Abstract
Previous studies have suggested that murine T cells are tolerant to epitopes derived from germ line variable regions of immunoglobulin (Ig) heavy (VH) or light chains. This has lead to the prediction that germ line VH-region epitopes found in neoplastic B cells cannot be used to provoke an antitumor immune response. To test these assumptions and address the question of how such a vaccine may alter the normal B-cell response, an antibody-forming B-cell hybridoma (1H6) expressing a conserved germ line VH gene with specificity for dextran was generated and used as a tumor model. Using algorithms for predicting major histocompatibility complex (MHC) binding, potential MHC class I and II binding peptides were identified within the 1H6 VH region, synthesized, and tested for MHC binding and immunogenicity. We show that germ line VH peptides, when presented by dendritic cells, are immunogenic in vitro and provoke a tumor-specific protective immune response in vivo. We conclude that (1) it is possible to induce a T-cell response to germ line VH peptides; (2) such peptides can be used to generate a B-cell tumor-specific vaccine; and (3) a vaccine targeting VH peptides expressed by the dominant dextran-specific B-cell clonotype had no effect upon the magnitude of the normal B-cell response to dextran.
Introduction
B-cell malignancies express a well-defined tumor antigen in the form of the tumor-associated immunoglobulin (Ig).1 The variable regions of the Ig heavy chain (VH) and light chain (VL) are subdivided into the hypervariable complementarity determining regions (CDR) and the more conserved framework regions (Fr), based on their degree of amino acid diversity. This diversity arises by several mechanisms including the juxtaposition of the variable, diversity and joining regions during B-cell ontogeny, and the somatic hypermutation that occurs subsequent to the response to antigenic stimulation.2-4 The idiotype of the Ig is the sum total of these individual regions of diversity and functions as a unique tumor antigen capable of eliciting both humoral and cellular responses directed toward the malignant B cell.5-18
Vaccination using the tumor-specific Ig or VH as the immunogen has been shown to elicit both protective and therapeutic immunity in murine models.8-11,13,19-24 In addition, both protein- and dendritic cell (DC)–based vaccines that use the patient-specific VH have resulted in clinically significant tumor-specific cellular responses.12,14,25-27 The need to generate patient-specific vaccines, however, may limit the general usefulness of this approach. In addition, using the entire idiotype as the immunogen may result in a limited T-cell response directed toward a single or a few immunodominant epitopes.
We hypothesize that major histocompatibility complex (MHC) class I and II binding peptides derived from germ line sequences of the VH, when removed from the suppressive effects of immunodominant epitopes simultaneously presented using the whole protein, can elicit T-cell responses to subdominant and cryptic germ line VH epitopes. If so, a vaccine using DCs pulsed with multiple MHC class I and II binding peptides derived from germ line Fr regions would be expected to elicit a broad T-cell response that can be used for multiple patients whose lymphomas use a common VH family. In this regard, most lymphomas use the VH3 and VH4 families.28,29 Indeed, the potential of using conserved VH framework region peptides as a vaccination approach recently has been suggested by the work of Trojan and colleagues.30
Previous studies, however, suggest that tolerance exists to germ line–derived VH sequences.31-35 For example, T-cell hybridomas generated from mice immunized with the whole Ig responded only to somatically mutated MHC class II–restricted VH peptides.31,32,35 In addition, an MHC class I–restricted VL peptide had to be somatically mutated to be immunogenic.34 In these studies, T-cell hybridomas specific for germ line VH peptides were not found, suggesting that tolerance or nonresponsiveness existed to such peptides.31-35 In these studies, however, an intact protein was used as the immunogen, and as such, it is possible that the T-cell response to the protein was skewed toward a limited number of immunodominant somatically mutated epitopes, thus preventing a response to germ line subdominant epitopes.
To test our hypothesis, we established a B-cell tumor model that used a germ line VH region by generating a dextran-specific hybridoma (1H6) derived from BALB/c primary response spleen cells obtained after immunization with the T-cell–independent antigen dextran B-1355.36 The 1H6 cell line therefore expresses a germ line VH region identical to that expressed in the dextran-specific B-cell clonotype dominating the humoral response to dextran in BALB/c mice.36,37 Nine- and 15- to 17-mer peptides derived from the VH, chosen based on their predicted binding affinity to MHC class I and class II molecules, respectively, of the H-2d haplotype, were synthesized, and their MHC binding was assessed. VH peptide-pulsed DCs were then used to test for peptide immunogenicity in vitro and in vivo. Our results establish that germ line VH region peptides are immunogenic and that immunization of mice with DCs pulsed with a combination of these peptides provides protection from challenge with a tumor expressing the target VH region. Our studies also address what effect a vaccination strategy that targets a B-cell tumor–associated Ig has upon the response capacity of normal B cells that share a VH with the neoplastic B cell.
Materials and methods
Mice
Female 6- to 8-week-old BALB/c mice were maintained in the animal facility at the State University of New York at Buffalo. All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Cell lines and culture conditions
The T2 cell line is transporter associated with antigen processing (TAP) deficient, does not express HLA class II antigens, and expresses low amounts of surface HLA-A2.38 Transfected derivatives expressing the H-2 antigens Dd, Kd, and Ld were obtained from Dr Ted Hansen (Washington University School of Medicine, St Louis, MO). These T2 cell lines were grown in RPMI-10 medium (RPMI-1640 medium containing 10% fetal bovine serum). The hybridoma cell line, 1H6, is the clonal product of the fusion of the myeloma cell line P3.X63.Ag8.653 and spleen cells from a BALB/c mouse immunized with α1,3-dextran B1355 from Leuconostoc mesenteroides. The T3 derivative (1H6.T3) was obtained by 3 serial intraperitoneal passages in BALB/c female mice to select a derivative with increased tumorigenicity and was used in all studies in this report. This cell line was grown in serum-free medium (Hybridoma-SFM; Invitrogen, Grand Island, NY). The A20 cell line is a BALB/c lymphoma (ATCC, Manassas, VA). Prior to inoculation into mice, the cells were grown for several generations in Hybridoma-SFM.
T2 stabilization assay
T2 cells (1 × 106) were incubated overnight at 37° C in 1 mL of serum-free RPMI 1640 in the presence of 50 μg of synthetic peptide dissolved in dimethyl sulfoxide (DMSO). The cells were washed in phosphate-buffered saline (PBS) and suspended in 100 μL PBS on ice. Ten μL of mouse immunoglobulin (3 μg/mL) were added followed by fluorescein isothiocyanate (FITC)–conjugated mouse anti–H-2 monoclonal antibody (eBioscience, San Diego, CA). The cells were washed and fixed in 10% formaldehyde. Anti–H-2 binding was assessed using a FACSCalibur flow cytometer, and the results were analyzed using the WinList software program (Verity Software House, Topsham, ME). Results are expressed as the ratio of the mean fluorescence intensity (MFI) for a given peptide to that obtained with the DMSO negative control, that is, MFI peptide/MFI DMSO.
Competition binding assay
The H-2 molecules Kd and Ld were purified from the mouse mastocytoma P815. The B-cell lymphoma A20-1.11 cell line was used as the source for IAd and IEd. MHC molecule purification and quantitative binding assays were performed as previously described.39 Briefly, purified MHC molecules were co-incubated with unlabeled peptide inhibitors at doses ranging from 12 μg/mL to 120 pg/mL and an excess of 125I-radiolabeled probe peptides for 48 hours in the presence of protease inhibitors. Following the incubation period, MHC-peptide complexes were separated from unbound radiolabeled peptide by size-exclusion gel-filtration chromatography. The percent of bound radioactivity was then determined. The concentration of unlabeled peptide required to inhibit binding of the labeled peptide by 50% (IC50) was determined by plotting dose versus percent inhibition. Under conditions where [label] < [MHC] and IC50 ≥ [MHC], the measured IC50 values are reasonable approximations of true Kd values.
Selection of peptides
The amino acid sequence of the 1H6 VH region was deduced from the DNA sequence determined using standard reverse transcriptase–polymerase chain reaction (RT-PCR) methodology40 with the forward primer 5′GGATGGAGCTGGATCTTTCTC and the reverse primer 5′ACATCGAAGTACCAGTCGTAA. The VH region sequence was subjected to bio-informatics analysis to identify peptides predicted to bind to MHC class I and II molecules using several web-based algorithms (Parker BIMAS: http://bimas.dcrt.nih.gov/molbio/hla_bind/; Rammensee SYFPEITHI algorithms: http://syfpeithi.bmi-heidelberg.com/Scripts/MHCServer.dll/EpitopePrediction.htm) and published binding motifs for predicted MHC class II binding.41-43 Peptides predicted to bind to H-2d class I and class II molecules with a high affinity were selected for this study and synthesized by Multiple Peptide Systems (San Diego, CA).
Preparation of DCs
DCs were generated using a modification of the procedure of Lutz et al.44 Mature DCs were obtained by incubation in RPMI-10 containing 1 μg/mL lipopolysaccharide (LPS; Sigma, St Louis, MO). The generation of DCs was verified by flow cytometry using antibodies to CD80, CD83, CD86, and MHC class II (data not shown). After LPS maturation, DCs displayed up-regulation of CD80 and MHC class II (data not shown).
In vitro stimulation
Six × 106 splenocytes from naive BALB/c mice were suspended in 2 mL RPMI-10 medium, plated in 24-well plates, and incubated with 3 × 105 irradiated (2500 rad) DCs, freshly prepared or thawed, unpulsed or pulsed for 2 to 4 hours with 50 μg of various peptides (and 5 μg β2-microglobulin for class I peptides). After 24 hours, cultures were supplemented with 20 U/mL recombinant human interleukin-2 (rhIL-2, Endogen, Woburn, MA). Cultures were incubated at 37° C, 5% CO2 for 7 days. At weekly intervals, irradiated pulsed or unpulsed DCs were again added along with IL-2, and the cultures were incubated for another 7 days. Cells were harvested, washed in PBS, and monitored for immunogenicity by determining interferon-γ (IFN-γ) secretion in the enzyme-linked immunospot (ELISPOT) assay.
IFN-γ ELISPOT assay
The ELISPOT assay for production of mouse IFN-γ was a modification of the procedure of Asai and colleagues.45 Antibodies were purchased from MabTech (Mariemont, OH). The number of spots in each well was counted using computer-assisted video image analysis (Zeiss ELISPOT reader K-80; Zeiss, Oberkochen, Germany). Results were expressed as the number of spots produced per 5 × 104 cells. Immunogenic peptides were determined by comparing the mean number of spots induced by unpulsed DCs with the mean number of spots induced by VH peptide–pulsed DCs.
Immunization of BALB/c mice with DC peptide vaccine
The 5 × 105 freshly prepared or thawed mature DCs in 1 mL of PBS were exposed individually to 50 μg of each 15- to 17-mer peptide for 4 hours or with 50 μg of a 9-mer peptide plus 5 μg β2-microglobulin for 2 hours at 37° C. Resultant cell suspensions were combined, pelleted, and suspended in PBS at a concentration of 2.5 × 106 cells/mL, and 200 μL of the pooled pulsed DCs was injected intraperitoneally into each of 5 BALB/c mice. Control mice received 5 × 105 unpulsed DCs intraperitoneally or were left unimmunized. Inoculation was repeated at days 14 and 28.
Challenge with tumor cells
Seven days after the second DC injection (day 21), 100 μL of a suspension of 1H6.T3 at 2.5 × 105 cells/mL or A20 cells at 1 × 107 cells/mL was injected subcutaneously into the abdominal region of BALB/c mice. Tumor size was monitored daily and the dimensions of the tumor measured by caliper. Tumor volume is approximated by this formula: a × b2/2, where a is the longer dimension and b is the shorter dimension.
Dextran immunization
BALB/c mice were vaccinated twice, at 2-week intervals, with peptide-pulsed DCs (see “Immunization of BALB/c mice with DC peptide vaccine”) and immunized once by intraperitoneal injection with dextran, 1 week after the second peptide-pulsed DC vaccination. Mice were bled 6 and 18 days after dextran immunization, and the antibody response to dextran determined using a dextran enzyme-linked immunosorbent assay (ELISA).
Dextran ELISA
The production of anti-dextran antibodies was detected in serum using a standard ELISA procedure. Plates were coated with poly-l-lysine followed by the addition of oxidized dextran (prepared by treatment of dextran B1355 with 2 mM sodium periodate and reduction with 2 M sodium borohydride). The detection antibody used was horseradish peroxidase–conjugated polyclonal goat anti–mouse immunoglobulin (ICN/Cappel, a subdivision of ICN, Costa Mesa, CA). The optical density at 490 nm was monitored using a Bio-Tek ELISA Reader (Bio-Tek, Winooski, VT). The amount of anti-dextran antibody in serum samples was determined by extrapolation from a standard curve prepared using affinity-purified 1H6 protein.
Statistical analysis
Statistical analysis of data was performed using the Student t distribution test to compare results of various treatments. A P value less than .05 was considered to represent a statistically significant difference. The time to death of animals was estimated using the product limit method of Kaplan and Meier. Survival plots were plotted as a step function representing the proportion of animals surviving over time in weeks. The survival of the study groups was compared using the log-rank method testing the null hypothesis of equal survival of the animals in separate groups.
Results
1H6 tumor model development
An antibody-forming plasma cell, which is the progeny of a highly conserved B-cell clonotype in the BALB/c repertoire, was immortalized by the fusion of spleen cells (from a BALB/c mouse immunized with the bacterial dextran B1355) to a drug-resistant immunoglobulin-deficient plasmacytoma. From this fusion, a hybridoma specific for the α1-3 linkage group of dextran was identified and cloned and its Ig VH gene sequenced. The VH sequence of this clone (1H6) was in germ line configuration and essentially identical to the clonotype that dominates the BALB/c response to dextran (Figure 1). Sequence differences between the VH region of the dominant primary antidextran antibody response and the 1H6 VH region were restricted to the CDR3 and Fr4 regions (underlined sequences in Figure 1). The 1H6 cell line provides a well-defined model whose cells, when injected into syngeneic BALB/c mice, produce progressively growing tumors, therefore providing a viable model with which to test the immunogenicity of germ line VH region peptides and the ability of these peptides to induce a protective tumor-specific immune response. This model also makes it possible to determine if vaccination with germ line VH region peptides induces a T-cell response that results in the killing of normal B cells expressing these peptides, thereby diminishing the B-cell repertoire and its ability to respond to antigen stimulation.
VH region sequences. The amino acid sequence of the germ line BALB/c mouse heavy chain VH region used in the antidextran response is depicted together with the sequences of the VH region (including the D and J regions) expressed by MOPC104E and the 1H6 cell line.
VH region sequences. The amino acid sequence of the germ line BALB/c mouse heavy chain VH region used in the antidextran response is depicted together with the sequences of the VH region (including the D and J regions) expressed by MOPC104E and the 1H6 cell line.
MHC peptide binding motif search
The 1H6 VH sequence was screened for the presence of potential MHC binding peptides using the Parker and Rammensee algorithms for MHC class I peptide binding predictions and using published binding motifs for MHC class II peptide binding predictions.37-39 A list of the selected peptides that were synthesized is presented in Table 1. Peptides are named by the first amino acid, the length of the peptide, and the last amino acid (eg, D9L for the 9-mer peptide DYWGQGTTL). All MHC class I binding peptides had a predicted T1/2 of dissociation of more than 45 minutes using the Parker algorithm and a Rammensee score greater than 10. All candidate MHC class I and II binding peptides were of germ line sequence except for D9L and Y9Y, peptides derived in part from the CDR-3 region (Table 1).
Candidate MHC class I and II binding VH peptides
Position . | Residues . | Name . | Predicted MHC restriction* . | Parker/Rammensee score . | Sequence . | Position . | Germ line . |
|---|---|---|---|---|---|---|---|
| 105 | 9 | D9L | Kd | 2400/28 | DYWGQGTTL | CDR3/Fr4 | No |
| Ld | 5/11 | ||||||
| 79 | 9 | A9T | Kd | 144/13 | AYMQLNSLT | Fr3 | Yes |
| 43 | 9 | K9I | Kd | 115/16 | KSLEWIGDI | Fr2/CDR2 | Yes |
| Ld | 5/14 | ||||||
| 94 | 9 | Y9Y | Kd | 60/10 | YYCARDRFY | Fr3/CDR3 | No |
| 78 | 9 | T9L | Kd | 48/13 | TAYMQLNSL | Fr3 | Yes |
| Ld | 5/12 | ||||||
| 75 | 9 | S9L | Kd | 40/16 | SSSTAYMQL | Fr3 | Yes |
| Ld | 30/20 | ||||||
| 63 | 9 | K9T | Kd | 29/18 | KFKGKATLT | CDR2/Fr3 | Yes |
| 52 | 9 | N9Y | Ld | 78/13 | NPNNGGTSY | CDR2 | Yes |
| 1 | 16 | E16A | IAd | P | EVQLQQSGPELVKPGA | L/Fr1 | Yes |
| 8 | 16 | G16K | IAd | P | GPELVKPGASVKMSCK | L/Fr1 | Yes |
| 29 | 17 | F17L | IEd | P | FTDYYMKWVKQSHGKSL | Fr1/CDR1/Fr2 | Yes |
| 56 | 15 | G15L | IEd | P | GGTSYNQKFKGKATL | CDR2/Fr3 | Yes |
| 65 | 16 | K16Y | IAd | P | KGKATLTVDKSSSTAY | Fr3 | Yes |
| 79 | 15 | A15V | IAd | P | AYMQLNSLTSEDSAV | Fr3 | Yes |
Position . | Residues . | Name . | Predicted MHC restriction* . | Parker/Rammensee score . | Sequence . | Position . | Germ line . |
|---|---|---|---|---|---|---|---|
| 105 | 9 | D9L | Kd | 2400/28 | DYWGQGTTL | CDR3/Fr4 | No |
| Ld | 5/11 | ||||||
| 79 | 9 | A9T | Kd | 144/13 | AYMQLNSLT | Fr3 | Yes |
| 43 | 9 | K9I | Kd | 115/16 | KSLEWIGDI | Fr2/CDR2 | Yes |
| Ld | 5/14 | ||||||
| 94 | 9 | Y9Y | Kd | 60/10 | YYCARDRFY | Fr3/CDR3 | No |
| 78 | 9 | T9L | Kd | 48/13 | TAYMQLNSL | Fr3 | Yes |
| Ld | 5/12 | ||||||
| 75 | 9 | S9L | Kd | 40/16 | SSSTAYMQL | Fr3 | Yes |
| Ld | 30/20 | ||||||
| 63 | 9 | K9T | Kd | 29/18 | KFKGKATLT | CDR2/Fr3 | Yes |
| 52 | 9 | N9Y | Ld | 78/13 | NPNNGGTSY | CDR2 | Yes |
| 1 | 16 | E16A | IAd | P | EVQLQQSGPELVKPGA | L/Fr1 | Yes |
| 8 | 16 | G16K | IAd | P | GPELVKPGASVKMSCK | L/Fr1 | Yes |
| 29 | 17 | F17L | IEd | P | FTDYYMKWVKQSHGKSL | Fr1/CDR1/Fr2 | Yes |
| 56 | 15 | G15L | IEd | P | GGTSYNQKFKGKATL | CDR2/Fr3 | Yes |
| 65 | 16 | K16Y | IAd | P | KGKATLTVDKSSSTAY | Fr3 | Yes |
| 79 | 15 | A15V | IAd | P | AYMQLNSLTSEDSAV | Fr3 | Yes |
Position is the amino acid position of the VH sequence at which the peptides begin. Residues are the number of amino acids in the peptide. CDR indicates complementarity determining region; L, leader; and Fr, framework region. The Parker scores are estimated half-times of dissociation of the peptide from the MHC allele in minutes.41 The Rammensee scores are based on the relative contribution of amino acid residues and position on MHC binding.42 P indicates peptides selected using published binding motifs for MHC class II peptide-binding predictions.43
The MHC allele corresponding to the predicted binding score of Parker, Rammensee, or published motifs.
Peptide binding assays
A T2 stabilization assay, using T2 cells transfected with H2Kd, Dd, or Ld, was used to determine the binding affinity of the candidate MHC class I peptides (Table 2). In this assay, the effect of peptides on the stabilization of MHC class I is compared to that of control treatment with DMSO vehicle using flow cytometry. An MFI ratio (MFI peptide/MFI DMSO) greater than 1 represents peptide binding. All candidate MHC class I binding peptides showed binding to at least one H-2 locus. The binding affinity of peptides A9T and D9L to purified H2Kd was further evaluated using a competitive binding assay. Both of these peptides that showed binding in the T2 assay bound to purified H2Kd with intermediate affinity (IC50 of 72 nM and 564 nM for A9T and D9L, respectively).
T2 Kd, Dd and Ld binding assay
. | Mean fluorescence intensity ratio* . | . | . | ||
|---|---|---|---|---|---|
| Peptide . | T2Kd . | T2Dd . | T2Ld . | ||
| DMSO | 1.0 | 1.0 | 1.0 | ||
| D9L | 1.9 | 1.0 | 1.0 | ||
| A9T | 1.2 | 1.0 | 1.0 | ||
| K9I | 1.0 | 1.4 | 1.1 | ||
| Y9Y | 1.2 | 1.2 | 1.0 | ||
| T9L | 1.0 | 1.3 | 1.0 | ||
| S9L | 1.0 | 1.3 | 1.0 | ||
| K9T | 1.2 | 1.1 | 1.0 | ||
| N9Y | 1.2 | 1.1 | 1.0 | ||
| P876 (β-gal peptide) | 1.0 | 1.9 | 2.9 | ||
. | Mean fluorescence intensity ratio* . | . | . | ||
|---|---|---|---|---|---|
| Peptide . | T2Kd . | T2Dd . | T2Ld . | ||
| DMSO | 1.0 | 1.0 | 1.0 | ||
| D9L | 1.9 | 1.0 | 1.0 | ||
| A9T | 1.2 | 1.0 | 1.0 | ||
| K9I | 1.0 | 1.4 | 1.1 | ||
| Y9Y | 1.2 | 1.2 | 1.0 | ||
| T9L | 1.0 | 1.3 | 1.0 | ||
| S9L | 1.0 | 1.3 | 1.0 | ||
| K9T | 1.2 | 1.1 | 1.0 | ||
| N9Y | 1.2 | 1.1 | 1.0 | ||
| P876 (β-gal peptide) | 1.0 | 1.9 | 2.9 | ||
Mean fluorescence intensity (MFI) ratio = (MFI peptide/MFI DMSO).
The binding affinity of the candidate MHC class II binding VH peptides to purified IAd and IEd was also determined using the competitive binding assay. As shown in Table 3, F17L, A15V, and the ovalbumin peptide I15E (used as a positive control) bound to either IAd or IEd with intermediate affinity (IC50 50-500 nM); G16K with low affinity (IC50 500-5000 nM); and peptides E16A, G15L, and K16Y were found to not bind to either IAd or IEd (IC50 > 5000 nM).
Peptide-MHC class II binding assay
. | IC50 binding to purified MHC (nM) . | . | |
|---|---|---|---|
| Peptide . | IAd . | IEd . | |
| E16A | — | — | |
| G16K | 2054 | 8109 | |
| F17L | 5450 | 61 | |
| G15L | — | — | |
| K16Y | 7539 | — | |
| A15V | 474 | — | |
| I15E (OVA peptide) | 62 | — | |
. | IC50 binding to purified MHC (nM) . | . | |
|---|---|---|---|
| Peptide . | IAd . | IEd . | |
| E16A | — | — | |
| G16K | 2054 | 8109 | |
| F17L | 5450 | 61 | |
| G15L | — | — | |
| K16Y | 7539 | — | |
| A15V | 474 | — | |
| I15E (OVA peptide) | 62 | — | |
— indicates binding affinity of 20 000 nM or greater.
Evaluating the immunogenicity of MHC class I binding VH peptides
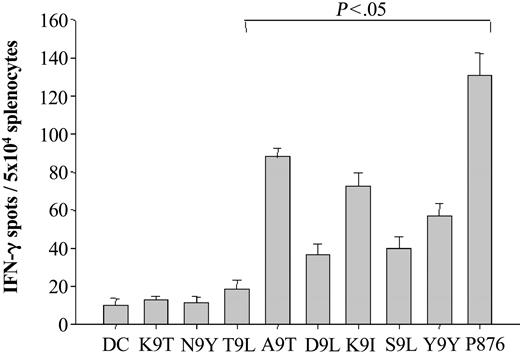
The ability of each peptide, when presented by DCs to provoke a T-cell response in vitro, was next determined (Figure 2). Monocyte-derived DCs were generated from BALB/c bone marrow using granulocyte-macrophage colony-stimulating factor (GM-CSF) and LPS for maturation. After pulsing with a VH peptide, a positive control peptide (peptide P876 from β-galactosidase), or no peptide, the DCs were irradiated and mixed with splenocytes at a DC-to-splenocyte ratio of 1:20. One week after the initial stimulation the splenocytes were restimulated with peptide-pulsed or control-irradiated DCs. One week after the second peptide-DC stimulation the spleen cells were assayed for IFN-γ production using an ELISPOT assay. Significant increases in the number of IFN-γ–producing cells (P < .05 compared to spleen cells stimulated with DCs without peptide) were observed in spleen cells stimulated with 5 different class I binding VH peptides and with the β-galactosidase–positive control peptide (Figure 2). Four of the VH region immunogenic peptides were of germ line sequence: A9T, K9I, S9L, and T9L. We conclude that MHC class I binding peptides derived from germ line–encoded VH regions are immunogenic in BALB/c mice when presented by autologous DCs.
Immunogenicity of MHC class I binding VH peptides. Murine DCs were generated using a modification of the method of Lutz et al, in which bone marrow mononuclear cells are treated with GM-CSF and matured with LPS.44 Mature murine DCs (1 × 105) were pulsed with either VH peptide (50 μg) or β-gal peptide (as a positive control) and β2-microglobulin (5 μg) for 2 hours. The peptide-pulsed DCs were washed, irradiated, and mixed with splenocytes at a DC-to-splenocyte ratio of 1:20 in the presence of IL-2 for 2 weekly in vitro stimulations. The number of antigen-reactive T cells was then assessed using an IFN-γ ELISPOT assay after an overnight restimulation in the presence of peptide. Spot numbers were automatically determined with the use of a computer-assisted video image analyzer. The numbers of antigen-reactive cells (per 5 × 104 cells) induced by MHC class I binding VH peptide-pulsed DCs were compared to those of non–peptide-pulsed DCs alone (bracket indicates a significant increase; P < .05). The results shown for the MHC class I binding VH peptides are representative of 3 independent experiments except for that of peptides K9I, K9T, N9Y, and Y9Y, which are representative of 4, 2, 2, and 2 independent experiments, respectively.
Immunogenicity of MHC class I binding VH peptides. Murine DCs were generated using a modification of the method of Lutz et al, in which bone marrow mononuclear cells are treated with GM-CSF and matured with LPS.44 Mature murine DCs (1 × 105) were pulsed with either VH peptide (50 μg) or β-gal peptide (as a positive control) and β2-microglobulin (5 μg) for 2 hours. The peptide-pulsed DCs were washed, irradiated, and mixed with splenocytes at a DC-to-splenocyte ratio of 1:20 in the presence of IL-2 for 2 weekly in vitro stimulations. The number of antigen-reactive T cells was then assessed using an IFN-γ ELISPOT assay after an overnight restimulation in the presence of peptide. Spot numbers were automatically determined with the use of a computer-assisted video image analyzer. The numbers of antigen-reactive cells (per 5 × 104 cells) induced by MHC class I binding VH peptide-pulsed DCs were compared to those of non–peptide-pulsed DCs alone (bracket indicates a significant increase; P < .05). The results shown for the MHC class I binding VH peptides are representative of 3 independent experiments except for that of peptides K9I, K9T, N9Y, and Y9Y, which are representative of 4, 2, 2, and 2 independent experiments, respectively.
Immunogenicity of MHC class II binding VH peptides
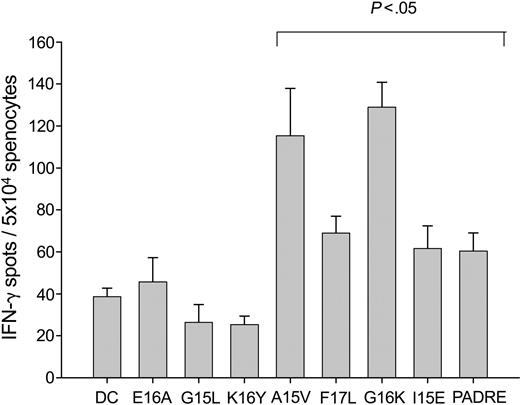
Six 15- to 17-mer peptides corresponding to sequences within the 1H6 VH were synthesized and tested for immunogenicity using the same protocol described above to test the MHC class I binding peptides. Three peptides were shown to be immunogenic: A15V, F17L, and G16K, corresponding to germ line 1H6 VH region sequences (Figure 3). All 3 of these peptides were shown to bind to MHC class II, while the 3 nonimmunogenic peptides E16A, G15L, and K16Y did not bind to MHC class II (Table 3). The positive control peptide I15E, shown in Table 3 to be an intermediate affinity binder to MHC class II, provoked a significant response in the ELISPOT assay, as did the pan-MHC class II binding peptide PADRE, also used as a positive control. These results establish that T cells are not tolerant to MHC class II binding peptides derived from germ line sequences within the VH region of 1H6.
Immunogenicity of MHC class II binding VH peptides. Murine DCs were generated using a modification of the method of Lutz et al, in which bone marrow mononuclear cells are treated with GM-CSF and matured with LPS.44 Mature murine DCs (3 × 105) were pulsed with either VH peptide (50 μg) or I15E, an ovalbumin-derived peptide, or PADRE, a pan-MHC class II binding peptide, as positive controls for 4 hours. The peptide-pulsed DCs were washed, irradiated, and mixed with splenocytes at a DC-to-splenocyte ratio of 1:20 in the presence of IL-2 for 2 weekly in vitro stimulations. The number of antigen-reactive T cells was then assessed using an IFN-γ ELISPOT assay after an overnight restimulation in the presence of peptide. Spot numbers were automatically determined with the use of a computer-assisted video image analyzer. The numbers of antigen-reactive cells (per 5 × 104 cells) induced by MHC class II binding VH peptide-pulsed DCs were compared to that of non–peptide-pulsed DCs alone (bracket indicates a significant increase; P < .05). The results shown for the MHC class II binding VH peptides are representative of 3 independent experiments except for peptides E16A and K16Y, which were representative of one experiment.
Immunogenicity of MHC class II binding VH peptides. Murine DCs were generated using a modification of the method of Lutz et al, in which bone marrow mononuclear cells are treated with GM-CSF and matured with LPS.44 Mature murine DCs (3 × 105) were pulsed with either VH peptide (50 μg) or I15E, an ovalbumin-derived peptide, or PADRE, a pan-MHC class II binding peptide, as positive controls for 4 hours. The peptide-pulsed DCs were washed, irradiated, and mixed with splenocytes at a DC-to-splenocyte ratio of 1:20 in the presence of IL-2 for 2 weekly in vitro stimulations. The number of antigen-reactive T cells was then assessed using an IFN-γ ELISPOT assay after an overnight restimulation in the presence of peptide. Spot numbers were automatically determined with the use of a computer-assisted video image analyzer. The numbers of antigen-reactive cells (per 5 × 104 cells) induced by MHC class II binding VH peptide-pulsed DCs were compared to that of non–peptide-pulsed DCs alone (bracket indicates a significant increase; P < .05). The results shown for the MHC class II binding VH peptides are representative of 3 independent experiments except for peptides E16A and K16Y, which were representative of one experiment.
DCs pulsed with a combination of immunogenic MHC class I and II binding 1H6 VH peptides induce a tumor-specific protective immune response in BALB/c mice
While the results presented above sustain the notion that germ line VH region peptides can induce a T-cell response in vitro, they do not address the question of whether a vaccine incorporating such peptides provokes a protective tumor immune response in vivo. To do so, these peptides would not only be required to elicit an in vivo T-cell response, but they must also be presented by the tumor in the context of MHC so as to render the tumor a target for the effector functions of such T cells. As an optimal tumor vaccine is likely to be one that elicits both MHC class I– and II–restricted T-cell responses directed against multiple tumor antigens, we tested the efficacy of these immunogenic VH peptides as a tumor vaccine using a combination of 3 germ line MHC class I binding peptides (A9T, K9I, and S9L) and 3 germ line MHC class II binding peptides (A15V, F17L, and G16K) shown to provoke a T-cell response in vitro. 5 × 105 peptide-pulsed DCs were injected intraperitoneally into BALB/c mice 3 times at 14-day intervals. Mice were challenged with 2.5 × 104 1H6 tumor cells, cultivated in serum-free medium, one week after the second DC vaccination. Mice were monitored weekly for evidence of tumor growth. VH peptide-pulsed DC vaccination resulted in significant protection against tumor challenge as shown in the Kaplan-Meier distributions of tumor-free mice in the VH vaccinated versus control groups (Figure 4; P < .001). The specificity of this protective immune response was demonstrated by the failure of the 1H6 VH peptide-pulsed DC vaccine to protect against a challenge with the A20 B-cell lymphoma, expressing a tumor-associated Ig derived from a nonrelated VH gene (P = .99; data not shown).
VH peptide–pulsed DC vaccination induces a protective immune response in vivo. This is a compilation of data from 3 independent experiments, each of which showed similar results. In the VH group (n = 15 mice) mice were vaccinated intraperitoneally with DCs pulsed with 3 germ line MHC class I peptides (A9T[Fr3], K9I[Fr2/CDR2], S9L[Fr3]) and 3 germ line MHC class II VH peptides (G16K[Leader/Fr1], F17L[Fr1/CDR1/Fr2], A15V[Fr3]) derived from the VH of the 1H6 murine lymphoma cell line and shown to be immunogenic in vitro, on days 1, 14, and 21. In the control group (n = 14 mice) mice were not vaccinated. On day 24, mice in all groups were challenged subcutaneously with 2.5 × 104 1H6 cells grown in serum-free hybridoma medium. Tumors were measured weekly, and the proportion of tumor-free mice is presented. The Kaplan Meier distributions of tumor-free mice for the VH group were significantly higher than that of the nonvaccinated control mice (control vs VH; P < .001). There was a significant decrease in the distribution of tumor-free mice in a group of mice (n = 15) treated with non–peptide-pulsed DCs alone, compared to that of the VH group (P = .007; data not shown).
VH peptide–pulsed DC vaccination induces a protective immune response in vivo. This is a compilation of data from 3 independent experiments, each of which showed similar results. In the VH group (n = 15 mice) mice were vaccinated intraperitoneally with DCs pulsed with 3 germ line MHC class I peptides (A9T[Fr3], K9I[Fr2/CDR2], S9L[Fr3]) and 3 germ line MHC class II VH peptides (G16K[Leader/Fr1], F17L[Fr1/CDR1/Fr2], A15V[Fr3]) derived from the VH of the 1H6 murine lymphoma cell line and shown to be immunogenic in vitro, on days 1, 14, and 21. In the control group (n = 14 mice) mice were not vaccinated. On day 24, mice in all groups were challenged subcutaneously with 2.5 × 104 1H6 cells grown in serum-free hybridoma medium. Tumors were measured weekly, and the proportion of tumor-free mice is presented. The Kaplan Meier distributions of tumor-free mice for the VH group were significantly higher than that of the nonvaccinated control mice (control vs VH; P < .001). There was a significant decrease in the distribution of tumor-free mice in a group of mice (n = 15) treated with non–peptide-pulsed DCs alone, compared to that of the VH group (P = .007; data not shown).
DCs pulsed with a combination of immunogenic MHC class I and II binding 1H6 VH peptides do not alter the humoral response of mice immunized with dextran B1355S
The 1H6 tumor model that we have developed allows us to address the fundamental question of what effect VH peptide-pulsed DC vaccination has on the host humoral response. In this model, the tumor-associated target of our VH peptide-pulsed DC vaccine, the 1H6 VH region, is expressed as the dominant B-cell clonotypic response to bacterial dextran. To address this question, BALB/c mice were immunized twice with DCs pulsed with the same 6 germ line peptides used in the tumor-protective vaccine described above. Control groups included untreated mice and mice injected with non–peptide-pulsed DCs. All 3 groups of mice were immunized with the dextran B1355S one week after the second injection of DCs. Mice were bled 6 days after immunization with dextran and the concentration of anti-dextran antibodies in the sera determined by ELISA (we had previously shown that the peak primary response to dextran is 6 days after challenge). No significant differences in the levels of anti-dextran antibodies were observed in any of the 3 groups of mice (Table 4). We also have evaluated the anti-dextran response 18 days after immunization, and there was still no statistically significant difference between the groups (data not shown). These results establish that the VH peptide-pulsed DC vaccine that provided a specific protective immune response against a tumor expressing the dominant antidextran clonotypic Ig had no effect upon the magnitude of the humoral immune response to dextran.
The effect of VH peptide vaccination on the antidextran humoral response
Vaccination group and mouse no. . | Antidextran Ab, μg/mL . |
|---|---|
| Control | |
| 1-1 | 264 |
| 1-2 | 342 |
| 1-3 | 263 |
| 1-4 | 125 |
| 1-5 | 201 |
| Mean | 239 |
| Dcs alone | |
| 2-1 | 221 |
| 2-2 | 239 |
| 2-3 | 272 |
| 2-4 | 351 |
| 2-5 | 240 |
| Mean | 265 |
| VH peptide vaccine* | |
| 3-1 | 263 |
| 3-2 | 219 |
| 3-3 | 365 |
| 3-4 | 160 |
| 3-5 | 163 |
| Mean | 234 |
Vaccination group and mouse no. . | Antidextran Ab, μg/mL . |
|---|---|
| Control | |
| 1-1 | 264 |
| 1-2 | 342 |
| 1-3 | 263 |
| 1-4 | 125 |
| 1-5 | 201 |
| Mean | 239 |
| Dcs alone | |
| 2-1 | 221 |
| 2-2 | 239 |
| 2-3 | 272 |
| 2-4 | 351 |
| 2-5 | 240 |
| Mean | 265 |
| VH peptide vaccine* | |
| 3-1 | 263 |
| 3-2 | 219 |
| 3-3 | 365 |
| 3-4 | 160 |
| 3-5 | 163 |
| Mean | 234 |
Ab indicates antibody.
DCs pulsed with the 3 germ line MHC class I peptides (A9T[Fr3], K9I[Fr2/CDR2], S9L[Fr3]) and 3 germ line MHC class II VH peptides (G16K[Leader/Fr1], F17L[Fr1/CDR1/Fr2], A15V[Fr3]) used in the tumor protection studies shown in Figure 4. P > .5 for all comparisons. These data are representative of 2 independent experiments.
Discussion
Our data establish that MHC class I and II binding peptides corresponding to germ line regions of an Ig VH are immunogenic. Previous studies have shown that somatic mutations within the VH or VL generate immunogenic T-cell epitopes, whereas peptides derived from germ line sequences are not immunogenic.31-35 The conclusion drawn from these studies was that murine T cells are tolerant to germ line V region sequences of the Ig. All of these previous studies, however, have similar experimental designs. In each case, mice were immunized with a protein antigen or were infected with a virus.31-35 These proteins must first gain access to antigen presenting cells (APCs), which then must process and present defined peptide epitopes in the context of MHC. T-cell hybridoma clones recognizing such peptide-MHC complexes were then generated and restimulated with peptide. However, T-cell responses to protein antigens are usually limited to only a small number of immunodominant epitopes, which may suppress the T-cell response to subdominant or cryptic epitopes.46-50 It is possible that the somatically mutated epitopes are immunodominant and, therefore, T-cell responses to the protein antigen are skewed toward such epitopes. This may explain why previous studies have failed to recognize the ability of T cells to respond to germ line epitopes in the immunoglobulin variable regions.
Our experimental plan was designed to eliminate the possible suppressive effects of dominant epitopes as well as to take into account the possibility that germ line peptides were not appropriately processed and presented by the DCs when pulsed with the whole protein. This was achieved by directly pulsing DCs with relatively high concentrations of synthetic germ line peptides that bind to MHC class I and II. Our findings under these conditions indicate that T cells can respond to germ line VH peptides and as such are not tolerant to these epitopes. It is interesting to note that whereas peptide G15L does not bind to I-Ad or I-Ed, a similar 12-mer peptide has been shown to bind to I-Ed but not to I-Ad.51 Although this seems paradoxical, the different binding behaviors can be attributed to the different flanking regions adjacent to the peptide core motif, as we have recently reported.52
It has previously been suggested that the lack of response to germ line VH peptides is due not to the immunodominance of somatically mutated peptides but rather to the existence of peripheral tolerance to germ line peptides.31 Recent work has suggested, however, that peripheral tolerance can be broken by stimulating low affinity, nontolerized T-cell clones.53 In this regard, if peripheral tolerance to germ line VH peptides does in fact exist, it is likely that ex vivo–matured DCs, expressing high levels of costimulatory molecules and pulsed with a high concentration of peptide so as to load a high frequency of MHC complexes as is done in the present studies, would stimulate such T cells and overcome peripheral tolerance.
These issues are particularly important with respect to the design of B-cell lymphoma vaccines using tumor-associated Ig as the antigen. We and others have previously established that the tumor-associated Ig can serve as a tumor-specific antigen capable of provoking a tumor-specific immune response resulting in both protective and therapeutic effects in animal models.8-11,13,19-24 Recent clinical studies suggest that the elicitation of a cellular immune response directed against the lymphoma idiotype is a feasible and clinically relevant immunotherapeutic approach.12,14,16,25-27 The major limitation of this is that a specific vaccine must be generated for each patient, a technically and logistically challenging process that may limit its general applicability due to the time and cost involved. The recognition that T cells can respond to germ line VH peptides may help to obviate this limitation. Since most human B-cell lymphomas express Ig with heavy chains corresponding to just 2 VH families, VH3 and VH4, it should be possible to select Fr region peptides that are present in most of these family members to be used as immunogens in a more globally applicable vaccine. Indeed, the immunogenicity of Ig Fr region–derived peptides and the potential of using such a vaccination strategy for patients with B-cell malignancies were previously described.30,54 By further selecting peptides that bind to multiple MHC alleles, one can construct a vaccine that would have greater population coverage than protein-based vaccinations.55 In addition, DCs pulsed with multiple MHC class I and II binding peptides would be expected to elicit a broader T-cell response than that elicited by vaccines using the intact protein as the antigen. Such a peptide vaccine would not be constrained by the immunodominance of somatically mutated epitopes that are likely to be associated with the response to protein vaccination.
To evaluate the feasibility of such an approach, we tested the ability of DCs pulsed with multiple MHC class I and II binding peptides derived from germ line VH sequences of the 1H6 Ig to elicit protective immunity against challenge with 1H6. We chose to evaluate this approach because there is evidence to suggest that an optimal vaccine is one that elicits a broad T-cell response as well as both an MHC class I– and II–restricted T-cell response.56,57 In this study we show that such a DC vaccine does elicit protection from 1H6 challenge. Although we have not determined which of the peptides in the vaccine contributed to the protective response, this initial test has established the feasibility of using germ line peptides to induce a protective immune response. It also established that one or more of these peptides are likely generated (ie, processed and presented) by and expressed on the target 1H6 tumor in vivo. Studies in our laboratory (Q.L., R.K., R.B.B., and S.H.B., unpublished, January 2000) indicate that the in vitro cultivation of DCs in serum-containing medium results in the uptake and presentation of serum-derived peptides, leading to nonspecific tumor protection by unpulsed DCs. For this reason we propagated the tumor target 1H6 cells in serum-free medium to minimize any effect of a T-cell response directed against serum-derived antigens.
To address the question of the specificity of this vaccination approach, vaccinated mice were challenged with another B-cell tumor. In contrast to the protection offered against 1H6, the peptide vaccine did not elicit any protection against challenge with the BALB/c A20 B-cell lymphoma line, the VH of which did not contain any of the peptide sequences of the 6 1H6 germ line–derived peptides used in the protection model.58 This selective protection against 1H6 suggests that the IH6 germ line VH peptide-pulsed DC vaccine elicited a tumor-specific adaptive response in vivo.
An important clinical concern for using vaccination strategies that target the B-cell Ig for patients with B-cell lymphoma is whether such an approach would adversely affect the humoral response to antigen exposure. The 1H6 model ideally lends itself to address this question, as the dominant B-cell clonotype in the dextran humoral response expresses the same VH region as the 1H6 tumor cell line. The data presented show that the 1H6 peptide-pulsed DC vaccine, while eliciting a protective immune response in vivo, had no significant quantitative effect on the antidextran antibody response. Several possible explanations for this exist. First, normal B cells of this dominant clonotype may not process and present the targeted peptides in the context of MHC sufficiently for T-cell recognition. This possibility is supported by recent studies showing negligible presentation of endogenous VH-derived peptides by high-density resting B cells.59 However, to be effective as a vaccine, the VH peptides must either be presented in the MHC of the malignant B cell or presented by antigen-presenting cells within the tumor microenvironment. A second possible explanation is that the dominant anti-dextran clonotype may be suppressed, but the level of anti-dextran antibody remains the same due to the increased expression of dextran-specific clonotypes using different VH regions. These possibilities will be explored in future studies.
The effect of VH peptide vaccination on the normal B-cell response also has been evaluated by Fan and Singh.60 In their studies, anti-VH cytotoxic T lymphocytes (CTLs), designed to eliminate autoreactive B cells in lupus-prone mice, killed only a fraction of normal B cells expressing these VH peptides after challenge with a hybridoma producing Ig using that VH. Taken together with the results presented here, targeting the Ig with a VH peptide-pulsed DC vaccine is likely to have little or no apparent effect upon the antigen response capacity of the B-cell repertoire, however, this needs to be studied further.
The VH peptide-pulsed DC vaccine did not elicit complete protection against 1H6 tumor challenge since a proportion of the vaccinated mice developed tumors. This was not surprising since the T-cell response to tumor antigens (particularly self-antigens) is not as robust as the response to viral antigens, for example. We are presently evaluating VH vaccines whereby modifications to certain amino acid residues of the peptide are generated to increase their immunogenicity. For example, fixed anchor analogs, whereby the main MHC anchor residues of the peptide are modified to increase peptide-MHC binding affinity, have been shown to increase peptide immunogenicity, including that of VH peptides.54,61 In addition, modifications of nonmain anchor residues result in subtle conformational alterations of the peptide, increasing the peptide/MHC affinity for the T-cell receptor.62 Indeed, we have previously shown that such heteroclitic peptides elicit stronger responses than that of the native epitope and can be used to overcome T-cell tolerance.62 As such, the use of heteroclitic peptides in a multi-epitope vaccine directed against a self-tumor antigen may increase its effectiveness and may represent a novel therapeutic approach for patients having a malignancy with a well-characterized tumor antigen, such as the VH for patients with B-cell malignancies
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2004-01-0105.
Supported by National Institutes of Health grant R01 CA 85518 (R.B.B. and S.H.B.) and a Leukemia and Lymphoma Society Translational Research Award (S.H.B).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Gary Lyman for his help in the statistical analyses and John Sidney for helpful discussions.


![Figure 4. VH peptide–pulsed DC vaccination induces a protective immune response in vivo. This is a compilation of data from 3 independent experiments, each of which showed similar results. In the VH group (n = 15 mice) mice were vaccinated intraperitoneally with DCs pulsed with 3 germ line MHC class I peptides (A9T[Fr3], K9I[Fr2/CDR2], S9L[Fr3]) and 3 germ line MHC class II VH peptides (G16K[Leader/Fr1], F17L[Fr1/CDR1/Fr2], A15V[Fr3]) derived from the VH of the 1H6 murine lymphoma cell line and shown to be immunogenic in vitro, on days 1, 14, and 21. In the control group (n = 14 mice) mice were not vaccinated. On day 24, mice in all groups were challenged subcutaneously with 2.5 × 104 1H6 cells grown in serum-free hybridoma medium. Tumors were measured weekly, and the proportion of tumor-free mice is presented. The Kaplan Meier distributions of tumor-free mice for the VH group were significantly higher than that of the nonvaccinated control mice (control vs VH; P < .001). There was a significant decrease in the distribution of tumor-free mice in a group of mice (n = 15) treated with non–peptide-pulsed DCs alone, compared to that of the VH group (P = .007; data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/3/10.1182_blood-2004-01-0105/6/m_zh80150464350004.jpeg?Expires=1769347550&Signature=EfNeAvZhApqzRlCkRCCWRfrM1wsOZLfecBHxLo1wU6gd6~~iMZ2BmohGgMrngWVbYWvyfKMrNz0TLOJVzXf5lgnAm33D09ZWR-C7lcF~IXqIskhogENXmu8oI2qz~4krt90pKNa9mwAHBDkgfgqTOHtTVBPGnftVp7YKRp7APryAri398TFr3MIm6NgJ9GnoG-TGb9sUet87r7GTFDf3Xco5gDUknoW1TGGb4m1sAPr6~I1wMFycAzVr1C-GbgTw826jVropVrh06lZ4RLSCKZwH5jYgOnRXNTsGzzVArdfmNvNJPVcGiuLTMd1LwH65hCG33kyuSSvLl5oYpn9PPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal