We read with interest the recently published article by Michel et al,1 which described their investigation of the prevalence of Helicobacter pylori infection (H pylori) in North American patients with idiopathic thrombocytopenic purpura (ITP) and the effect of H pylori eradication on platelet count. They reported that H pylori could not be associated with the pathogenesis of ITP because of the low prevalence of H pylori infection among ITP patients and the ineffectiveness of eradication therapy. Their negative results are similar to those reported in 2 earlier studies from France2 and Spain.3 On the other hand, studies carried out in Italy and Japan, including our own,4 have shown a higher response rate to H pylori eradication therapy among chronic ITP (cITP) patients. Indeed, official surveillance by Japan's Ministry of Health, Labour and Welfare recently showed a 64% rate of response to H pylori eradication among ITP patients.5 These successes strongly suggest that H pylori must be one of the causes of cITP, at least in these countries.
In their article, Michel et al commented that differences in H pylori strains or Lewis antigen expression might have contributed to the discrepancies between their observations and those from Italy and Japan.1 We previously reported that platelet eluates from cITP patients recognized H pylori cytotoxin-associated gene A (CagA) protein and that levels of anti-CagA antibody in eluates declined after eradication therapy in completely responsive patients.4 This result suggests that molecular mimicry between CagA and a platelet antigen might mediate the autoimmunity in some cITP patients. It is thus noteworthy that the CagA positivity of H pylori varies depending upon geographic location. In Japan, most H pylori strains express CagA, whereas the proportion of CagA-positive strains in Western countries is lower.6,7 Furthermore, the amino acid sequence of H pylori CagA protein is known to show significant diversity among the East Asian and Western strains.8 The fact that the difference in the clinical response to eradication therapy among these countries corresponds to the difference in the CagA status of the bacterium suggests to us that CagA plays a key role in the pathogenesis of a subset of cITP. We often use noninvasive 13C-urea breath tests to diagnose H pylori infection in cITP patients because of their thrombocytopenia. To determine whether bacterial strain is predictive of the efficacy of eradication therapy, however, it will be necessary to isolate the strains and investigate their CagA status using endoscopic examination in a prospective study.
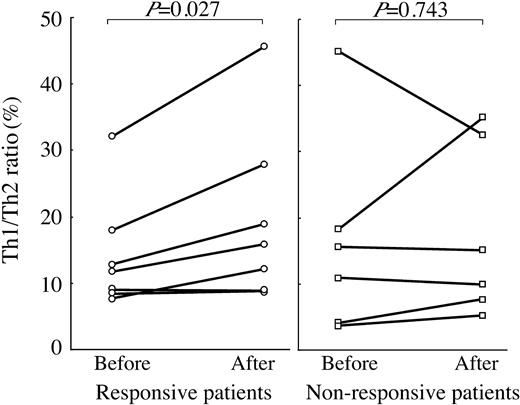
In the same issue of Blood, McCrae commented that H pylori-induced alterations in T helper 1 (Th1)-type immune responses and cytokine profiles might promote development of ITP.9 The Th balance in chronic ITP is uncertain, however. To assess any changes in Th balance before and after eradication therapy in patients with H pylori-positive cITP, we used flow cytometry to measure the ratio of cells positive for intracellular interferon-γ to those positive for interleukin-4 (Th1/Th2 ratio). We found that Th1/Th2 ratios were significantly increased after eradication therapy in the 7 responsive patients but not in the 6 nonresponsive patients who were successfully eradicated (Figure 1). This suggests that the Th balance in patients with H pylori-associated cITP tends to become Th1 dominant after clinical remission is brought on by H pylori eradication. Further investigation will be required to confirm this immunological response.
Changes in Th1/Th2 ratios resulting from successful eradication in H pylori-positive cITP patients. Th1/Th2 ratios were measured before and 4 months after eradication therapy. Values obtained before and after eradication were compared using paired t tests; significant increases were observed in the 7 responsive patients (P = .027).
Changes in Th1/Th2 ratios resulting from successful eradication in H pylori-positive cITP patients. Th1/Th2 ratios were measured before and 4 months after eradication therapy. Values obtained before and after eradication were compared using paired t tests; significant increases were observed in the 7 responsive patients (P = .027).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal