Abstract
The chemokine receptor CXCR4 and its functional ligand, CXCL12, are essential regulators of development and homeostasis of hematopoietic and lymphoid organs. Heterozygous truncating mutations in the CXCR4 intracellular tail cause a rare genetic disease known as WHIM syndrome (warts, hypogammaglobulinemia, infections, myelokathexis), whose pathophysiology remains unclear. We report CXCR4 function in 3 patients with WHIM syndrome carrying heterozygous truncating mutations of CXCR4. We show that CXCR4 gene mutations in WHIM patients do not affect cell surface expression of the chemokine receptor and its internalization upon stimulation with CXCL12. Moreover, no significant differences in calcium mobilization in response to CXCL12 are found. However, the chemotactic response of both polymorphonuclear cells and T lymphocytes in response to CXCL12 is increased. Furthermore, immunophenotypic analysis of circulating T and B lymphocytes reveals a decreased number of memory B cells and of naive T cells and an accumulation of effector memory T cells associated with a restricted T-cell repertoire. Based on our results, we suggest that the altered leukocyte response to CXCL12 may account for the pathologic retention of mature polymorphonuclear cells in the bone marrow (myelokathexis) and for an altered lymphocyte trafficking, which may cause the immunophenotyping abnormalities observed in WHIM patients. (Blood. 2004;104:444-452)
Introduction
WHIM syndrome is a rare disease characterized by warts, hypogammaglobulinemia, recurrent respiratory bacterial infections, and myelokathexis, defined as the presence of an increased proportion of mature myeloid cells with degenerative changes in the bone marrow, associated with severe neutropenia in the peripheral blood.1-3 This condition, most often inherited as an autosomal dominant trait, is caused by heterozygous truncating mutations in the C-terminus tail of the chemokine receptor CXCR4.4
CXCR4 is the only cognate receptor for the CXC-chemokine L12 (CXCL12, or stromal-derived factor-1α [SDF-1α]).5 CXCL12 is constitutively produced by stromal and endothelial cells (ECs) ubiquitously; the highest concentrations of CXCL12 are found in bone marrow, spleen red pulp, and lymph node medulla. CXCR4-CXCL12 interaction plays a key role in regulating bone marrow homeostasis6-9 and is involved in lymphocyte trafficking.10,11 Chemotaxis and integrin-mediated adhesion are the main cellular responses to CXCL129,12-16 ; in addition, CXCR4 signaling participates in several cellular activation and proliferation processes.17,18
Both CXCR4- and CXCL12-deficient mice display a lethal phenotype, with severe impairment of myeloid and B-cell generation, reduced proliferation of both triple-negative and double-positive thymocytes, and developmental defects in cerebellum, heart, and gut vascularization. These abnormalities illustrate that this pair of molecules plays an indispensable role in controlling cell migration and influences (either directly or indirectly) survival/proliferation of different cell types during embryogenesis.19-21 Mice reconstituted with progenitor cells infected with CXCL12 intrakine (which prevents surface CXCR4 expression) suffer from impaired hematopoiesis that involves both myeloid and lymphoid cell lineages.22 AMD3100, a pharmacologic CXCR4 antagonist, induces a rapid mobilization of hematopoietic progenitors and mature cells in a dose-dependent manner.23 In contrast, overexpression of CXCR4 in transgenic T lymphocytes induces their accumulation in the bone marrow and causes a reduction of these cells in peripheral blood.24
Most WHIM patients are lymphopenic and hypogammaglobulinemic and share a common history of recurrent respiratory bacterial infections but do not show increased occurrence of opportunistic infections or autoimmune manifestations. Warts are caused by common serotype human papilloma virus (HPV) infection and are unresponsive to treatment.3
The number of circulating B lymphocytes is most commonly reduced; the proportion of T-cell subsets is apparently preserved, but the ability to generate naive and memory lymphocytes has not been investigated. Recently, severe neutropenia has been possibly attributed to increased apoptosis in the bone marrow.25,26 Moreover, a significant increase in the neutrophil count can be observed in WHIM patients during acute systemic infections and in response to granulocyte colony stimulating factor (G-CSF).27
In spite of the recent identification of the molecular basis of WHIM,4 the functional properties of circulating leukocytes from WHIM patients have not been investigated, and the pathophysiology of the disease remains poorly characterized. In the present study we have investigated expression and function of the CXCR4 receptor in 3 patients with molecularly proven WHIM and have identified previously unreported abnormalities in both lymphoid and myeloid cells. These observations may help to explain the pathogenic basis of defects in bone marrow output of myeloid and lymphoid cells and immune surveillance in patients with WHIM syndrome.
Patients, materials, and methods
Patients
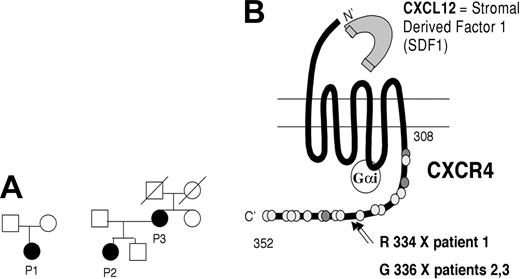
We have studied 3 patients with inheritance, clinical, and laboratory features of WHIM syndrome (Figure 1A; Table 1).
Characteristics of WHIM patients. (A) Pedigrees of 3 patients with WHIM syndrome. (B) Scheme of CXCR4 structure and localization of mutations in patients P1, P2, and P3. GαI indicates G protein; the dark gray dots correspond to threonine residues; and the light gray dots correspond to serine residues. Positions of proximal and terminal amino acid in the C-tail of the molecule are indicated.
Characteristics of WHIM patients. (A) Pedigrees of 3 patients with WHIM syndrome. (B) Scheme of CXCR4 structure and localization of mutations in patients P1, P2, and P3. GαI indicates G protein; the dark gray dots correspond to threonine residues; and the light gray dots correspond to serine residues. Positions of proximal and terminal amino acid in the C-tail of the molecule are indicated.
Main clinical and laboratory features of the 3 WHIM patients
. | P1 . | P2 . | P3 . |
|---|---|---|---|
| Age, y | 7 | 17 | 36 |
| Warts | − | + | +++ |
| IgG, g/L | 4.25 | 3.50 | 4.38 |
| IgA, g/L | 0.12 | 0.20 | 0.25 |
| IgM, g/L | 0.33 | 0.35 | 0.42 |
| WBCs, × 109/L | 1.5 | 0.4 | 1.3 |
| Neutrophils, % | 20 | 20 | 32 |
| Lymphocytes, % | 67 | 65 | 61 |
| Myelokathexis | + | + | + |
. | P1 . | P2 . | P3 . |
|---|---|---|---|
| Age, y | 7 | 17 | 36 |
| Warts | − | + | +++ |
| IgG, g/L | 4.25 | 3.50 | 4.38 |
| IgA, g/L | 0.12 | 0.20 | 0.25 |
| IgM, g/L | 0.33 | 0.35 | 0.42 |
| WBCs, × 109/L | 1.5 | 0.4 | 1.3 |
| Neutrophils, % | 20 | 20 | 32 |
| Lymphocytes, % | 67 | 65 | 61 |
| Myelokathexis | + | + | + |
− indicates absent; +, present; and +++, numerous.
Our youngest patient (P1), 7 years old, is the only child of healthy nonconsanguineous parents. None of her relatives have symptoms suggestive of WHIM syndrome. She was born with Fallot tetralogy, which was surgically corrected at 2 years of age. On that occasion, she was found to be severely neutropenic. She experienced 3 episodes of pneumonia. Myelokathexis was documented on bone marrow aspirate at the age of 4 years. Since then, she has been treated with G-CSF and has not experienced any further severe infections. Her leukocyte absolute number remains above 1.5 × 109/L (1500/μL) with 5 μg/kg of daily G-CSF, and she has not yet developed warts. She displays mild hypogammaglobulinemia and has never received treatment with intravenous immunoglobulin.
Patient P2, 17 years old, presents the full-blown picture of the syndrome. She has a positive family history, since her mother (P3) is affected as well. She is severely leukopenic and hypogammaglobulinemic and suffered from bacterial meningitis at 1 year of age, when she was diagnosed with WHIM. Since then, she has been treated with intravenous immunoglobulin and has not suffered from other serious infections. Warts appeared when she was 3 years old and have since remained localized.
Her mother (P3) has a history of recurrent respiratory infections and suffered from one episode of meningitis at the age of 24 years. She presents extensive warts since the age of 17 years. Neither patient P2 nor P3 have ever received treatment with G-CSF.
Cell preparation
Upon informed consent and approval by the Spedali Civili hospital ethics committee, ethylenediaminetetraacetic acid (EDTA) or heparin blood samples were collected from 3 WHIM patients and age-matched controls. In addition, bone marrow aspirate was obtained from patient P3. Ficoll-Hypaque (Amersham-Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation was used to isolate both peripheral blood mononuclear cells (PBMCs) and polymorphonuclear leukocytes (PMNs), and bone marrow mononuclear cells (BMMNCs). PBMCs were cultured in RPMI 1640 and interleukin-2 (IL-2; 250 U/mL) for 3 weeks. PMNs were immediately used after ammonium chloride erythrocyte lysis. Human endothelial cells (ECs) were obtained from umbilical vein (HUVEC) and cultured as previously described.28 For the experimental procedures, we have routinely used confluent cells (105/2-cm2 culture well), obtained between the first and the fourth passage, that were maintained in 199 medium with 20% bovine serum (Hyclone, Logan, UT) supplemented with endothelial cell growth supplement (50 μg/mL; Collaborative Research, Lexington, MA) and heparin (100 μg/mL; Sigma Chemical, St Louis, MO). The purity of EC cultures was checked by expression of von Willebrand factor and found to be more than 99% positive.
Reagents
Human recombinant CXCL12, CCL2, CCL21, and CXCL8 were obtained from Pepro Tech (Rocky Hill, NJ). All reagents and media, which were tested by the Endotoxin kit (Sigma Chemical), contained endotoxin at concentrations below the detection levels (12 pg/mL).
Mutational analysis
Genomic DNA was isolated from whole blood cells. The cells were lysed in extraction buffer (10 mM Tris [tris(hydroxymethyl)aminomethane], 0.1 M EDTA, 0.5% sodium dodecyl sulfate (SDS), and 10 mg/mL proteinase K) overnight at 37°C and were subjected to extraction with phenol and chloroform. The DNA was then precipitated in ethanol. Polymerase chain reaction (PCR) amplification was performed with 100 ng of genomic DNA as the template and 50 μL of a reaction mixture consisting of 10 mM Tris-HCl, 2 mM MgCl2, 50 mM KCl, 0.2 mM deoxynucleoside triphosphate (dNTPs), and 1.25 U Taq DNA polymerase (Perkin-Elmer Instruments, Norwalk, CT). The primers and conditions used for PCR amplification of the 2 coding exons of CXCR4 are available upon request. Amplified PCR products were analyzed by gel electrophoresis in a 2% agarose gel and purified by centrifugation through Amicon (Millipore, Bedford, MA). PCR products were sequenced by dideoxynucleotide termination with the BigDye terminator kit (Applied Biosystems, Foster City, CA). Sequences were analyzed on an ABI Prism 310 apparatus (Applied Biosystems).
Flow cytometry
Whole-blood cells (100 μL) were stained for 20 minutes at 4°C with a mixture of the appropriate combination of the following commercial monoclonal antibodies (mAbs): CD3 fluorescein isothiocyanate (FITC), CD4 PerCP (peridinin chlorophyll A protein), CD19 PerCP, CD20 PerCP, CD23 phycoerythrin (PE), CD27 FITC, CD27 PE (Becton Dickinson, Mountain View, CA), CD21 PE, anti-human IgD FITC, and CD45R0 FITC (PharMingen, San Diego, CA). After staining, red blood cells were lysed with 2 mL lysing buffer (Becton Dickinson) for 5 minutes at room temperature and then washed twice with phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Purified anti-CXCR4 clone 12G5 and anti-CCR7 clone 2H4 (PharMingen) antibodies were used to stain 100 μL of fresh whole-blood cells for 30 minutes at 4°C at an optimal concentration of 1 μg/mL. After 2 washes with PBS-1% BSA, blood cells were stained for 15 minutes at 4°C with 0.7 μg biotin-SP-conjugated AffiniPure F(ab')2 fragment goat anti-mouse IgG + IgM (H+L) (Jackson ImmunoResearch, West Grove, PA), and then for 15 minutes at 4°C with 50 ng PE-streptavidin conjugated (Jackson ImmunoResearch). Before staining with the purified antibodies, blood samples were pre-incubated with 50 μg human immunoglobulins for 30 minutes at 4°C. Sample acquisition was performed with FACSCalibur (Becton Dickinson). Data analysis was performed with CellQuest software (Becton Dickinson) gating by forward versus side scatter. Flow cytometric analysis of bone marrow B-cell compartment was performed with different combinations of monoclonal antibodies (CD45, CD22, CD10, CD38, CD34, TdT) in order to differentiate B-cell precursors (CD45lo, CD22lo, CD10hi, CD38+, CD34+, TdT+ cells) from immature (CD45+, CD22+, CD10+, CD38+, CD34-, TdT-) and mature (CD45hi, CD22hi, CD10-, CD38+, CD34-, TdT-) cells as previously described.29
CXCR4 internalization
Analysis of CXCR4 down-regulation upon CXCL12 treatment was performed as previously described.30 Briefly, fresh whole-blood samples (100 μL) from WHIM patients and controls were placed at 37°C and incubated with or without addition of CXCL12 (1000 ng/mL). After 15 minutes, the samples were cooled in ice, and CXCR4 expression was evaluated by flow cytometry according to the procedure described above. Whole-blood samples from patient P3 and a control subject also were incubated for 5 or 15 minutes at 37°C with 1000 ng/mL CXCL12 or with phorbol myristate acetate (PMA; Sigma Chemical) at 10 ng/mL. Then cells were washed with ice-cold PBS and incubated with anti-CXCR4 mAb for 30 minutes. After this period, cells were stained with biotinylated anti-mouse mAb and PE-streptavidin as described above.
Chemotaxis and transendothelial migration
PMN migration was evaluated with a chemotaxis microchamber technique (NeuroProbe, Pleasanton, CA), as previously described.31 Chemoattractants, diluted in assay medium (RPMI 1640 with 1% BSA and 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]), were placed in the lower wells of the chemotaxis chamber. A polyvinylpyrrolidone (PVP)-free polycarbonate filter (5-μm pore size; NeuroProbe) was placed over the chemoattractants. A 50-μL aliquot of PMN suspension at 1.5 × 106 cell/mL was placed in the upper wells. The chamber was incubated at 37°C in air with 5% CO2 for 30 minutes. Migrating cells in 5 high-power oil-immersion fields were counted.
T-cell chemotaxis was evaluated by using a Transwell system (Costar, Cambridge, MA). Cell suspensions (3 × 106 cell/mL) were incubated for 120 minutes at 37°C in air with 5% CO2. The number of migrated cells in the bottom wells was counted. Percentage of spontaneous T-cell migration ranged from 2.5% to 10% of input cells. The results are expressed as chemotactic index (fold increase of cell response to stimulants over the response to medium alone) and are representative of at least 2 experiments.
Transmigration and adhesion assays were performed essentially as previously described.28 In brief, HUVEC were grown to confluence on polycarbonate Transwell inserts (5-μm pore; Corning, Costar). Sample inserts were stained with Diff-Quik (Dade Behring, Düdingen, Switzerland) to assess monolayer confluence. Assay media consisted of RPMI 1640 plus 10% fetal calf serum (FCS). CXCL12, at various concentrations, was seeded in the lower compartment. 51Cr-labeled T cells were seeded in the upper compartment (2 × 104 cells/insert) and co-incubated with EC monolayers for 3 hours at 37°C. After incubation, the upper compartment was removed and nonadherent cells were gently washed away. HUVEC and adherent nontransmigrated T cells were scraped from the top filter side and the radioactivity in the lower side of the filter and in the lower compartment referred to as transmigrated cells. The adherent cells were considered to comprise cells bound to HUVEC as well as those that had transmigrated. Results were expressed as the percentage of the total seeded cells minus the cells that had transmigrated to media alone.
Calcium mobilization assays
Changes in intracellular calcium concentration ([Ca2+]i) were monitored using the fluorescent probe fura-2 as previously described.32 Briefly, T cells (107/mL), expanded in culture with IL-2 (250 U/mL) for 3 weeks, were resuspended in RPMI 1640 and incubated with 1 μM fura-2 acetoxymethyl ester (Calbiochem, San Diego, CA) at 37°C for 30 minutes. After incubation, cells were washed and resuspended in Hanks balanced salt solution (HBSS; Biochrom, Berlin, Germany) containing 1.2 mM CaCl2 and kept at room temperature until used. Fura-2 fluorescence was measured with a Perkin-Elmer LS 50B spectrophotometer (Perkin-Elmer Instruments, Shelton, CT) at 37°C with cells (3 × 106/mL) continuously stirred. Samples were excited at 340 and 380 nm, and emission was continuously recorded at 487 nm. Results were calibrated as previously described.32
TCRVB repertoire and TRECs analysis
Heteroduplex analysis was performed on PCR products, obtained after amplification with T-cell receptor β chain (TCRB)-specific primers, from PBMC of patients P2 and P3 as well as from 2 controls of similar age. Amplicons were heated to 95°C for 5 minutes, cooled to 50°C for 1 hour, and loaded on 12% nondenaturing polyacrylamide gel electrophoresis (PAGE; 29:1 acrylamide-bisacrylamide). Gels were run for 5 to 6 hours at 200 V at room temperature and stained, in the dark, for 30 to 60 minutes in a solution containing 0.75 μg/mL ethidium bromide in 200 mL Tris Borate EDTA buffer (TBE).33 Homoduplex and heteroduplex bands (indicative of monoclonal and oligoclonal expansions, respectively) as well as the smears (resulting from the amplification of polyclonal populations) were detected by UVP's Gel Documentation System GDS8000 and were analyzed using GelWorks 1D Analysis software (both from Ultraviolet Products, Cambridge, MA). The appearance of dominant peaks suggests the presence of oligoclonal or clonal T-cell populations, while polyclonal T-cell populations are detected as more or less bent lines rising above the threshold.
The quantification of the signal-joint T-cell receptor (TCR) rearrangement excision circles (TRECs) was done by means of real-time PCR on DNA prepared from PBMCs of the 3 patients and 11 age-matched controls and was expressed as copies/106 PBMCs as previously described34 or are adjusted for the concentration of lymphocytes and are expressed as copies/mL; that is, TRECS/mL = (TRECS/106 cells) × (cells/μL) × 10-3.
Statistical analysis
All the experiments were performed at least 2 times. Comparison between WHIM patients and control subjects was performed by Student t test for paired or unpaired data, as appropriate. For comparison among multiple groups, analysis of variance (ANOVA) test followed by Bonferroni correction test were used. Results of statistical analysis of the single experiment shown in figures are reported in the legend as P values at the .05 significance level to identify which of the means were significantly different from the others.
Results
CXCR4 gene mutation analysis
Mutational analysis of the CXCR4 gene, performed on genomic DNA, has shown that all 3 patients carry heterozygous mutations that result in partial truncation of the CXCR4 carboxyl-terminus (Figure 1B). P1 has a point mutation at nucleotide 1000 (C/T), leading to premature termination at codon 334, whereas P2 and P3 are heterozygous for a novel mutation of nucleotide 1006 (G>T), resulting in 17-residue truncation of CXCR4. None of the healthy family members share the same mutation.
Flow cytometric analysis of CXCR4 expression and internalization in leukocytes of WHIM patients
Since CXCR4 is widely expressed by leukocytes,6,7 we asked whether heterozygosity for truncating mutations of CXCR4 may affect the levels of receptor expression of peripheral blood leukocytes. As assessed by flow cytometry on whole-blood samples, CXCR4 surface expression was comparable between WHIM patients and controls (data not shown).
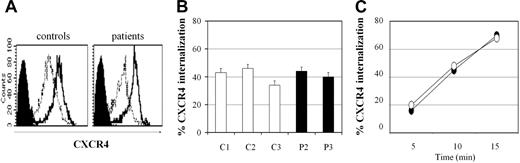
CXCL12 and PMA are known to modulate the levels of surface expression of CXCR4 by inducing receptor internalization within minutes of stimulation. This process involves phosphorylation of CXCR4 serine and threonine residues in the intracytoplasmic tail, followed by β-arrestin recruitment and clathrin-mediated endocytosis.35 Since the heterozygous truncating mutations detected in WHIM patients result in the loss of serine and threonine residues of the CXCR4 C-tail, we asked whether the mutations might affect CXCR4 internalization following in vitro stimulation with CXCL12 or PMA. We found that after incubation of fresh blood for 15 minutes with CXCL12 (1000 ng/mL), the extent of CXCR4 that was subjected to internalization was not significantly different between WHIM patient P3 and a control subject (Figure 2A-B). Time course analysis of CXCR4 internalization in peripheral blood lymphocytes demonstrated that down-regulation of CXCR4 cell surface level, already detectable after 5 minutes of incubation with CXCL12 (1000 ng/mL), was observed at comparable extent between cells of the control subject and WHIM patient (Figure 2C).
Flow cytometric analysis of CXCR4 internalization upon CXCL12 stimulation. Blood samples from WHIM patients and controls were placed at 37°C and incubated with or without addition of CXCL12 (1000 ng/mL). After 15 minutes, the samples were cooled in ice and CXCR4 expression evaluated by flow cytometry using anti-CXCR4 antibody. (A) Difference between CXCR4 mean intensity of fluorescence (on lymphogate) before stimulation (100%, bold line) and after CXCL12 stimulation (thin line). Filled black histograms indicate isotype control fluorescence. (B) Percentages of CXCR4 internalization in controls (C1, C2, C3; □) and WHIM patients (P2, P3; ▪). Error bars indicate SD. (C) Time course of CXCR4 internalization: control 1 (○) and patient P3 (•). Shown is 1 representative experiment of 2 performed.
Flow cytometric analysis of CXCR4 internalization upon CXCL12 stimulation. Blood samples from WHIM patients and controls were placed at 37°C and incubated with or without addition of CXCL12 (1000 ng/mL). After 15 minutes, the samples were cooled in ice and CXCR4 expression evaluated by flow cytometry using anti-CXCR4 antibody. (A) Difference between CXCR4 mean intensity of fluorescence (on lymphogate) before stimulation (100%, bold line) and after CXCL12 stimulation (thin line). Filled black histograms indicate isotype control fluorescence. (B) Percentages of CXCR4 internalization in controls (C1, C2, C3; □) and WHIM patients (P2, P3; ▪). Error bars indicate SD. (C) Time course of CXCR4 internalization: control 1 (○) and patient P3 (•). Shown is 1 representative experiment of 2 performed.
Furthermore, a similar pattern of CXCR4 internalization was observed in cells from both WHIM and controls' cells upon in vitro stimulation with PMA (data not shown).
T-cell and PMN chemotaxis in response to CXCL12 in patients with WHIM syndrome
CXCL12 acts as a potent chemoattractant for hematopoietic stem cells and early progenitors. However, mature white blood cells (WBCs) such as lymphocytes and PMNs also display responsiveness to the chemokine.15,16
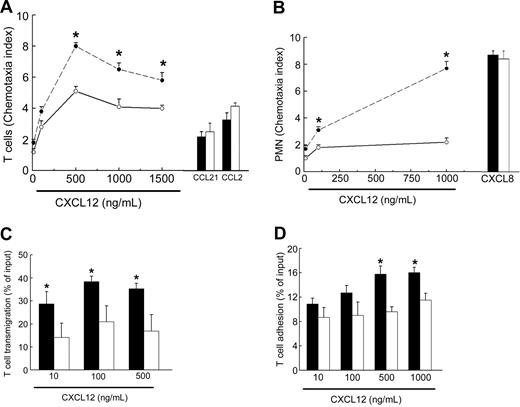
To evaluate the mechanism of white blood cell retention in bone marrow of WHIM patients, we assessed the chemotactic response of T cells and PMNs to CXCL12. Because of the severe leukopenia, we were unable to perform chemotaxis assays in freshly isolated T cells, thus we relied upon T lymphocytes that had been expanded in vitro with IL-2 for 3 weeks. Under these experimental conditions, T cells from WHIM patients or control subjects displayed expression of CXCR4 at a comparable extent (data not shown). For these experiments, we used CXCL12, CCL21, or CCL2 that are respectively ligands for CXCR4, CCR7, and CCR2.11 In both patients P2 and P3 and in controls the strongest response was detected at concentrations above 500 ng/mL of CXCL12, but the response to this chemokine was again much higher in WHIM patients (Figure 3A). In contrast, chemotaxis in response to CCL2 or to CCL21 was not different between WHIM patients and healthy controls. Analysis of cell surface expression of CXCR4, CCR2, and CCR7 in T cells by flow cytometry showed that cells from WHIM patients P2 and P3 displayed comparable levels of chemokine receptors, suggesting that the increased chemotaxis of WHIM T cells is not due to higher levels of CXCR4 expression (data not shown).
Increased chemotactic response to CXCL12 of PMNs and T lymphocytes in WHIM patients. (A) T-cell chemotaxis in response to CXCL12 (10, 100, 500, 1000, 1500 ng/mL), CCL21 (50 ng/mL), or CCL2 (50 ng/mL) was assessed in WHIM patient P3 (• and ▪) or healthy subject (○ and □) as described in “Patients, materials, and methods.” (B) PMN chemotaxis in response to CXCL12 (10, 100, 1000 ng/mL) or to CXCL8 (50 ng/mL) was assessed in WHIM patient P3 (• and ▪) or healthy subject (○ and □) as described in “Patients, materials, and methods.” (C-D) T-cell transendothelial migration (C) and T-cell adhesion (D) in response to CXCL12 (10, 100, 500 ng/mL) were assessed in WHIM patient P1 (▪) and in a healthy subject (□) as described in “Patients, materials, and methods.” CXCL12-induced transmigration and adhesion at the net of spontaneous activity are presented as percent of input cells. In panels A and B, results are presented as chemotaxis index and are representative of 2 other experiments performed in patients P2 and P3 with similar results. Asterisks (*) indicate a significant difference in chemotactic response of cells from a WHIM patient in comparison to cells from a control subject (P < .05). Error bars indicate SD.
Increased chemotactic response to CXCL12 of PMNs and T lymphocytes in WHIM patients. (A) T-cell chemotaxis in response to CXCL12 (10, 100, 500, 1000, 1500 ng/mL), CCL21 (50 ng/mL), or CCL2 (50 ng/mL) was assessed in WHIM patient P3 (• and ▪) or healthy subject (○ and □) as described in “Patients, materials, and methods.” (B) PMN chemotaxis in response to CXCL12 (10, 100, 1000 ng/mL) or to CXCL8 (50 ng/mL) was assessed in WHIM patient P3 (• and ▪) or healthy subject (○ and □) as described in “Patients, materials, and methods.” (C-D) T-cell transendothelial migration (C) and T-cell adhesion (D) in response to CXCL12 (10, 100, 500 ng/mL) were assessed in WHIM patient P1 (▪) and in a healthy subject (□) as described in “Patients, materials, and methods.” CXCL12-induced transmigration and adhesion at the net of spontaneous activity are presented as percent of input cells. In panels A and B, results are presented as chemotaxis index and are representative of 2 other experiments performed in patients P2 and P3 with similar results. Asterisks (*) indicate a significant difference in chemotactic response of cells from a WHIM patient in comparison to cells from a control subject (P < .05). Error bars indicate SD.
Next, we evaluated the chemotactic response of CXCR4 in freshly isolated PMNs from WHIM patients P2 and P3 and healthy control subjects. We placed increasing concentrations of CXCL12 in the lower wells of a chemotaxis microchamber and PMNs in the upper wells. In all subjects analyzed, CXCL12 induced a significant PMN migration at a concentration of 1000 ng/mL in comparison to medium response. However, the extent of chemotaxis, measured as chemotaxis index, was always stronger for WHIM cells than for normal cells (P < .05). Moreover, compared with healthy controls, PMN from WHIM patients P2 and P3 displayed a higher chemotactic response also at lower concentrations of CXCL12 (Figure 3B).
In contrast, the chemotactic response of PMNs to the proinflammatory chemokine CXCL8 (50 ng/mL) was comparable between the control subject and the WHIM patient. These results suggest that CXCL12 induces migration of WHIM leukocytes with an increased efficacy (ie, number of migrated cells) but similar potency (ie, peak concentration). The study of B-cell migration was hampered by the severe lymphopenia that is typical of these patients.
We next investigated the capacity of T cells obtained from WHIM patient P1 to migrate across an EC monolayer. Also in this assay, the percentage of input cells that transmigrated across the EC monolayer in response to CXCL12 (500 ng/mL) was much higher in the WHIM patient (35.27% ± 2.34%) than in a healthy control (16.96% ± 7.03%) (Figure 3C). The increased transmigration of WHIM patient cells over control cells was observed at all of the CXCL12 concentrations tested (10, 100, 500 ng/mL) (P < .05). Conversely, the total fraction of T cells adherent to ECs was increased in WHIM patients at the highest concentrations of CXCL12 (Figure 3D). These results suggest that CXCR4 truncation increases both migration and adhesion of leukocytes in response to CXCL12.
Calcium mobilization in lymphocytes of WHIM patients
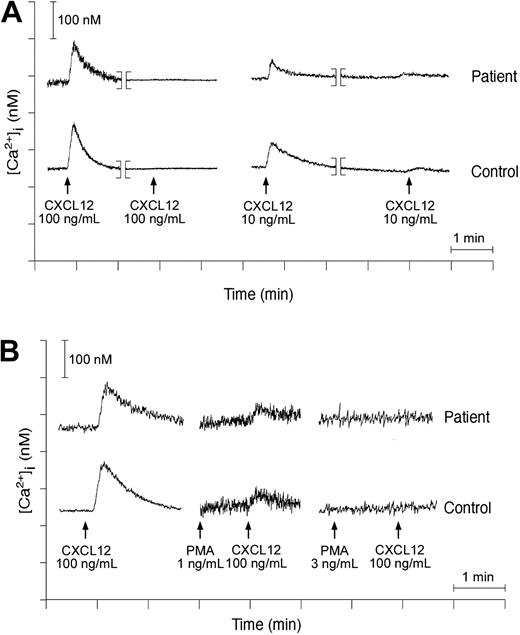
Stimulation of CXCR4 induces a cascade of signaling events, including early G protein-dependent calcium mobilization.30 In view of the increased ability of T cells to migrate in response to CXCL12, intracellular calcium fluxes were evaluated in these cells. We investigated the chemotactic response of T lymphoblasts cultured in vitro with IL-2. Under these conditions, stimulation of T cells from patients P2 and P3 or from healthy control subjects with CXCL12 (up to 100 ng/mL) induced a dose-related increase in intracellular calcium concentration that was comparable between patients and controls (Figure 4A).
Calcium transients in activated T cells from WHIM patients. Fura-2-loaded lymphocytes of WHIM patient P3 or healthy subject stimulated with CXCL12 at 100 ng/mL. The calcium concentration (y-axis) obtained following normalization of fluorescence ratio as described in “Patients, materials, and methods” is plotted against time (x-axis). (A) Homologous desensitization of lymphocytes to CXCL12 was investigated by analysis of calcium transients in response to CXCL12 at 100 ng/mL or 10 ng/mL in cells previously exposed to the same concentration of the chemoattractant. (B) Lymphocyte desensitization in response to PMA was analyzed in patients and healthy subjects by stimulation of cell suspension with PMA at 1 ng/mL or 3 ng/mL following addition of CXCL12, as indicated in the figure.
Calcium transients in activated T cells from WHIM patients. Fura-2-loaded lymphocytes of WHIM patient P3 or healthy subject stimulated with CXCL12 at 100 ng/mL. The calcium concentration (y-axis) obtained following normalization of fluorescence ratio as described in “Patients, materials, and methods” is plotted against time (x-axis). (A) Homologous desensitization of lymphocytes to CXCL12 was investigated by analysis of calcium transients in response to CXCL12 at 100 ng/mL or 10 ng/mL in cells previously exposed to the same concentration of the chemoattractant. (B) Lymphocyte desensitization in response to PMA was analyzed in patients and healthy subjects by stimulation of cell suspension with PMA at 1 ng/mL or 3 ng/mL following addition of CXCL12, as indicated in the figure.
Next, we have evaluated both homologous and heterologous desensitization of CXCR4 by calcium assays. Treatment of IL-2-cultured T lymphocytes with CXCL12 at 100 ng/mL completely desensitized cells in response to a secondary stimulation with the same chemokine both in WHIM patients and in controls (Figure 4A). Lower concentrations of CXCL12 (10 ng/mL) were not sufficient to fully desensitize lymphocytes, but again, no differences in calcium mobilization were observed between WHIM patients and controls (Figure 4A). Finally, when PMA was used as a first stimulus at 1 or at 3 ng/mL (followed by challenge with CXCL12), a similar degree of CXCR4 desensitization was observed in WHIM patients and in controls (Figure 4B).
Immunophenotypic analysis of peripheral blood lymphocytes in WHIM patients
Previous studies have shown that WHIM patients are lymphopenic, but the distribution of the main lymphocyte subsets (CD4/CD8/CD19/CD16) and the proliferative responses to mitogens are generally conserved. Furthermore, although patients display a variable degree of hypogammaglobulinemia and have a reduced number of B cells, they are able to respond to active immunizations.2,36
The identification of the molecular and functional defects of CXCR4 in leukocytes from WHIM patients prompted us to seek possible abnormalities in T- and B-cell development and trafficking.
B lymphocytes
All the patients investigated (P1, P2, and P3) presented a low percentage of circulating CD19+ lymphocytes (3.8%, 2.4%, 1.8% of PBMCs, respectively), suggesting a potential defect in the generation of B cells in the bone marrow. However, investigation of bone marrow B-cell subsets in one patient (P3) showed that B-cell precursors comprised 0.29% of BMMNCs (normal value [nv], 0.17 ± 0.15), immature B cells were 0.62% (nv, 0.75 ± 0.51), and mature B lymphocytes were 2.49% of BMMNC (nv, 2.20 ± 0.97), demonstrating that their distribution was comparable between patient P3 and an age-matched control (data not shown). Further characterization of bone marrow leukocytes could not be extended to all 3 patients for ethical reasons.
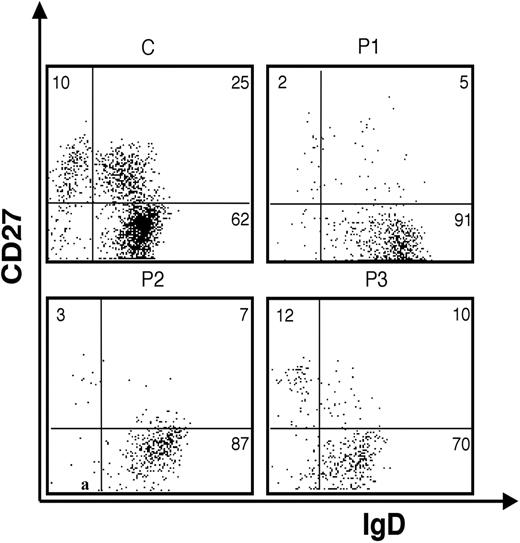
In addition, immunophenotypic analysis of circulating B lymphocytes showed important abnormalities in WHIM patients as compared to age-matched controls. In particular, a severe reduction of circulating CD27+ memory B cells that affected both un-switched (IgD+) and switched (IgD-) CD19+ B lymphocytes was observed (Figure 5; Table 2).
Fluorescence analysis of B cells derived from the peripheral blood (PB) of WHIM patients (P1, P2, P3) and a healthy control subject (C). Anti-IgD/anti-CD27 2-color staining of peripheral blood B cells gated on CD19+.
Fluorescence analysis of B cells derived from the peripheral blood (PB) of WHIM patients (P1, P2, P3) and a healthy control subject (C). Anti-IgD/anti-CD27 2-color staining of peripheral blood B cells gated on CD19+.
Reduction of circulating B-cell percentages in WHIM patients
. | % PBLs . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Patients . | CD19+ . | CD27−IgD+ . | CD27+IgD+ . | CD27+IgD− . | |||
| P1 | 3.8 | 3.45 | 0.19 | 0.07 | |||
| P2 | 2.4 | 2.08 | 0.07 | 0.16 | |||
| P3 | 1.8 | 1.26 | 0.18 | 0.21 | |||
| Healthy control | 7.7 ± 2.7 | 4.3 ± 1.6 | 1.6 ± 1.1 | 1.6 ± 0.6 | |||
. | % PBLs . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Patients . | CD19+ . | CD27−IgD+ . | CD27+IgD+ . | CD27+IgD− . | |||
| P1 | 3.8 | 3.45 | 0.19 | 0.07 | |||
| P2 | 2.4 | 2.08 | 0.07 | 0.16 | |||
| P3 | 1.8 | 1.26 | 0.18 | 0.21 | |||
| Healthy control | 7.7 ± 2.7 | 4.3 ± 1.6 | 1.6 ± 1.1 | 1.6 ± 0.6 | |||
Percentages of peripheral blood B cells (PBLs) are reduced in WHIM patients. The analysis shown in table is representative of 2 distinct experiments involving 9 healthy control subjects. Data are presented as mean ± standard deviation for healthy controls and as means for patients.
In spite of these abnormalities and of low serum immunoglobulins levels, we could detect a normal production of specific antibodies upon immunization with tetanus-toxoid (TT) vaccine in patient P1. Before vaccination, serum titers of anti-tetanus Ig were low (anti-TT antibodies < 0.03 IU/mL), but rapidly increased to 2.7 IU/mL after 10 weeks. However, analysis of anti-tetanus toxoid Ig one year after immunization showed titers were again below detection levels (< 0.03 IU/mL), suggesting that this subject has a normal capacity to mount an IgG response against protein antigens, but production of these antibodies is not maintained over time.
T lymphocytes
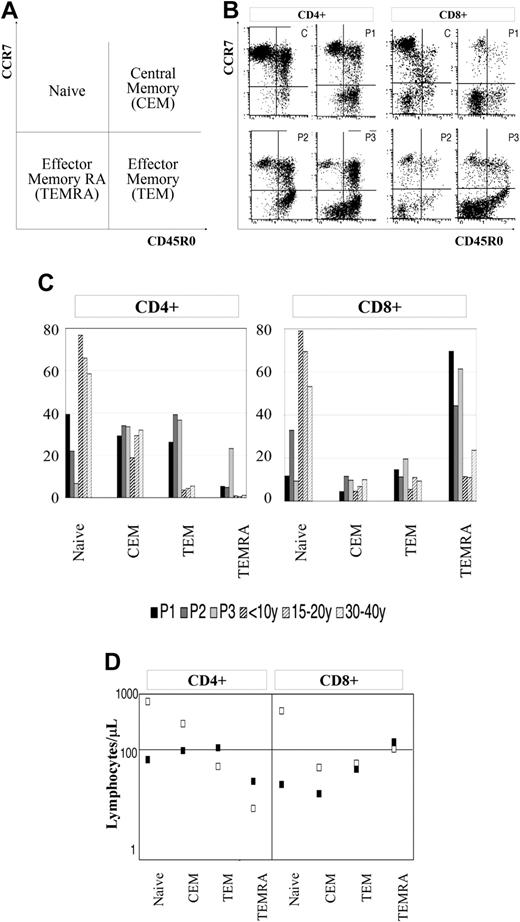
Based on surface expression of CD45 isoforms and CCR7, a homing chemokine receptor, both CD4+ and CD8+ T cells can be divided into 4 groups. CD45R0-/CCR7+ cells identify naive cells, whereas CD45R0+/CCR7+, CD45R0+/CCR7-, and CD45R0-/CCR7- represent central memory T cells, effector memory T cells, and terminal effector RA+ T cells, respectively.37,38 We found that WHIM patients have a reduced number of circulating naive T cells, whereas the proportion of CCR7- effector memory T cells was significantly increased as compared to age-matched controls (Figure 6B-C). The latter difference was more pronounced among CD8+ cells than among CD4+ cells. In addition, analysis of the absolute number of cells showed that WHIM patients presented a 10-fold reduction of naive cells, a 3-fold reduction of central memory cells, and a 2- to 3-fold increase in effector memory cells (Figure 6D).
Peripheral blood T-lymphocyte phenotype of WHIM patients. (A) Naive, central memory (CEM), effector memory (TEM), and effector memory RA+ (TEMRA) T lymphocytes, whole-blood cells from WHIM patients (P1, P2, P3), and 9 age-matched controls identified by triple staining for the expression of CD4 or CD8 and both CCR7 and CD45R0. (B) FACS analysis was performed by gating on CD4+ or CD8+ cells. (C) Bars represent the relative proportions of the 4 T-cell subsets obtained by flow cytometry. (D) Squares indicate the average absolute number of lymphocytes per microliter for each subset in WHIM patients (▪) and controls (□).
Peripheral blood T-lymphocyte phenotype of WHIM patients. (A) Naive, central memory (CEM), effector memory (TEM), and effector memory RA+ (TEMRA) T lymphocytes, whole-blood cells from WHIM patients (P1, P2, P3), and 9 age-matched controls identified by triple staining for the expression of CD4 or CD8 and both CCR7 and CD45R0. (B) FACS analysis was performed by gating on CD4+ or CD8+ cells. (C) Bars represent the relative proportions of the 4 T-cell subsets obtained by flow cytometry. (D) Squares indicate the average absolute number of lymphocytes per microliter for each subset in WHIM patients (▪) and controls (□).
Next, we analyzed whether the increase of effector memory T cells was associated with a restricted heterogeneity of T-cell repertoire. To this purpose, we performed heteroduplex analysis of the T-cell receptor variable β chain (TCRBV) chains that are most commonly expressed by circulating T cells. When analyzed with appropriate software, homoduplex and heteroduplex bands result in peaks of different sizes, indicating the presence of clonal or oligoclonal T-cell populations. The absence of relevant peaks suggests lack of cells expressing a specific subfamily of TCR genes. As shown in Figure 7, lymphocytes from age-matched healthy donors give rise to few small peaks, indicating minor oligoclonal expansions, which can be the result of mild antigenic stimulation. On the contrary, several discrete peaks in lymphocytes from patients P2 and P3 suggest a highly restricted T-cell diversity.
Oligoclonal TCRBV repertoire in WHIM patients. TCRBV diversity profiles of the indicated TCRBV segments obtained after heteroduplex analysis of TCRBV-specific PCR products prepared from lymphocytes from patients P2 and P3 and from 2 age-matched control subjects. Subsequent analysis with the GelWorks 1D software was performed. The threshold is indicated with dotted lines.
Oligoclonal TCRBV repertoire in WHIM patients. TCRBV diversity profiles of the indicated TCRBV segments obtained after heteroduplex analysis of TCRBV-specific PCR products prepared from lymphocytes from patients P2 and P3 and from 2 age-matched control subjects. Subsequent analysis with the GelWorks 1D software was performed. The threshold is indicated with dotted lines.
Finally, to evaluate whether the lymphocyte abnormalities (lymphopenia, reduced number of circulating naive T cells, and the restricted T-cell repertoire) observed in WHIM patients were related to a defective thymic output, we evaluated in PBMCs of patients and age-matched control subject the TCR excision circles (TRECs), which are considered markers of recent thymic immigrants.34 Analysis of TRECS measured by a standard real-time PCR-based method from total lymphocytes demonstrated that WHIM patients presented a substantial number of TRECs (P1, 6984; P2, 247 423; P3, 64 825 copies/106 PBMCs). These values are in accordance with those of age-matched healthy subjects (35 523 ± 17 919 circles/106 PBMCs). But, upon adjustment for the absolute number of circulating lymphocytes, the concentration of TRECS per milliliters of blood was lower in WHIM patients (P1, 7019; P2, 64 330; P3, 51 406 copies/mL) than in control subjects (14 092 ± 71 676), thus reflecting the lymphopenic state of patients. Therefore, it is uncertain whether that the abnormal increase of the memory subset is due to defective thymic output or to disturbances of T-cell trafficking and homeostasis related to the increased chemotactic response of CXCR4 in WHIM patients.
Discussion
Although genetic heterogeneity is suggested by the absence of mutation in CXCR4 in one of the pedigrees analyzed by Hernandez et al,4 mutations implying a loss of the very terminal portion of CXCR4 tail have been described in every other WHIM patient tested so far (9 pedigrees), indicating a causal role for these type of mutations in the pathogenesis of the disease. We have shown that CXCR4 gene mutations in WHIM patients result in enhanced chemotactic response to CXCL12 both in PMNs and lymphocytes.
Truncated CXCR4 mutants transfected into different cell lines that do not express endogenous CXCR4 present reduced internalization, increased GTP-ase activity, sustained calcium flux, and selective resistance to heterologous desensitization.30 Using a single Epstein-Barr virus (EBV)-transformed B-cell line derived from a WHIM patient, Hernandez et al4 have reported increased calcium flux in response to CXCL12 stimulation. In contrast, we have not found significant differences in calcium flux induction and desensitization and in CXCR4 internalization between T cells from WHIM patients and controls. The discrepancy between our data and experimental data obtained with artificial mutants may have different explanations. Experiments with transfection of mutants do not reflect the condition of heterozygosity for CXCR4 mutation observed in WHIM cells. Moreover, the truncating mutations used in the transfection experiments are more severe than those identified in WHIM patients.
In this study we aimed to investigate blood PMNs and T cells for their ability to migrate in vitro in response to CXCL12. The results reported show that both PMNs and T cells from WHIM patients displayed an increased response to CXCL12 in term of number of migrating cells (efficacy), without changes in the maximal effective chemotactic concentration (potency). However, the biochemical mechanisms that account for the increased chemotactic responses to the ligand CXCL12 in WHIM remain elusive. CXCL12 is known to activate numerous signaling pathways, including receptor-associated trimeric G proteins, phospholipase Cγ, PI3K, and small G proteins. Signaling through these molecules ultimately leads to an increase of intracellular calcium concentration, cytoskeleton reorganization, and cellular migration.35,39,40 Several modulating factors such as phosphatases, RGSs (regulator of G-protein signaling), adaptor proteins, and ubiquitin may affect signaling and/or chemotactic response of CXCR4 to its ligand.41-47 Interestingly, heterozygous mutations in the phosphatase and tensin homologue (PTEN), a negative regulator of CXCR4 activation, result in increased responsiveness to CXCL12 in mice.42
We have reported for the first time that the heterozygous mutations of CXCR4 that are associated with WHIM syndrome confer an increased responsiveness of mature PMN and T cells to CXCL12. This observation might account for the peculiar association of myelocathexis and leukopenia that is observed in these patients. The role of CXCL12 and of its receptor in maintaining a normal hematopoiesis was originally postulated on the basis that CXCR4- and CXCL12-null mice display limited expansion of myeloid precursors.6,7 In addition, inhibition of G-protein-coupled receptors signaling by treatment of mice with pertussis toxin, a molecule that blocks the function of Gi proteins, or following administration of the CXCR4 antagonists AMD-3100, results in progressive increase of circulating hematopoietic stem cells.9,23
CXCL12 is constitutively expressed by stromal cells and by bone marrow endothelium and cooperates with integrins for retention of circulating progenitors at the site of hematopoiesis and for inducing arrest of circulating stem cells on vascular endothelium.7,12 In our study, we observed that T cells from WHIM patients display increased adherence and potent transendothelial migratory properties in comparison to healthy donors. These data support the notion that circulating leukocytes in WHIM patients may be captured in bone marrow because of their increased chemotactic response to CXCL12. There is evidence that the process of emigration of mature PMNs to the bloodstream is accompanied by decreased responsiveness of these cells to CXCL12, and the ability of G-CSF to mobilize hematopoietic precursors is partly due to modulation of CXCR4 and CXCL12 expression in the bone marrow.48,49 In contrast, senescent PMNs display an increased expression of CXCR4 and an enhanced response to CXLC12, thereby leading to their preferential homing to the bone marrow, where these cells can be removed from circulation.50 These recent observations by Martin et al50 may account for the abnormal accumulation of senescent PMNs in the bone marrow that is typically observed in WHIM patients.
To explain the characteristic immune dysfunction of WHIM syndrome (hypogammaglobulinemia, lymphopenia, warts), we sought to obtain a detailed immune phenotype of WHIM patients. Analysis of B- and T-cell subsets in WHIM patients has shown important abnormalities in the distribution of memory and naive lymphocytes, such as a significant decrease in the number of circulating memory (CD27+) and memory-switched (CD27+ IgD-) B cells and a reduction of the circulating naive T cells.
We failed to detect abnormalities in B-cell development in the bone marrow of one of our WHIM patients. Moreover, the ability to produce specific antibodies upon active immunization and the high rate of somatic hypermutation among IgG CD19+ cells in patient P1 argue against gross defects in germinal center reaction. Postgerminal center B cells consist of plasma cells and memory cells. Both of them express CXCR4 and move in response to CXCL12 gradient concentration toward lymph nodes medullary cords, splenic red pulp, and bone marrow. Altered responsiveness to CXCL12 may impair physiologic recirculation and homing to supportive niches of these cells. Indeed, plasma cells and memory B cells display substantial migration to CXCL12, thus proving a model to explain the mechanism of hypogammaglobulinemia in WHIM patients.51,52 In keeping with this hypothesis, we observed that the number of circulating memory B cells is reduced in WHIM syndrome. Based on this observation, WHIM patients seem to present a unique defect in maintaining, rather than in generating, B-cell memory. To further support this hypothesis, we found that WHIM patient P1 failed to maintain protective titers of anti-tetanus toxoid antibodies over time.
Immunophenotyping of circulating T lymphocytes revealed novel abnormalities in patients with WHIM. In particular, we have found that the disease is characterized by a marked reduction of circulating naive T cells and an increase of effector memory T cells. The accumulation of the latter subset in blood circulation is not simply the consequence of chronic papillomavirus infection, since it was also observed in patient P2, who remains wart-free. The pathogenesis of lymphopenia in WHIM might reflect a reduced thymic output or aberrant trafficking of T lymphocytes as a consequence of the disturbed responsiveness to CXCL12. We hypothesize that the perturbed homeostasis of cell trafficking due to CXCR4-altered signaling may account for the abnormal distribution of leukocyte subpopulations in blood circulation of WHIM patients. Although most circulating lymphocytes express CXCR4, there are important differences among T-cell subsets. Indeed, naive T cells display high expression of CXCR4 and CCR7, whereas these are markedly reduced in central and effector memory T cells.53 In keeping with these data, naive T cells are more responsive than memory T cells to CXCL12.54 The higher levels of CXCL12 expressed in lymph nodes and spleen, together with the increased responsiveness to this chemokine observed in WHIM, may cause preferential retention of naive T cells in lymphoid organs and thus contribute to the reduced number of CD45RA+/CCR7+ T cells in peripheral blood. On the other hand, the lymphopenic state of WHIM patients may determine homeostatic expansion of effector memory oligoclonal T cells, as also observed in other lymphopenic conditions such as in the elderly and in HIV-infected subjects.38
Alternatively, one may speculate that effector cells of WHIM patients are retained in the bloodstream because of disturbances in lymphocyte trafficking between lymphoid organs that affect their program of coordinated changes and the set of surface chemokine receptors required for their recruitment to target tissues. Based on these premises, the aberrant distribution of naive, central, and effector memory T cells observed in WHIM patients is likely to have functional and clinically relevant implications and may in part account for the observed susceptibility to infections. At the same time, since CXCR4 is also expressed by other cell types, including dendritic and natural killer (NK) cells, it is likely that disregulation in the homeostasis and trafficking of these cell also may contribute to the determination of the WHIM phenotype. A better comprehension of WHIM syndrome will further clarify the role of CXCR4-CXCL12 interaction in the regulation of bone marrow and lymphoid homeostasis and will provide new insights in several aspects of immune surveillance and memory. Finally, pharmacologic manipulation of CXCR4 signaling may represent a novel and effective form of treatment for WHIM.
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2003-10-3532.
Supported by grants from the Ministero Istruzione Universita Ricerca (MIUR)-Cofin 2002 (E.M., S.S.), MIUR-FIRB (Fondo Investimenti Ricerca di Base) (Luigi D. Notarangelo, L.I.), MIUR (Centro per l'Innovazione Diagnostica e Terapeutica [IDET]), PF Ministry of Health by Joint Program CNR-MIUR (Law 449/97), Progetto Strategico Minute Salute-Bambin Gesù 2002, Minute Salute-Burlo 2001 (Luigi D. Notarangelo), and FIRCA (National Institutes for Health) (S.S.).
Presented as an oral presentation at the Special Symposium on Primary Immunodeficiencies of the Third Annual Meeting of the Federation of Clinical Immunological Societies (FOCIS), Paris, France, May 15, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Ji Ming Wang for his comments and suggestions and to Dr Antonietta Silini for reviewing the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal