 5 minutes preincubation with 5 μg/mL CB. MPO release was determined and shown as percentage of total cellular lysate values. (B) Neutrophils were stimulated with increasing doses of LTB4 for 1 hour following 5 minutes preincubation with 5 μg/mL CB. (C) Elastase activity in supernatants of neutrophils stimulated with CB/fMLP. *P < .05. **P < .02. ***P < .01. Error bars indicate SEM.
Absence of primary granule release in chemoattractant-stimulated Rac2-/- neutrophils. Freshly prepared BMNs from WT and rac2-/- neutrophils were examined for their ability to release the primary granule marker MPO in response to increasing doses of fMLP (A) for 15 minutes following 5 minutes preincubation with 5 μg/mL CB. MPO release was determined and shown as percentage of total cellular lysate values. (B) Neutrophils were stimulated with increasing doses of LTB4 for 1 hour following 5 minutes preincubation with 5 μg/mL CB. (C) Elastase activity in supernatants of neutrophils stimulated with CB/fMLP. *P < .05. **P < .02. ***P < .01. Error bars indicate SEM.
We were unable to obtain significant MPO release in WT or Rac2-/- BMNs in response to murine recombinant TNF (50 ng/mL, 5 minutes) and fMLP (0 μM-40 μM, 15 minutes; data not shown). Fibronectin adhesion (1 hour) induced insignificant MPO release from WT (5% ± 2%) and Rac2-/- (0.2% ± 0.15%) BMNs. Addition of fMLP (0 μM-40 μM) to fibronectin-adhered BMNs did not enhance MPO release in WT (4.8% ± 0.5%) or Rac2-/- (0.8% ± 0.75%) BMNs. This is in contrast to human neutrophils, which release elastase in response to fibronectin.34 These findings suggest that cytochalasin B pretreatment is necessary to induce primary granule release from neutrophils, as supported by previous studies.32,35 We also tested the effect of 2 Ca2+ ionophores, A23187 and ionomycin, on MPO release from BMNs, but these failed to induce significant release from WT or Rac2-/- cells at 1 μM to 10 μM (data not shown). The inability of murine BMNs to release MPO in response to these stimuli may indicate a species difference, since human neutrophils release primary granule products in response to similar conditions (data not shown).32,34,36
In order to confirm that primary granule mediator release was deficient in Rac2-/- BMNs, we measured elastase activity as an additional primary granule marker. Elastase release was also abolished in CB/fMLP-stimulated Rac2-/- BMNs compared with WT cells (Figure 1C). Similar results were obtained in cells stimulated with CB/LTB4 (data not shown). These observations suggest that Rac2 gene deletion leads to loss of primary granule mediator release in neutrophils.
The responsiveness of Rac2-/- neutrophils to respiratory burst stimulation was confirmed by testing BMNs from rac2-/- and WT mice using a conventional cytochrome c reduction assay. BMNs from Rac2-/- mice generated 53% less
CD63 translocation in Rac2-/- neutrophils is deficient in response to CB/fMLP
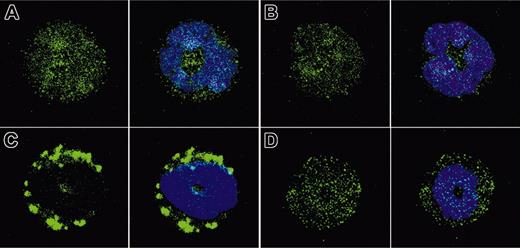
To determine if primary granules translocate to the membrane in response to CB/fMLP in Rac2-/- neutrophils, cytospins of resting and stimulated WT and Rac2-/- BMNs were prepared and labeled for CD63 immunoreactivity. Resting WT and Rac2-/- BMNs exhibited a homogeneous cytoplasmic staining, suggesting the presence of primary granules in the cytoplasm (Figure 2A-B). Following stimulation with CB/fMLP (5 μM, 15 minutes) of WT BMNs, large aggregates of CD63 immunoreactivity formed in submembranous regions, suggesting that CD63+ granules had translocated to the cell membrane (Figure 2C). In contrast, Rac2-/- BMNs did not display any change in CD63 labeling from that of resting cells (Figure 2D), suggesting that granule trafficking may be deficient in Rac2-/- neutrophils. It was not possible to resolve the structure of individual CD63+ granules by confocal analysis, as these are smaller than the limit of resolution of this technique (∼0.3 μm in diameter).3
CD63+ granule translocation is inhibited in Rac2-/- neutrophils. Cytospins were prepared from freshly isolated BMNs treated with CB/fMLP (5 μM) for 15 minutes, stained with 10 μg/mL anti-CD63 as a primary granule marker (green), and counterstained with DAPI (blue) to show nuclear structure. Panels show representative images from (A) resting WT BMNs, (B) resting Rac2-/- BMNs, (C) CB/fMLP-stimulated WT BMNs, and (D) CB/fMLP-stimulated Rac2-/- BMNs, with the left panel in each case showing CD63 alone and the right panel showing combined images of CD63 immunoreactivity and DAPI nuclear stain. Original magnification, ×40.
CD63+ granule translocation is inhibited in Rac2-/- neutrophils. Cytospins were prepared from freshly isolated BMNs treated with CB/fMLP (5 μM) for 15 minutes, stained with 10 μg/mL anti-CD63 as a primary granule marker (green), and counterstained with DAPI (blue) to show nuclear structure. Panels show representative images from (A) resting WT BMNs, (B) resting Rac2-/- BMNs, (C) CB/fMLP-stimulated WT BMNs, and (D) CB/fMLP-stimulated Rac2-/- BMNs, with the left panel in each case showing CD63 alone and the right panel showing combined images of CD63 immunoreactivity and DAPI nuclear stain. Original magnification, ×40.
Granular morphology in WT and Rac2-/- neutrophils is identical
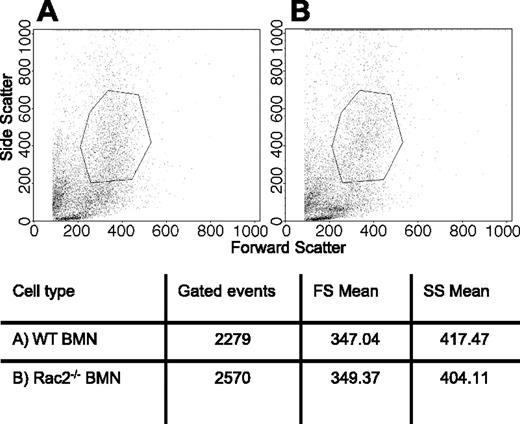
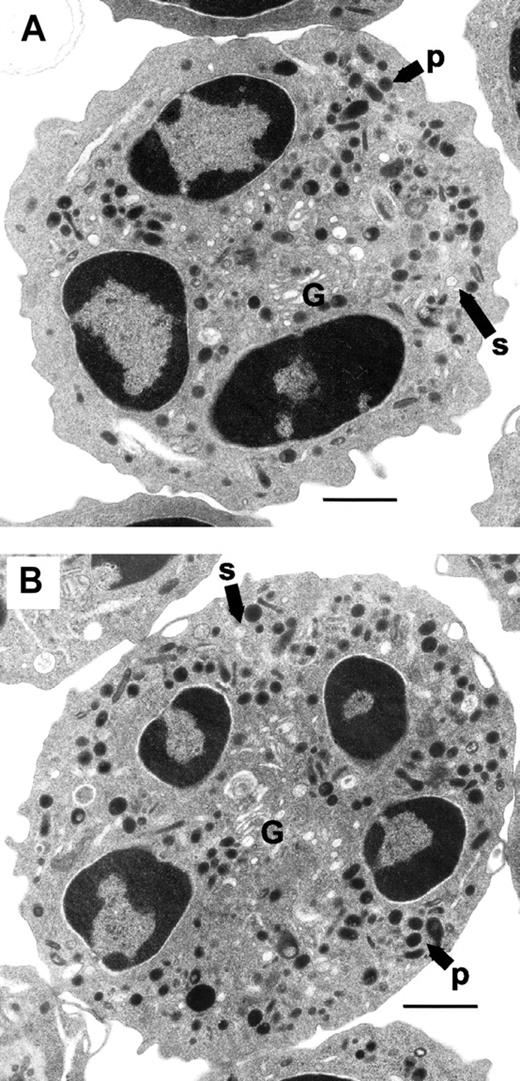
Changes in granule morphology in Rac2-/- neutrophils may explain the defect in primary granule release, such as an overall reduction in granule volume or numbers. To test whether neutrophil morphology from rac2-/- mice was altered in comparison with WT cells, BMNs were subjected to analysis by flow cytometry and assessed for their size and granularity. There were no significant differences in mean forward and side scatter values obtained from gated BMNs from WT and rac2-/- mice (Figure 3), suggesting that cell sizes and granularity of BMNs were similar. BMNs from WT and rac2-/- animals were further analyzed by EM using a conventional osmium tetroxide–based fixation technique coupled with DAB staining to enhance the electron density of peroxidase-containing organelles. Both WT and Rac2-/- neutrophils exhibited identical granule morphology in BMNs (Figure 4), with no apparent difference in granule volume, numbers, or electron density. The similarity in cellular morphology was evident in all cells observed. These findings suggest that rac2 gene deletion did not affect granulogenesis in BMNs, or any other apparent aspects of cellular morphology in resting cells.
Similar granularity in WT and Rac2-/- neutrophils. BMNs were isolated from (A) WT and (B) Rac2-/- mice and subjected to flow cytometric analysis to analyze their side scatter properties. Each scatter plot shows 10 000 events. Gates in the scatter plots are arbitrarily selected to show representative means of forward scatter (FS) and side scatter (SS), as indicated in the table beneath the panels.
Similar granularity in WT and Rac2-/- neutrophils. BMNs were isolated from (A) WT and (B) Rac2-/- mice and subjected to flow cytometric analysis to analyze their side scatter properties. Each scatter plot shows 10 000 events. Gates in the scatter plots are arbitrarily selected to show representative means of forward scatter (FS) and side scatter (SS), as indicated in the table beneath the panels.
Normal granular morphology in neutrophils from rac2-/- mice. Neutrophils were pelleted from bone marrow and sectioned for EM analysis. Panels show typical cells from (A) WT and (B) rac2-/- mice. Labels indicate primary granule (p), secondary granule (s), and Golgi apparatus (G). The bar indicates 1 μm. Original magnification, ×7000.
Normal granular morphology in neutrophils from rac2-/- mice. Neutrophils were pelleted from bone marrow and sectioned for EM analysis. Panels show typical cells from (A) WT and (B) rac2-/- mice. Labels indicate primary granule (p), secondary granule (s), and Golgi apparatus (G). The bar indicates 1 μm. Original magnification, ×7000.
Loss of fMLP-induced primary granule release in Rac2-/- neutrophils is not rescued by in vitro priming with TNF
In order to determine if in vitro priming may reconstitute the release of primary granule release in Rac2-/- neutrophils, TNF was preincubated with cells prior to their activation with CB/fMLP. Pretreatment of Rac2-/- BMNs with TNF (50 ng/mL) failed to correct the defect in MPO release following CB/fMLP stimulation (Figure 5A). Separate experiments were done to confirm that Rac2-/- neutrophils were responsive to TNF stimulation by carrying out flow cytometric analysis for surface expression of adhesion markers CD11b and CD18. In Rac2-/- neutrophils stimulated by TNF at 50 ng/mL for 10 minutes, CD11b and CD18 were up-regulated to a similar degree as that seen in WT neutrophils, indicating that cells were effectively primed by TNF incubation (data not shown).
Priming of Rac2-/- neutrophils fails to reverse the degranulation defect. WT and Rac2-/- BMNs were stimulated with CB/fMLP (5 μM) with or without preincubation of 50 ng/mL TNF for 5 minutes. (A) MPO release from WT and Rac2-/- BMNs in the presence or absence of TNF preincubation. ***P < .01 compared with TNF-preincubated WT controls at the same doses of fMLP. (B) Phosphorylation of p38 MAP kinase in Rac2-/- neutrophils is normal in response to TNF and CB/fMLP. Western blot analysis was carried out for phospho-p38 and p38 MAP kinase in cellular lysates. (C) PENs were treated with increasing doses of fMLP for 15 minutes following 5 minutes preincubation with 5 μg/mL CB. MPO release was determined and shown as percentage of total cellular lysate values. ***P < .01. Error bars indicate SEM.
Priming of Rac2-/- neutrophils fails to reverse the degranulation defect. WT and Rac2-/- BMNs were stimulated with CB/fMLP (5 μM) with or without preincubation of 50 ng/mL TNF for 5 minutes. (A) MPO release from WT and Rac2-/- BMNs in the presence or absence of TNF preincubation. ***P < .01 compared with TNF-preincubated WT controls at the same doses of fMLP. (B) Phosphorylation of p38 MAP kinase in Rac2-/- neutrophils is normal in response to TNF and CB/fMLP. Western blot analysis was carried out for phospho-p38 and p38 MAP kinase in cellular lysates. (C) PENs were treated with increasing doses of fMLP for 15 minutes following 5 minutes preincubation with 5 μg/mL CB. MPO release was determined and shown as percentage of total cellular lysate values. ***P < .01. Error bars indicate SEM.
p38 MAP kinase phosphorylation in Rac2-/- neutrophils is induced by TNF and CB/fMLP
TNF is a potent stimulus for p38 MAP kinase phosphorylation in human neutrophils, and may be required for fMLP-induced primary and secondary granule release as well as interleukin 8 (IL-8) secretion.14,37 We determined whether TNF might also activate p38 kinase in murine BMNs in order to prime these cells for degranulation. Phosphorylation of p38 was investigated in WT and Rac2-/- BMNs before and after stimulation with TNF (50 ng/mL, 5 minutes), CB/fMLP, and TNF plus CB/fMLP (Figure 5B). In WT neutrophils, TNF and CB/fMLP induced p38 phosphorylation, although TNF was less potent in this regard. This is in contrast to human neutrophils, where TNF was shown to be a potent inducer of p38 phosphorylation.37 Addition of TNF to CB/fMLP did not enhance phosphorylation in response to CB/fMLP. Interestingly, Rac2-/- neutrophils exhibited nearly normal levels of p38 phosphorylation in response to TNF and/or CB/fMLP compared with WT cells. These findings suggest that p38 MAP kinase activation alone is not sufficient for triggering primary granule exocytosis.
Defect in fMLP-induced primary granule release is not due to an in vivo priming deficiency in Rac2-/- neutrophils
In vivo priming of neutrophils through peritoneal elicitation is likely to be a stronger stimulus for cell priming than in vitro treatment with cytokines. In peritoneal lavage cells, WT neutrophils exhibited a significant dose-dependent release of primary granule MPO in response to CB/fMLP, with responses reaching maximal values at 5 μM fMLP (78% ± 4% of total cellular MPO), as shown in Figure 5C. In contrast, PENs from rac2-/- mice demonstrated a lack of MPO release under similar conditions (Figure 5C). Rac2-/- PENs showed no increase above baseline levels of spontaneous MPO release (14% ± 2%) in response to all concentrations of fMLP tested in this study. MPO release from WT PENs was considerably greater than that of WT BMNs, presumably due to their primed state. These observations rule out the possibility that the loss of primary granule release may be due to a priming defect in Rac2-/- neutrophils.
Secondary granule release is normal in Rac2-/- neutrophils
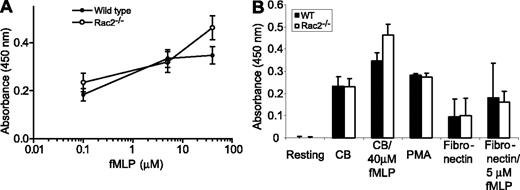
We next tested whether the loss of Rac2 function might also affect the release of another important granule population in neutrophils, secondary granules, which release bacteriostatic mediators such as LTF during phagocytosis and degranulation. The release of LTF from BMNs was measured in response to stimulation with CB/fMLP. There was normal release of LTF from Rac2-/- BMNs in response to CB/fMLP stimulation (Figure 6A), which was equivalent to that of WT neutrophils. However, treatment of WT and Rac2-/- BMNs with CB alone resulted in significant release of LTF (0.23 ± 0.04 OD for WT and Rac2-/- cells, after subtracting background of untreated resting cells). This represented between 50% and 67% of the total releasable LTF in response to maximal stimulation (40 μM fMLP). To determine the release of LTF under more physiologic conditions, freshly prepared BMNs were adhered to fibronectin-coated plates for 1 hour and their supernatants assayed for LTF release. Both WT and Rac2-/- BMNs released LTF following adhesion, representing approximately 24% to 26% of the total releasable LTF (Figure 6B). This release was not significantly enhanced by addition of increasing doses of fMLP (0 μM-40 μM) or coincubation with TNF (10 ng/mL). Addition of 500 ng/mL PMA induced approximately 59% to 81% of total releasable LTF, which did not differ in WT or Rac2-/- BMNs, suggesting that LTF secretion in response to protein kinase C (PKC) activation is similar in both WT and Rac2-/- BMNs. In our hands, this assay was relatively insensitive for detecting LTF release. However, we were able to determine that there was no significant difference in LTF release in WT and Rac2-/- BMNs, indicating that this pathway of exocytosis is independent of Rac2.
Secondary granule LTF release is equivalent in WT and Rac2-/- neutrophils. BMNs were stimulated with increasing doses of (A) fMLP (after CB preincubation). Release was significant (P < .05) at the highest dose of agonist. (B) Comparison of the effects of CB, CB with 40 μM fMLP, PMA (500 ng/mL), fibronectin adhesion (1 hour), or fibronectin (1 hour) followed by 5 μM fMLP on LTF release determined by immunoassay. Error bars indicate SEM.
Secondary granule LTF release is equivalent in WT and Rac2-/- neutrophils. BMNs were stimulated with increasing doses of (A) fMLP (after CB preincubation). Release was significant (P < .05) at the highest dose of agonist. (B) Comparison of the effects of CB, CB with 40 μM fMLP, PMA (500 ng/mL), fibronectin adhesion (1 hour), or fibronectin (1 hour) followed by 5 μM fMLP on LTF release determined by immunoassay. Error bars indicate SEM.
Tertiary granule release is not affected in Rac2-/- neutrophils
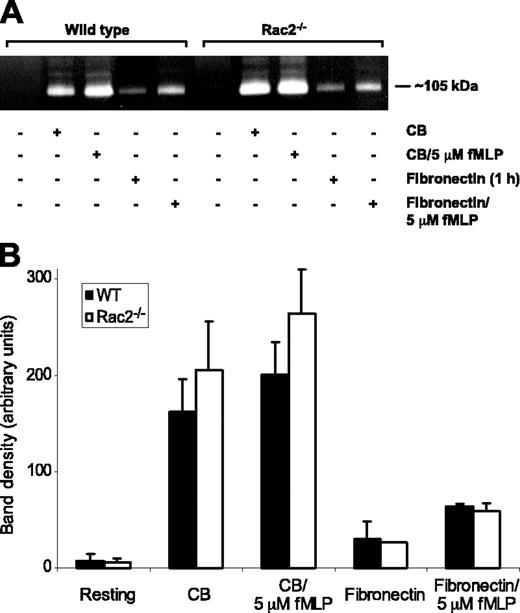
Supernatants of CB/fMLP-stimulated WT and Rac2-/- BMNs were subjected to zymography analysis to determine the release of gelatinase. Unstimulated cells released negligible quantities of gelatinase activity, whereas cells stimulated with CB alone or CB plus 5 μM fMLP released a significant amount of gelatinase activity migrating at approximately 105 kDa (Figure 7A). This molecular weight corresponds with the expected size of murine matrix metalloprotease-9 (MMP-9),38 a marker for the tertiary granules in neutrophils.1 Stimulation of BMNs with CB/fMLP did not enhance the release of MMP-9 over CB alone (Figure 7). Total cell MMP-9 was equivalent in WT and Rac2-/- BMNs based on densitometric analysis of zymography bands from cell lysates, suggesting that Rac2-/- BMNs were not deficient in MMP-9 synthesis and storage (data not shown). Similarly to LTF release, CB alone induced significant gelatinase release in BMNs from WT and rac2-/- mice (78%-80% of total releasable MMP-9). However, we found that MMP-9 release occurred under physiologic conditions in WT and Rac2-/- BMNs in response to adhesion to fibronectin alone (1 hour) and fibronectin (1 hour) plus 5 μM fMLP (Figure 7). Addition of fMLP enhanced the release of MMP-9 when added to fibronectin-adhering cells. Cells released between 22% and 32% of maximal MMP-9 release in response to fibronectin adhesion followed by fMLP (5 μM) stimulation for 15 minutes.
Release of tertiary granule MMP-9 is similar in WT and Rac2-/- neutrophils. (A) Supernatants of WT and Rac2-/- BMNs, either resting or stimulated with CB, CB with 5 μM fMLP, fibronectin (1 hour), or fibronectin (1 hour) with 5 μM fMLP, were separated by gel electrophoresis and analyzed by zymography to detect gelatinase activity, most of which migrated at approximately 105 kDa. (B) Graph showing the average optical density of MMP-9 activity in supernatants of cells stimulated by CB, CB with 5 μM fMLP, fibronectin adhesion (1 hour), or fibronectin (1 hour) followed by 5 μM fMLP. Error bars indicate SEM.
Release of tertiary granule MMP-9 is similar in WT and Rac2-/- neutrophils. (A) Supernatants of WT and Rac2-/- BMNs, either resting or stimulated with CB, CB with 5 μM fMLP, fibronectin (1 hour), or fibronectin (1 hour) with 5 μM fMLP, were separated by gel electrophoresis and analyzed by zymography to detect gelatinase activity, most of which migrated at approximately 105 kDa. (B) Graph showing the average optical density of MMP-9 activity in supernatants of cells stimulated by CB, CB with 5 μM fMLP, fibronectin adhesion (1 hour), or fibronectin (1 hour) followed by 5 μM fMLP. Error bars indicate SEM.
Discussion
Rho GTPases are critical regulators of cellular activation events, which modulate the function of important intracellular effector molecules.5 In this report, we show that Rac2 is required for primary granule release in neutrophils in response to chemoattractant stimulation. Rac2-/- neutrophils exhibited a profound defect in their ability to release 2 different primary granule mediators, MPO and elastase, in response to CB/fMLP and CB/LTB4. In support of these findings, CD63+ granules in Rac2-/- neutrophils failed to translocate in response to CB/fMLP, whereas WT cells exhibited intense granule mobilization to submembranous regions. Addition of the potent priming cytokine TNF did not rescue the degranulation defect in Rac2-/- neutrophils in response to CB/fMLP, although studies have shown that TNF augmented PMA-induced superoxide release and fMLP-induced F-actin formation in these cells.19 Peritoneal exudate neutrophils from rac2-/- animals also showed no MPO release upon stimulation with CB/fMLP. Therefore, the defect in degranulation is not related to deficiencies in neutrophil priming. This is also the first report, to our knowledge, that describes primary granule mediator release in murine neutrophils.
The loss of primary granule release in Rac2-/- BMNs is unrelated to granulogenesis defects, since their side scatter properties resembled that of WT BMNs, and thin sections of Rac2-/- BMNs examined by EM exhibited identical granularity to that of WT cells. Therefore, Rac2 appears to be specifically required for the intracellular machinery dedicated to neutrophil primary granule translocation prior to docking and fusion. Granule translocation is likely to be dependent on highly specific cytoskeletal remodelling events. Moreover, the release of secondary and tertiary granules was not defective in CB/fMLP-treated Rac2-/- neutrophils, suggesting that cytoskeletal reorganization associated with exocytosis in these granule populations was intact in these cells. The addition of CB (an inhibitor of actin filament formation that acts as a priming reagent in neutrophils) is a prerequisite for chemoattractant-induced primary granule release. However, CB alone was able to induce secondary and tertiary granule release, indicating that degranulation can occur by CB-induced microfilament depolymerization.39 Since CB is a nonphysiologic agent, we carried out measurements of LTF and MMP-9 release in response to adhesion to fibronectin and fMLP, and found that the release of secondary and tertiary granules was similar in WT and Rac2-/- BMNs. Our findings suggest that secondary and tertiary granule exocytosis is unimpeded in Rac2-/- BMNs during physiologic stimulation. Although it was not possible to compare the release of mediators from all 3 granule types in response to the same stimulus, the data show that primary granule release in Rac2-/- neutrophils was abolished even during artificial or physiologic priming.
These findings also indicate a lack of functional overlap between Rac1 and Rac2 in neutrophil primary granule exocytosis. Another study has recently suggested divergent roles for Rac1 and Rac2 in cell spreading and motility, respectively.40 This is in contrast to previous studies which suggested overlapping roles for Rho GTPases in cytoskeletal reorganization, cell motility, and gene transcription.5 Murine BMNs from rac2-/- animals express Rho, Rac1, and Cdc42,19 as determined by Western blot analysis (data not shown), and generate similar amounts of Rac1 and Rac2,9 in contrast to human neutrophils which express predominantly Rac2.8 No other Rho GTPase substituted for Rac2 in mediating primary granule release in spite of being expressed at levels similar to WT neutrophils. Thus, downstream signals of Rac2 in this pathway are unlikely to cross-react with other GTPases. Based on our experimental data reported in this paper, we were unable to discriminate whether the defect in primary granule release in Rac2-/- neutrophils was in the signaling pathway adjacent to the receptor or in the final steps leading up to exocytosis, which will be important to determine in future investigations.
Rac2 has been shown to activate MAP kinases in other cell types.41 In particular, p38 and ERK1/2 kinases have been proposed to act as effectors downstream of Rac2 signaling during fMLP stimulation. Moreover, studies have demonstrated that p38 MAP kinase is an obligatory effector molecule required for fMLP-induced IL-8 release in human neutrophils,37 and that inhibition of p38 MAP kinase by SB203980 led to decreased primary and secondary granule release in CB/fMLP-stimulated human neutrophils.14 Similarly, p38 MAP kinase activity was slightly decreased in fMLP-stimulated Rac2-/- neutrophils, suggesting that p38 MAP kinase may function downstream of Rac2.19 In this study, we found that CB/fMLP induced strong p38 phosphorylation in Rac2-/- BMNs, whereas TNF did not augment the phosphorylation signal induced by CB/fMLP. These findings suggest that activation of p38 MAP kinase is not related to the degranulation defect in Rac2-/- neutrophils, and that p38 activation may not be sufficient for primary granule release.
In contrast to the effects of rac2-/- gene deletion on primary granule release, secondary and tertiary granule release was intact in Rac2-/- BMNs in response to CB/fMLP and adhesion to fibronectin. In addition, PMA induced LTF release from both WT and Rac2-/- BMNs. The diacylglycerol-sensitive C1 domain serves as a target for phorbol ester stimulation, and proteins containing this domain include conventional isoforms of PKC.42 PMA induces superoxide production from neutrophils by activation of the NADPH oxidase complex through PKC stimulation.43,44 However, PMA did not evoke primary granule release from murine bone marrow neutrophils. The discrepancy in the effects of PMA on superoxide production and primary granule exocytosis, and the observation that Rac2 is important in both processes, suggests that diacylglycerol-sensitive proteins, such as conventional PKC isoforms, may represent a split in signaling pathways downstream of Rac2 that have not yet been identified for primary granule exocytosis.
In summary, these findings indicate that Rac2 is a critical regulatory GTPase in primary granule exocytosis. The morphology of neutrophils from rac2-/- mice was similar to that of WT animals, suggesting that granulogenesis was normal in these cells. Priming of neutrophils and expression of other homologous Rho GTPases in these cells failed to substitute for the degranulation defect. These findings provide important insights into pathways regulating neutrophil exocytosis, which may serve as targets for anti-inflammatory therapy.
Prepublished online as Blood First Edition Paper, April 8, 2004; DOI 10.1182/blood-2003-07-2624.
Supported by CIHR MOP grant 43 913 (P.L.), CIHR MOP grant 12 660 (G.A.F.), and Riley Children's Foundation and grant P01 HL069 974 (M.C.D.). D.A.L. receives an Alberta Heritage Foundation for Medical Research Studentship, G.A.F. is a Scholar of the Alberta Heritage Foundation for Medical Research, and P.L. is a Canadian Lung Association/CIHR Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ms Jody Seewalt and Mr Richard Sherburne for technical support. We are also grateful to Dr Redwan Moqbel and Dr Tom DeCoursey for helpful comments on the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal