Abstract
To investigate the role of phospholipase D (PLD) in FcϵRI signaling, the wild-type or the catalytically inactive forms of PLD1 or PLD2 were stably overexpressed in RBL-2H3 mast cells. FcϵRI stimulation resulted in the activation of both PLD1 and PLD2. However, PLD1 was the source of most of the receptor-induced PLD activity. There was enhanced FcϵRI-induced degranulation only in cells that overexpressed the catalytically inactive PLD1. This dominant-negative PLD1 enhanced FcϵRI-induced tyrosine phosphorylations of early signaling molecules such as the receptor subunits, Syk and phospholipase C-γ which resulted in faster release of Ca2+ from intracellular sources. Therefore, PLD1 negatively regulates signals upstream of the Ca2+ response. However, FcϵRI-induced PLD activation required Syk and was downstream of the Ca2+response, suggesting that basal PLD1 activity rather than that activated by cell stimulation controlled these early signaling events. Dominant-negative PLD1 reduced the basal phosphatidic acid formation in unstimulated cells, which was accompanied by an increase in FcϵRI within the lipid rafts. These results indicate that constitutive basal PLD1 activity by regulating phosphatidic acid formation controls the early signals initiated by FcϵRI aggregation that lead to mast cell degranulation. (Blood. 2004;104:4122-4128)
Introduction
Phospholipase D (PLD) cleaves the terminal phosphodiester bond of phosphatidylcholine (PC) to yield phosphatidic acid (PA) and choline. The PA can then form important lipid mediators, including lysoPA, arachidonic acid, and diacylglycerol, which act as second messengers in many cellular responses.1,2 Two mammalian PLD genes, PLD1 and PLD2, have been identified. Although both PLD enzymes require phosphatidylinositol 4,5-bisphosphate for activity, PLD1 is regulated by protein kinase C (PKC), and Arf and Rho small G protein families, whereas PLD2 can be activated by PKC, Arf, and phosphatidylinositol 4-phosphate 5-kinase α.3-7 PLD activation may be involved in many cellular responses such as actin stress fiber formation,8 exocytosis,9,10 and endocytosis.11 PLD is activated by many cell surface receptors; in immune cells, it is activated by antigen receptors present on T and B cells, monocytes, and mast cells.12,13
Aggregation of the high-affinity immunoglobulin E (IgE) receptor (FcϵRI) on basophils and mast cells results in the rapid phosphorylation of the tyrosine residues in the immunoreceptor tyrosine-based activation motif of the β and γ subunits of the receptor.14 This then recruits the protein tyrosine kinase Syk that is essential for phospholipase C-γ (PLC-γ) activation and the increase in intracellular Ca2+ ([Ca2+]i) and degranulation.15-18 The mediators that are released cause allergic inflammation and anaphylactic reactions.19-21 By mRNA analysis PLD1b and PLD2 are present in the RBL-2H3 cells that have been used as an in vitro model for mast cells.22 In RBL-2H3 cells, PLD1b is predominantly in secretory granules and lysosomes,23 whereas PLD2 is in the plasma membrane.22 Stimulation of FcϵRI activates PLD in both rat mast cells24 and RBL-2H3 cells.25 Transient expression of PLD isoforms in RBL-2H3 cells suggest that both play a role in secretion although expressed at different subcellular locations.22 The FcϵRI-induced PLD activation requires extracellular Ca2+.26 PLD is also activated in these cells by thapsigargin, or calcium ionophore, that increases intracellular Ca2+ and PMA that activates PKC.27-29 These results suggest that the FcϵRI-induced activation of PLD is downstream of the receptor-induced activation of PKC and the increase in [Ca2+]i.
In the presence of primary alcohols PLD catalyzes not only the hydrolysis of PC to form PA but also a transphosphatidylation reaction that yields phosphatidylalcohol. This reaction has been used to quantify the activation of PLD and to demonstrate the requirement of the PA generated by this pathway. Addition of primary alcohols to RBL-2H3 cells has suggested an essential role for PLD in FcϵRI-induced mast cell degranulation30 and membrane ruffling.31 To study the role of the different PLD isoforms in mast cell activation we established cloned cell lines that overexpressed wild-type or a dominant-negative form of PLD1b and PLD2. The results, contrary to the conclusions based on chemical inhibition, found that PLD1, but not PLD2, is a negative regulator of mast cell degranulation.
Materials and methods
Materials and antibodies
L-1-[palmitoyl-1-14C]lysopalmitoyl PC ([14C]lysoPC; 55.8 mCi/mmol [2064.6 MBq/mmol]) was from NEN Life Science (Boston, MA). Silica Gel 60 was purchased from EM Science (Gibbstown, NJ). Medium 199 (H) was from Biofluid (Rockville, MD). Thapsigargin was from EMD Biosciences (San Diego, CA). Mouse anti-FLAG (M2) monoclonal antibody (mAb) was purchased from Sigma (St Louis, MO). The specificity and source of rabbit polyclonal antibodies used were as follows: N-terminal specific anti-PLD1 and anti-PLD2 from Biosource International (Camarillo, CA) and anti-linker for activation of T cells (LAT) from Upstate Biotechnology (Lake Placid, NY). Sheep anti-rat mast cell protease II (RMCPII) was from Moredun Scientific (Scotland, United Kingdom). The source of other materials was as described previously.32
Construction of plasmids and stable transfection
FLAG-tagged rat wild-type and catalytically inactive PLD1b and PLD2 cDNAs in the pCMV5 mammalian expression vector were kindly provided by Dr Taro Okada (Kobe University, Japan). For stable transfection, 20 μg linearized expression constructs together with 2 μg pSV2-neo vector were cotransfected into 5 × 106 RBL-2H3 cells by electroporation (960 μF, 310 V) and selected with 400 μg/mL active G418 as described previously.16 Cell lines were screened for the level of FLAG-PLD expression by immunoblotting with anti-FLAG (M2) antibody (Ab) using blotting with anti-FcϵRIβ Ab as an internal control. By flow cytometry the expression level of FcϵRI was similar on the different cell lines.
Cell culture, activation, and analysis
RBL-2H3 cells, the Syk-negative variant (TB1A2), and the Syk reconstituted (3A5) cells were cultured as described previously.16,33 For activation, cells were cultured overnight with or without the antigen-specific anti-trinitrophenyl IgE. For histamine release assays, the cell monolayers were washed twice with Eagle Minimum Essential Media (MEM) containing 0.1% bovine serum albumin (BSA) and 10 mM tris(hydroxymethyl)amino-methane (Tris; pH 7.4). The cells incubated with IgE were then stimulated with antigen (35 molecules of dinitrophenyl coupled per molecule of human serum albumin), and the nonsensitized cells were stimulated with the calcium ionophore A23187 or thapsigargin in the same medium. After 45 minutes at 37°C, the medium was removed for histamine analysis as described previously.34
Immunoprecipitation and immunoblotting were performed as described previously, except that cell lysates for PLD immunoblotting were prepared without boiling.35 Where indicated, the blots were scanned and the densitometric quantitation of the bands were used to calculate the ratio of the phosphorylated proteins in the transfected compared with the control cells.
PLD assay
Cells (2 × 105/well) were seeded in 12-well tissue culture plates (Corning, Corning, NY). After overnight culture, monolayers were washed twice with Eagle MEM and labeled with [14C]lysoPC (0.25 μCi [0.00925 MBq]/106 cells) in Eagle MEM for 90 minutes at 37°C. The cells were washed twice with Eagle MEM, and then incubated for 24 hours with or without the antigen-specific IgE in regular culture medium. For cell activation, monolayers were washed twice with Eagle MEM containing 0.1% BSA and 10 mM Tris (pH 7.4), and stimulated with 1 mL PLD-medium (Eagle MEM containing 0.1% BSA, 10 mM Tris [pH 7.4], 0.25% 1-butanol, with or without antigen or calcium ionophore A23187) for the indicated time at 37°C. The reaction was stopped by rapidly removing the reaction mixture and immediately adding 0.4 mL ice-cold methanol/HCl mixture (1:0.006, vol/vol). The cells were scraped into tubes, and 0.4 mL chloroform was added. After phase separation and lipid extraction, the quantity of [14C]phosphatidylbutanol (PBut) formed was determined by autoradiography as described previously.36 Paired Student t test was used for statistical analysis.
Measurement of basal PA formation
Cells were labeled with [14C]lysoPC as described for the PLD assay. After labeling the monolayers were washed twice and cultured for 24 hours in regular culture medium. The medium was then removed, and ice-cold methanol/HCl mixture and chloroform were added as described for the PLD assay. After phase separation and lipid extraction, [14C]PA formed was determined by autoradiography as described previously.37 PA formation in 24 hours was calculated from the radioactivity in [14C]PA as a percentage of the total radioactivity of all spots in that lane.
Measurement of single cell Ca2+ response
Cells (2 × 105/well) were cultured overnight with or without IgE in 24-well culture plates. The monolayers were then washed twice with loading medium (medium 199, supplemented with 2 mM CaCl2 0.1% BSA and 250 μM sulfinpyrazone) and loaded with 2 μM Fura-2 am for 1 hour at 37°C. After loading, cells were transferred to room temperature for 30 minutes and then washed and stimulated in working medium (medium 199 containing 2 mM CaCl2, 10 mM Tris pH 7.4, 0.01% BSA and 250 μM sulfinpyrazone). For the extracellular Ca2+-free experiments, monolayers were washed just before stimulation with ethylene glycol tetraacetic acid (EGTA) medium-A (working medium with 1 mM EGTA replacing the 2 mM CaCl2) and stimulated in EGTA medium-B (EGTA reduced to 0.1 mM in EGTA medium-A). Fura-2 fluorescence in single cells was measured using a Tillvision imaging system (Till Photononic GmbH, Grafelfing, Germany) as described previously.35
Subcellular fractionation and FcϵRI distribution
Subcellular fractionation was performed as described previously.9 Detergent-insoluble glycolipid-enriched microdomains (GEMs) were prepared essentially as described previously except that the lysates were not precleared before the sucrose density gradient centrifugation.38 Ten fractions were collected from the top of the gradient. Under these conditions around fraction 3 at the 5%/30% sucrose interface contains detergent-insoluble GEMs, and fractions 7 to 10 contain detergent-soluble fractions. For analysis of FcϵRI in the membrane, cells were sensitized with biotinylated IgE for 2 hours (0.5 μg/106 cells) before lysis for sucrose density gradient centrifugation.
Results
Generation of cell lines overexpressing the wild-type and the catalytically inactive forms of PLD1 or PLD2
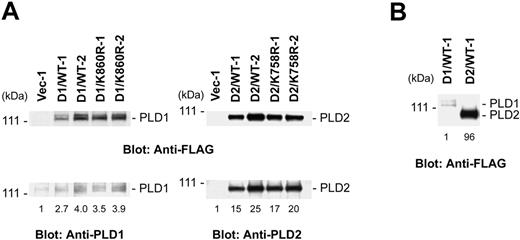
To investigate the participation of PLD subtypes in FcϵRI-induced mast cell activation we transfected the FLAG-tagged wild-type or catalytically inactive forms of rat PLD1b or PLD2 into the RBL-2H3 cells. The catalytically inactive forms have a mutation of a residue in 1 of the 2 catalytic domains of each PLD subtype (Lys860 in rat PLD1b or Lys758 in PLD2 to Arg, respectively).3 After transfection the clones selected with G418 were screened by immunoblotting with anti-FLAG antibodies. More than 4 independent cloned cell lines were isolated with each transfection of wild-type PLD1b (D1/WT), catalytically inactive PLD1b (D1/K860R), wild-type PLD2 (D2/WT), or catalytically inactive PLD2 (D2/K758R). As controls, cloned cell lines (Vec) were isolated after transfection with empty vectors. The expression level, as measured by immunoblotting with both anti-FLAG and subtype specific anti-PLD antibodies, of the 2 highest expressing clones from each transfection are shown in Figure 1A. By densitometric analysis of the immunoblots, there was an approximately 3.5-fold increase in the expression of PLD1b and an approximately 20-fold increase of PLD2 with similar levels for the expression of the wild-type and the catalytically inactive forms. The anti-FLAG immunoblots indicated that there was an approximately 100-fold greater expression of the wild-type and catalytically inactive PLD2 than that of PLD1 (Figure 1B). This low expression of transfected PLD1 was observed after multiple transfections (data not shown).
Generation of stable cell lines overexpressing the wild-type or catalytically inactive forms of PLD1b or PLD2. (A) Two cloned lines with the highest levels of each of the different forms of PLD were selected for detailed analysis. Total lysates from the cells expressing the wild-type PLD1b (D1/WT), catalytically inactive PLD1b (D1/K860R), wild-type PLD2 (D2/WT), catalytically inactive PLD2 (D2/K758R), or empty vector (Vec-1) were immunoblotted with anti-FLAG Ab, anti-PLD1 Ab, or anti-PLD2 Ab (2.5 × 105 cell equivalents/lane for PLD1 and 105 cell equivalents/lane for PLD2). (B) Overexpressed exogenous PLD1b and PLD2 levels were compared by immunoblotting with anti-FLAG Ab (105 cell equivalents/lane). The numbers at the bottom of the lanes are the normalized densitometric analysis.
Generation of stable cell lines overexpressing the wild-type or catalytically inactive forms of PLD1b or PLD2. (A) Two cloned lines with the highest levels of each of the different forms of PLD were selected for detailed analysis. Total lysates from the cells expressing the wild-type PLD1b (D1/WT), catalytically inactive PLD1b (D1/K860R), wild-type PLD2 (D2/WT), catalytically inactive PLD2 (D2/K758R), or empty vector (Vec-1) were immunoblotted with anti-FLAG Ab, anti-PLD1 Ab, or anti-PLD2 Ab (2.5 × 105 cell equivalents/lane for PLD1 and 105 cell equivalents/lane for PLD2). (B) Overexpressed exogenous PLD1b and PLD2 levels were compared by immunoblotting with anti-FLAG Ab (105 cell equivalents/lane). The numbers at the bottom of the lanes are the normalized densitometric analysis.
PLD1, but not PLD2, regulates the FcϵRI-induced degranulation
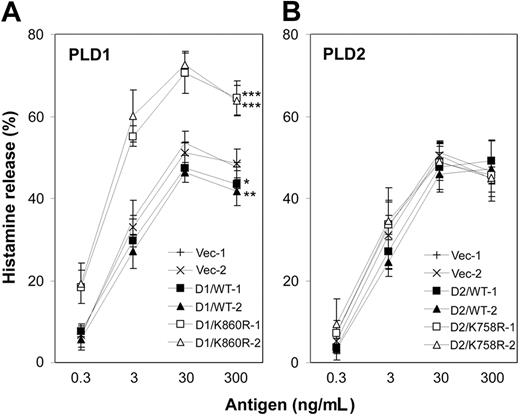
To study the role of PLD1 and PLD2 in FcϵRI-induced degranulation we tested the receptor-induced histamine release of the different transfected cell lines (Figure 2). The FcϵRI-induced histamine release was markedly enhanced by the expression of the dominant-negative D1/K860R, while overexpression of the wild-type PLD1 resulted in a modest inhibition compared with the control cells (Figure 2A). Although the expression of PLD2 in D2/WT or D2/K758R cells was much higher than was achieved by transfection with PLD1, the histamine release from the D2/WT or D2/K758R cells was similar to the controls and to the parental RBL-2H3 cells (Figure 2B and data not shown). These results demonstrate that PLD1, but not PLD2, negatively regulates FcϵRI-induced degranulation.
Overexpression of catalytically inactive PLD1 enhances FcϵRI-induced histamine release. Two cell lines of each type transfected with PLD1 (A) or PLD2 (B) were stimulated for 45 minutes with the indicated concentrations of antigen. Histamine release into the supernatants is expressed as the percentage of the total content of the cells. Data represent the mean ± SD from 4 independent experiments. The paired t test for the percentage of release curves was statistically significant compared with the 2 controls as indicated: *P < .05, **P < .005, ***P < .0005.
Overexpression of catalytically inactive PLD1 enhances FcϵRI-induced histamine release. Two cell lines of each type transfected with PLD1 (A) or PLD2 (B) were stimulated for 45 minutes with the indicated concentrations of antigen. Histamine release into the supernatants is expressed as the percentage of the total content of the cells. Data represent the mean ± SD from 4 independent experiments. The paired t test for the percentage of release curves was statistically significant compared with the 2 controls as indicated: *P < .05, **P < .005, ***P < .0005.
FcϵRI stimulation activates both PLD1 and PLD2; however, PLD1 is the major contributor to the receptor-induced PLD activation
The FcϵRI-induced PLD activation was measured in the different cell lines to establish the relative contribution of PLD1 and/or PLD2 (Figure 3A-B). Receptor stimulation significantly increased PLD activation more in the cells that overexpressed wild-type PLD1 or PLD2 than in the control cells (Figure 3A). However, the approximately 3.3-fold increase in PLD1 protein expression resulted in an approximately 3.5-fold enhancement in FcϵRI-induced PLD activation, whereas the approximately 20-fold increase in PLD2 protein levels caused only an approximately 3.5-fold rise in PLD activation. These results strongly suggested that PLD1 was the major but not only source of PLD activation after FcϵRI stimulation. This conclusion was supported by the results with the cells overexpressing the catalytically inactive forms of these 2 PLD enzymes that act as dominant-negative inhibitors (Figure 3B). The FcϵRI-induced PLD activation was dramatically inhibited only in the cells that overexpressed the catalytically inactive PLD1. The mutated PLD2, even though expressed at much higher levels than PLD1, did not function as a dominant-negative inhibitor of receptor-induced PLD activation. Therefore, although both PLD1 and PLD2 are activated, PLD1 was the major source of the increased PLD activity after FcϵRI stimulation and this correlated with the results of its regulation of degranulation.
FcϵRI-induced PLD activation requires Syk and is enhanced by overexpression of wild-type PLD1 and PLD2, but is inhibited only by catalytically inactive PLD1. Cells expressing wild-type PLD1 and PLD2 (A) or their catalytically inactive forms (B), were labeled with [14C]lysoPC for 90 minutes, cultured overnight with antigen-specific IgE, then stimulated for 20 minutes with the indicated concentrations of antigen in the presence of 0.25% 1-butanol. Production of [14C]PBut induced by antigen is expressed as the percentage of total labeled lipids. Significant change in PLD activity compared with the corresponding control-treated cells is indicated as follows: *P < .05, **P < .005. (C) Syk-deficient RBL-2H3 cells (SykNeg) and Syk-reconstituted cells (Sykwt) were used to measure PLD activation. Stimulation was with 100 ng/mL antigen or 300 nM A23187. Significant change in PLD activity compared with SykNeg cells is indicated as follows: **P < .005. Data in all panels are the mean ± SD from 3 independent experiments.
FcϵRI-induced PLD activation requires Syk and is enhanced by overexpression of wild-type PLD1 and PLD2, but is inhibited only by catalytically inactive PLD1. Cells expressing wild-type PLD1 and PLD2 (A) or their catalytically inactive forms (B), were labeled with [14C]lysoPC for 90 minutes, cultured overnight with antigen-specific IgE, then stimulated for 20 minutes with the indicated concentrations of antigen in the presence of 0.25% 1-butanol. Production of [14C]PBut induced by antigen is expressed as the percentage of total labeled lipids. Significant change in PLD activity compared with the corresponding control-treated cells is indicated as follows: *P < .05, **P < .005. (C) Syk-deficient RBL-2H3 cells (SykNeg) and Syk-reconstituted cells (Sykwt) were used to measure PLD activation. Stimulation was with 100 ng/mL antigen or 300 nM A23187. Significant change in PLD activity compared with SykNeg cells is indicated as follows: **P < .005. Data in all panels are the mean ± SD from 3 independent experiments.
FcϵRI-induced PLD activation requires Syk
Syk is an essential molecule in FcϵRI-induced mast cell activation that functions upstream of most of the cellular tyrosine phosphorylations and the increase in [Ca2+]i.16,39 Reconstitution studies of Syk-deficient DT40 B cells has also demonstrated that Syk is required for B-cell receptor-induced Ca2+ mobilization and PLD1 activation.40,41 To study PLD function in FcϵRI signaling, we tested receptor-induced PLD activation in the Syk-deficient and the Syk-reconstituted variant of the RBL-2H3 cells (Figure 3C). FcϵRI stimulation did not induce detectable PLD activation in the Syk-deficient cells; this defect was corrected in the Syk-reconstituted cells. In contrast, the calcium ionophore A23187-induced activation of PLD was not dependent on Syk. These results indicate that FcϵRI-induced PLD activation is downstream of Syk.
Dominant-negative PLD1 enhances the FcϵRI-induced tyrosine phosphorylation of early signaling molecules
To determine whether PLD1 regulates early or late steps in degranulation, we tested the different cell lines for calcium ionophore A23187- or thapsigargin-induced histamine release. The extent of degranulation with both of these stimulants was similar in the vector transfected and the cells that overexpressed wild-type or catalytically inactive PLD1 (data not shown). The inhibitory effect of PLD1 on FcϵRI-induced, but not A23187- or thapsigargin-induced, degranulation suggested that PLD1 regulated an early step after FcϵRI aggregation, probably upstream of the Ca2+ response. We therefore investigated the receptor-induced cellular protein tyrosine phosphorylation, which is a very early event critical for downstream signal propagation.15 The antigen-induced total cellular protein tyrosine phosphorylation was increased in D1/K860R cells when compared with the control cells (data not shown).
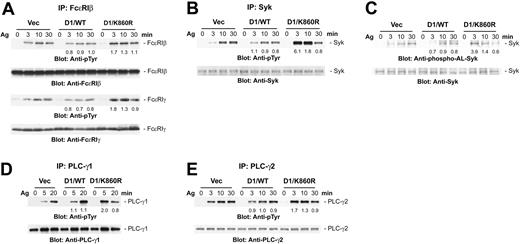
Aggregation of FcϵRI results in the rapid tyrosine phosphorylation of the β and γ subunits of the receptor. The receptor-induced tyrosine phosphorylation of both the β and γ chains of FcϵRI (Figure 4A) and subsequent Syk tyrosine phosphorylation (Figure 4B) were rapid and increased in D1/K860R cells compared with the wild-type PLD1 or vector-transfected cells. Similarly, the phosphorylation of the activation loop tyrosines of Syk, which is essential for the activation of Syk,39,42 was increased at the early time points in the D1/K860R cells (Figure 4C). As expected, there was stronger tyrosine phosphorylation of both PLC-γ1 and PLC-γ2 at the early time points in the D1/K860R cells compared with the control cells (Figure 4D-E). These results indicated that PLD1 acts as a negative regulator of the tyrosine phosphorylation of the FcϵRI → Syk → PLC-γ pathway which is critical for downstream signals for degranulation.
Overexpression of catalytically inactive PLD1 enhances FcϵRI-induced tyrosine phosphorylation of early signaling molecules. The indicated cell lines were sensitized, then stimulated with antigen (100 ng/mL). Lysates were immunoprecipitated with anti-FcϵRIβ Ab (A), anti-Syk Ab (B), anti-PLC-γ1 Ab (D), or anti-PLC-γ2 Ab (E), then analyzed by immunoblotting with the indicated Abs. (C) Total cell lysates were analyzed by immunoblotting with antibodies to the phosphorylated activation loop tyrosines of Syk (anti-phospho-ALSyk). The result shown is representative of 3 independent experiments, and similar results were obtained with the other cell lines. The changes in phosphorylation in the PLD-transfected cells were compared with the controls by densitometry after normalization for protein loading. The numbers are the average from the different experiments.
Overexpression of catalytically inactive PLD1 enhances FcϵRI-induced tyrosine phosphorylation of early signaling molecules. The indicated cell lines were sensitized, then stimulated with antigen (100 ng/mL). Lysates were immunoprecipitated with anti-FcϵRIβ Ab (A), anti-Syk Ab (B), anti-PLC-γ1 Ab (D), or anti-PLC-γ2 Ab (E), then analyzed by immunoblotting with the indicated Abs. (C) Total cell lysates were analyzed by immunoblotting with antibodies to the phosphorylated activation loop tyrosines of Syk (anti-phospho-ALSyk). The result shown is representative of 3 independent experiments, and similar results were obtained with the other cell lines. The changes in phosphorylation in the PLD-transfected cells were compared with the controls by densitometry after normalization for protein loading. The numbers are the average from the different experiments.
Expression of dominant-negative PLD1 results in faster FcϵRI-induced [Ca2+]i response
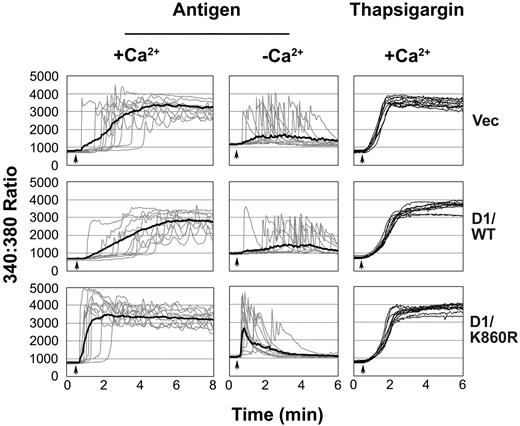
An increase in [Ca2+]i is essential for mast cell activation. The more rapid FcϵRI-induced tyrosine phosphorylation of signaling molecules in D1/K860R cells suggests that there should also be a faster Ca2+ response. As expected, the FcϵRI-induced Ca2+ release from intracellular stores and the influx from the extracellular medium were faster in D1/K860R cells (Figure 5). In contrast, the thapsigargin-induced response due to the emptying of intracellular Ca2+ stores was unchanged. Interestingly, the “lag-times” (time between antigen addition and initiation of the response) was similar with or without extracellular Ca2+ in all the cell lines and were much shorter in the cells expressing D1/K860R. The faster [Ca2+]i response in the cells expressing the dominant-negative PLD1 correlates with the rapid tyrosine phosphorylation of signaling molecules and suggests that PLD1 by regulating receptor tyrosine phosphorylation is controlling these later events. The more rapid rise of [Ca2+]i in the D1/K860R cells in the absence of extracellular Ca2+ indicates that this cannot be due to receptor-induced PLD activation which requires Syk and extracellular Ca2+.
FcϵRI-but not thapsigargin-induced Ca2+ responses are faster in cell lines expressing catalytically inactive PLD1. Cells were stimulated at the time indicated by the arrow with antigen (100 ng/mL) or thapsigargin (100 nM). Experiments were in the presence (+Ca2+) or absence (-Ca2+) of extracellular Ca2+. The graphs shown are the Ca2+ response of 10 individual cells for each cell line (faint lines), representative of the 60 cells within each experiment, and the average of the 60 cells (thick line in antigen stimulation). The result shown is representative of 3 independent experiments, and similar results were obtained when the other cell line of each transfection was examined.
FcϵRI-but not thapsigargin-induced Ca2+ responses are faster in cell lines expressing catalytically inactive PLD1. Cells were stimulated at the time indicated by the arrow with antigen (100 ng/mL) or thapsigargin (100 nM). Experiments were in the presence (+Ca2+) or absence (-Ca2+) of extracellular Ca2+. The graphs shown are the Ca2+ response of 10 individual cells for each cell line (faint lines), representative of the 60 cells within each experiment, and the average of the 60 cells (thick line in antigen stimulation). The result shown is representative of 3 independent experiments, and similar results were obtained when the other cell line of each transfection was examined.
Dominant-negative PLD1 reduces basal PA formation in unstimulated RBL-2H3 cells
The enhanced tyrosine phosphorylation of early signaling molecules and the more rapid [Ca2+]i response indicate that PLD1 regulates the initiation of early receptor-induced signaling events. However, the observation that the receptor-induced PLD activation requires extracellular Ca2+ and is downstream of Syk would indicate that the regulation of early signaling events is due to the constitutive activity of PLD1. Therefore, we investigated the effect of the expression of the different molecules on the basal PLD enzymatic activity in unstimulated cells (Figure 6A). By the transphosphatidylation assay there was increased basal PLD activity in unstimulated cells that overexpressed wild-type PLD1 or PLD2, with a decrease only in the cells with the dominant-negative PLD1. The formation of PA from lysoPC as a measure of PLD activity in unstimulated cells was increased by overexpression of both wild-type PLD1 or PLD2 but decreased only by dominant-negative PLD1 (Figure 6B). As the expression level of PLD1 in D1/WT cells was much lower than that of PLD2 in D2/WT cells, most basal PLD activity as well as the FcϵRI-induced PLD activation is controlled by PLD1. Therefore, PLD1 is constitutively activated and involved in PA formation in resting RBL-2H3 cells. Taken together these data suggest that changes in basal PA formation regulate degranulation.
Inhibition of basal PLD1 activity correlates with reduced PA formation and morphologic changes. (A) Basal PLD activity; (B) basal PA formation. The indicated cell lines were labeled with [14C]lysoPC for 90 minutes and then cultured for 24 hours. For the transphosphatidylation, cells were incubated with 0.25% 1-butanol for another 1 hour, and the labeled PBut (A) or PA (B) was then separated on thin-layer chromatography (TLC). Data represent the mean ± SD from at least 5 independent experiments. Statistically significant change compared with the 2 control cells is indicated as follows: *P < .05, **P < .005.
Inhibition of basal PLD1 activity correlates with reduced PA formation and morphologic changes. (A) Basal PLD activity; (B) basal PA formation. The indicated cell lines were labeled with [14C]lysoPC for 90 minutes and then cultured for 24 hours. For the transphosphatidylation, cells were incubated with 0.25% 1-butanol for another 1 hour, and the labeled PBut (A) or PA (B) was then separated on thin-layer chromatography (TLC). Data represent the mean ± SD from at least 5 independent experiments. Statistically significant change compared with the 2 control cells is indicated as follows: *P < .05, **P < .005.
Some PLD1 localizes in GEMs/lipid rafts in unstimulated RBL-2H3 cells
Because of these effects of PLD1 on receptor-induced signaling and degranulation, we investigated the cellular distribution of these proteins. The tagged PLD1 in the transfected cells could not be precisely localized by microscopy but by differential centrifugation, and immunoblotting most of the PLD1 was in the cytoplasmic fraction although a small amount (∼15%) of both the wild-type and the catalytically inactive PLD1 was present in the crude membrane fraction (data not shown). The detergent-resistant lipid raft domains in the plasma membrane contain proteins that are important in signal transduction and therefore may play an important role in FcϵRI-induced signaling.43,44 When Triton-X-solubilized cells were fractionated by sucrose density ultracentrifugation, a small fraction of the tagged PLD1 colocalized to the same fractions as LAT, a marker for the GEM fraction (Figure 7A). In contrast, rat mast cell protease II (RMCPII), which is a granular marker, was only detected in fractions with higher sucrose density. Catalytically inactive PLD1 tagged with FLAG showed a similar distribution (data not shown). These data strongly suggest that some PLD1 localizes in the GEM fraction and by regulating basal PA production controls the initiation of FcϵRI-induced signaling.
Some PLD1 constitutively localizes to the plasma membrane, especially in GEMs/lipid rafts, and changes FcϵRI distribution in the membrane. (A) Lysates from unstimulated D1/WT cells were fractionated by sucrose density gradient centrifugation, and fractions were analyzed by immunoblotting with anti-FLAG, LAT, and RMCPII Abs. The percentage of the total protein in the indicated fractions was calculated from the densitometric analysis and is the average ± standard deviation from 3 independent experiments. Similar results were obtained when the other cell line of each transfection was examined. (B) FcϵRI distribution in the membrane. Lysates from unstimulated cells presensitized with biotinylated IgE were fractionated by sucrose density gradient centrifugation. Biotinylated IgE was detected with horseradish peroxidase (HRP)-conjugated streptavidin. The percentage of the biotinylated IgE localized to the lipid rafts was calculated from the densitometric analysis and is the average ± standard deviation from 3 different experiments. Statistically significant change compared with the control cells or D1/WT cells is indicated as follows: *P < .05. Similar results were obtained when the other cell line of each transfection was examined.
Some PLD1 constitutively localizes to the plasma membrane, especially in GEMs/lipid rafts, and changes FcϵRI distribution in the membrane. (A) Lysates from unstimulated D1/WT cells were fractionated by sucrose density gradient centrifugation, and fractions were analyzed by immunoblotting with anti-FLAG, LAT, and RMCPII Abs. The percentage of the total protein in the indicated fractions was calculated from the densitometric analysis and is the average ± standard deviation from 3 independent experiments. Similar results were obtained when the other cell line of each transfection was examined. (B) FcϵRI distribution in the membrane. Lysates from unstimulated cells presensitized with biotinylated IgE were fractionated by sucrose density gradient centrifugation. Biotinylated IgE was detected with horseradish peroxidase (HRP)-conjugated streptavidin. The percentage of the biotinylated IgE localized to the lipid rafts was calculated from the densitometric analysis and is the average ± standard deviation from 3 different experiments. Statistically significant change compared with the control cells or D1/WT cells is indicated as follows: *P < .05. Similar results were obtained when the other cell line of each transfection was examined.
Decreased basal PLD1 activity correlates with changes in FcϵRI distribution in the membrane
In unstimulated RBL-2H3 cells very little of the FcϵRI localizes in the detergent insoluble fractions/lipid rafts.38,43 Interestingly, there was an increase of the FcϵRI in the lipid raft fractions in unstimulated D1/K860R cells (Figure 7B). These changes in the distribution of FcϵRI in the membrane correlate with the more rapid receptor aggregation-induced signals in the D1/K860R cells.
Discussion
In this study we demonstrate a role for PLD1 in regulating degranulation in mast cells by showing the following. (1) Dominant-negative PLD1 enhances receptor-induced degranulation. (2) This effect correlates with a decrease in PA formation in unstimulated cells due to basal PLD1 activity. This regulates the initiation of signal transduction in cells. (3) There is also FcϵRI-induced activation of PLD which is downstream of Syk. (4) Although both PLD1 and PLD2 are activated after receptor aggregation, the major contributor to PLD activity is PLD1.
FcϵRI stimulates both PLD1 and PLD2; however, PLD1 is the major contributor of PLD activation. Similarly, PLD1, but not PLD2, is activated downstream of FcγRI in U937 monocytes, suggesting PLD1 is an important source of PLD activation for immune receptors.45 PLD2, but not PLD1, is activated by the platelet-derived growth factor (PDGF) or angiotensin II receptors in vascular smooth muscle cells.2 Mastoparan, which is thought to directly activate Gi, stimulates only PLD2 but not PLD1 in RBL-2H3 or COS7 cells.46 Therefore, different receptors primarily stimulate 1 of the 2 PLD isoforms, which for FcϵRI appears to be PLD1.
A major source for the production of PA in cells is PLD that hydrolyzes PC to generate PA and choline. Although PA constitutes only approximately 2.2% of the total phospholipids in RBL-2H3 cells, it regulates important processes, such as protein phosphorylation and membrane trafficking.2,47 Overexpression of wild-type PLD1 and PLD2 resulted in increased PA formation in unstimulated cells (Figure 6B). However, only dominant-negative PLD1 induced a decrease in basal PA formation in unstimulated cells even though it was expressed at much lower levels than PLD2. The decreased PA generation increased the fraction of the receptor in lipid rafts and enhanced FcϵRI-induced degranulation. However, PLD1 may also act by mechanisms other than PA generation. There were morphologic changes in the PLD1-overexpressing cells (T.H., L.M.N., M.C.J., C.O., R.P.S., unpublished observation, 2004) which could be due to interactions of PLD1 with Rho family proteins that control the actin cytoskeleton.
Several reports suggest that the phospholipid content of the plasma membrane affects mast cell degranulation; addition of PC or sphingomyelin-containing vesicles to RBL-2H3 cells enhances FcϵRI-stimulated but not ionophore-induced degranulation.48 Addition of bacterial PLC, PLD, or phospholipase A2 triggered degranulation of permeabilized RBL-2H3 cells, suggesting that lipid products produced by these enzymes or breakdown of phospholipid substrates may play a role in exocytosis.37 Therefore, the phospholipid content of resting cells is strictly regulated to critically control mast cell signal initiation and propagation.
The detergent-resistant lipid raft domains in the plasma membrane may play an important role in FcϵRI-induced signaling.43 These domains are enriched in sphingolipids, cholesterol, and dually acylated proteins such as Lyn. Modification of the lipid content of the plasma membrane can disrupt lipid rafts and interfere with signal transduction.49-51 Such treatments also effect the number of FcϵRI on the cell surface.52 Although the total number of FcϵRI was similar in the different PLD-transfected cell lines, the decrease in PA changed the distribution of FcϵRI in the plasma membrane with an increase of the FcϵRI in the lipid raft fractions in the D1/K860R cells. Many proteins that are important in signal transduction are present in lipid rafts, a microenvironment conducive to signal initiation.43 As has been reported in other cells,53 some PLD1 was present in lipid rafts, suggesting that it could regulate the PA level in these membrane microdomains. Such regulation of PA levels by the basal PLD1 activity can control signal initiation for antigen-induced mast cell degranulation.
Although some PLD1 is in the plasma membrane, most of it is associated with granules. In RBL-2H3 cells, transiently transfected PLD1 localizes primarily to secretory vesicle and may translocate to the plasma membrane after FcϵRI stimulation.22,23,31 This antigen-induced translocation requires catalytically active PLD1; however, surprisingly, primary alcohols did not inhibit this movement of PLD1 to the plasma membrane. PLD1 once in the plasma-membrane is associated with activators such as PKCα, ARF6, or Rac1.54 Therefore, the effects we observed may be due to PLD1 in either the granules or plasma membrane.
Primary alcohols act as alternative substrates to water in the transphosphatidylation reaction, resulting in the formation of phosphatidylalcohols and the inhibition of the generation of PA. The effects of primary alcohols in cells may not only be due to this decrease in PA generation but also due to direct toxic effects. The use of primary alcohols has suggested that PLD activation is an enhancer of receptor-induced degranulation in hematopoietic cells.28 In antigen-stimulated RBL-2H3 cells these alcohols block PA generation25 and degranulation,30 suggesting an essential role for PLD. However, our results using overexpression of either the wild-type or the dominant-negative forms of PLD suggest that PLD1 has a negative regulatory role in signal transduction and degranulation. Even though PLD1 is expressed and activated to various levels in the different transfected cell lines, addition of primary alcohol decreased FcϵRI-induced degranulation to a similar extent in all these cells (T.H. and R.P.S., unpublished observations, 2004). This suggests that decreased exocytosis by these alcohols may not be due to inhibition of PLD activation but due to their lipophilicity and effects on membrane organization.55
In summary, the results presented here demonstrate the importance of basal PLD1 activity in regulating signaling from FcϵRI. The similarity of FcϵRI to other immune receptors on monocytes, T cells, and B cells suggest that PLD may also play an important role in the control of the initiation of signaling from those receptors.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-06-2091.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Taro Okada of the Kobe University Graduate School of Medicine for providing plasmids encoding FLAG-tagged rat wild-type and catalytically inactive PLD1b and PLD2. We also thank Dr Kyungduk Moon for reviewing this manuscript; Dr Juan Rivera, Dr Kiyonao Sada, Dr Katsuhiro Suzuki, and Dr Zhi-Hui Xie for helpful discussion; and Greta Bader for excellent technical help with the histamine analysis.

![Figure 3. FcϵRI-induced PLD activation requires Syk and is enhanced by overexpression of wild-type PLD1 and PLD2, but is inhibited only by catalytically inactive PLD1. Cells expressing wild-type PLD1 and PLD2 (A) or their catalytically inactive forms (B), were labeled with [14C]lysoPC for 90 minutes, cultured overnight with antigen-specific IgE, then stimulated for 20 minutes with the indicated concentrations of antigen in the presence of 0.25% 1-butanol. Production of [14C]PBut induced by antigen is expressed as the percentage of total labeled lipids. Significant change in PLD activity compared with the corresponding control-treated cells is indicated as follows: *P < .05, **P < .005. (C) Syk-deficient RBL-2H3 cells (SykNeg) and Syk-reconstituted cells (Sykwt) were used to measure PLD activation. Stimulation was with 100 ng/mL antigen or 300 nM A23187. Significant change in PLD activity compared with SykNeg cells is indicated as follows: **P < .005. Data in all panels are the mean ± SD from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2091/6/m_zh80240471200003.jpeg?Expires=1767750736&Signature=A21ZnoXJw1u0gWvSdM9s37Bas6LBHVs9Fcc1H9b5R3KM66DaWgUfwQQg8uU0xRp-6OJMiYyl8wuvHCr~zW5tG1HdhzjG4nsBfmQZrP3SjvDeZ~XDE3dbmRb1-cYr4GLELYjcMxN7eum8nlLZlEW19pxBRpVqu2ZVrOZBDVSrh2KLNgzWpwPtvyJamMr3erm8fFCsY~sOxAyJe0cUre-tUFEOjBlOLNYGbv54qDeDlRliGQlVAcgf7zj41Y92ktx0l7TaINOiS~K1c8vA5Ln4oAk7c-UblbL6ptzlJyQk3y5twOoFTUhfzlXCxI5-UROvAhVdtl71FNXErNGaNMfkzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Inhibition of basal PLD1 activity correlates with reduced PA formation and morphologic changes. (A) Basal PLD activity; (B) basal PA formation. The indicated cell lines were labeled with [14C]lysoPC for 90 minutes and then cultured for 24 hours. For the transphosphatidylation, cells were incubated with 0.25% 1-butanol for another 1 hour, and the labeled PBut (A) or PA (B) was then separated on thin-layer chromatography (TLC). Data represent the mean ± SD from at least 5 independent experiments. Statistically significant change compared with the 2 control cells is indicated as follows: *P < .05, **P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2091/6/m_zh80240471200006.jpeg?Expires=1767750736&Signature=sfccsrvaOwsGFFo~Z2mYoygbUxDbI7HWsLct2v8myH-BzBFOr5UWzRZeCUblK9z~lyeZocGIabs7BKiIXxpsdL85bkjlaVHda3P7zYxjnSPUOCeCLKS3EVzct8cwZm07W4BItbDz62QA1zyJwqcpU3~bx1bQz0ZeHPXEHYUb~vyaM5YyO77l7kLGnKo7RJnqt9E936n~NOabiXxj9gzH8pDbdj0JtLSxiXyD49c0-pssM7NlQ0f4L7vUoh85zdMsH5luiOPfaVY9mUJB6WRJY3qL5sBs~K-CqjF3IzTOds-BOcmhY1e6avQPFEWCmqRjpPiZhxxTgEiyB-1SL598jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal