Abstract
Mast cells (MCs) initiate immune responses from mucosal surfaces and perivascular spaces. Stem cell factor (SCF) regulates MC development and viability, but the role of innate serum factors in MC development is unexplored. Cultured cord blood-derived human MCs (hMCs) express mRNA transcripts for all 4 known receptors for lysophosphatidic acid (LPA), an abundant serum-associated lipid growth factor. In an SCF-dependent serum-free culture system, LPA (2.5-10 μM) increased the total number of hMCs by approximately 10-fold compared with cultures maintained in the absence of LPA under otherwise identical conditions. LPA was comitogenic with SCF but did not prolong MC survival. LPA-mediated proliferation was blocked by VPC-32179, a competitive antagonist of LPA1 and LPA3 receptors, and by pertussis toxin, and it was also attenuated by GW9662, a selective antagonist of peroxisome proliferator-activated receptor (PPAR)-γ. LPA accelerated the acquisition of hMC granules and increased Kit expression. hMCs derived in the presence of LPA were functional, as evidenced by their immunoglobulin E (IgE)-dependent histamine release and by their characteristic proliferative responses to interleukin-3 (IL-3), IL-4, and IL-9 in combination with SCF. Thus, LPA acts through LPA receptor and PPAR-γ-dependent pathways to accelerate hMC proliferation and differentiation, and it modulates their phenotype without providing cytoprotection. LPA could facilitate MC hyperplasia in inflammation associated with either innate or adaptive immunity. (Blood. 2004; 104:4080-4087)
Introduction
Mast cells (MCs) reside in perivascular locations in all tissues and organs. This distribution facilitates their direct role in innate immune responses1 and their hyperplasia in antihelminthic adaptive immunity.2 MCs develop in peripheral tissues from bone marrow-derived, circulating committed progenitors,3 with subpopulations that vary in the composition of their preformed secretory granule proteases, proteoglycans, and profiles of secreted mediators. Normal MC development requires the stromal cell-derived cytokine stem cell factor (SCF), which is a ligand for the receptor tyrosine kinase (RTK) Kit.4 Several cytokines from T cells (interleukin-3 [IL-3], IL-4, IL-5, and IL-9) are comitogenic with SCF for cord blood-derived cultured human MCs (hMCs) in vitro,5 and some of them are also cytoprotective for hMCs.6 Although not required for MC development in connective tissues, T cell-derived cytokines are required for MC development in the intestinal mucosa of mice2 and humans7 and for the reactive MC hyperplasia observed at the gastrointestinal tract mucosa in TH2-type inflammation.2 However, MC hyperplasia also occurs at foci of fibrosis, remodeling, and wound healing, even in the connective tissues, where their development is typically T cell-independent.8-12 It is thus likely that additional T cell-independent growth factors exist for MCs that have yet to be identified.
Lysophosphatidic acid (LPA) is a multifunctional phospholipid mediator produced and released extracellularly by platelets, adipocytes, and fibroblasts through phospholipase D-mediated reduction of lysophosphatidyl choline.13 LPA is constitutively present in the sera of most species at concentrations of 5 to 25 μM, with most of it bound to albumin and gelsolin.14,15 It is metabolized to phosphatidic acid by LPA-acyltransferase, to monoacylglycerol by phosphatidate phosphohydrolase, or to glycerol-3-phosphate by lysophospholipases (for a review, see Goetzl et al16 ). In inflammation, local vascular leakage and cell activation result in increased tissue concentrations of LPA. LPA is unique among lipids for its activity as a growth factor.16 LPA exerts many of its activities through a group of G protein-coupled receptors (GPCRs) formerly referred to as endothelial differentiation and growth (Edg) receptors 2, 4, and 7, now termed LPA receptors (LPARs) 1 to 3.17-19 Recently, the orphan GPCR p2y9/GPR23 (now LPAR4) was reported to be an LPAR.20 LPARs belong to a structurally homologous family of GPCRs (formerly designated Edg receptors) that include 5 other members with specificity for a related lipid, sphingosine 1-phosphate (S1P).21 LPARs are widely distributed, and a critical role for LPAR1 in neurodevelopment has been confirmed in mice lacking this receptor.22 In some cell types, LPA can also induce proliferation in an LPAR-independent manner,23 likely reflecting its recently recognized capacity to serve as a transcellular agonist of the nuclear transcription factor peroxisome proliferator-activated receptor (PPAR)-γ.24 The fact that T lymphocytes25 and macrophages26 each express several LPARs, as well as PPAR-γ,27,28 suggests the potential for diverse and complex effects of LPA acting through either receptor system in immune or inflammatory responses.
Consistent with their roles in host defense and their wide perivascular distribution, MCs express receptors for a wide range of soluble innate signals, including complement fragments and other serum constituents.29 We hypothesized that MCs would respond developmentally to LPA, given its abundance in serum and its function as a stromal-active growth factor. Using the cytokine triad of SCF, IL-6, and IL-10 with a serum-free medium,5,30 we now demonstrate that LPA accelerates the development and maturation of cord blood-derived hMCs. hMCs express mRNA for all 4 LPARs, and LPA induces increments in hMC mitogenesis, numbers, tryptase content, metachromasia, and surface expression of Kit, but not in viability. The effect of LPA on hMC proliferation is blocked by VPC-32179, a high-affinity, dual-selective antagonist of LPAR1 and LPAR3,31 and is sensitive to inhibition by pertussis toxin (PTX), but it is also partly attenuated by the PPAR-γ- selective antagonist GW9662. Thus, LPA is a novel growth factor for hMCs in vitro and could act synergistically with SCF in a T cell-independent mechanism for self-limited MC hyperplasia in response to injury or inflammation.
Materials and methods
Cell cultures
Mononuclear cells harvested from umbilical cord blood5 were suspended at concentrations of 1 × 106/mL in serum-free AIM V medium (Invitrogen, Grand Island, NY) or RPMI 1640 (Gibco BRL, Gaithersburg, MD) containing 10% fetal calf serum (FCS), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 100 U/mL penicillin, 2 μg/mL gentamicin (all from Sigma-Aldrich, St Louis, MO), and 0.2 μM 2-mercaptoethanol (2-ME) (Gibco BRL). Both media were supplemented with 100 ng/mL SCF (R&D Systems, Minneapolis, MN), 50 ng/mL IL-6 (R&D Systems), and 10 ng/mL IL-10 (Endogen, Woburn, MA). Various concentrations (1.25-10 μM) of the 18-carbon form of LPA containing a single double bond (LPA, 18:1; Sigma Aldrich) were added to the AIM V medium. Cultures were maintained at 37°C and 5% CO2. Medium and cytokines were changed weekly to maintain the cells at 1 × 106/mL concentrations.
To assess the effect of depletion of endogenous serum-associated LPA (and other serum constituents) on hMC growth, some cultures were maintained in the RPMI-based medium detailed here, with standard FCS or with FCS that had been delipidated by charcoal filtration, as detailed elsewhere.32 In these experiments, replicate samples of cells maintained in the presence of delipidated serum received additional supplementation with LPA 18:1 (5 μM) to evaluate the contribution of LPA to serum-associated growth factor activity. Cell numbers were assessed at weekly intervals, and the total numbers of toluidine blue-positive cells were assessed by staining with toluidine blue (as noted in “Cytochemistry and immunocytochemistry”), while total numbers of Kit-positive cells were assessed in each condition as detailed in “Flow cytometry.”
Cytochemistry and immunocytochemistry
Aliquots of 2 × 104 cells immobilized to glass slides in a cytocentrifuge (Cytospin 4; Thermo Shandon, Pittsburgh, PA) were stained with antibodies for tryptase, chymase, and cathepsin G33 (Chemicon International, Carlsbad, CA), as described.5 Replicate slides were stained with toluidine blue (which elicits a metachromatic reaction in the granules of MCs) and for chloroacetate esterase (CAE) activity.5 Cells were visualized at 10 to 400 × magnification with a DM LB2 microscope with a Leica 50× Plan objective with a 0.90 oil aperture (Leica, Northvale, NJ) and photographed with a WILD MPS52 camera (Leica). Images were scanned with a flatbed scanner (Canon, Lake Success, NY) and processed with Adobe Photoshop software (Adobe, San Jose, CA). The number of cells staining strongly with each stain was expressed as a percentage of 200 cells counted on each slide. The percentages of cells staining positively were tabulated for each experiment and were used to calculate the average purity of cells over several experiments.
Flow cytometry
Cytofluorographic staining was performed using mouse anti-human monoclonal antibodies against Kit, CD13, and CD14 (all from Biosource International) and isotype-matched controls, as detailed elsewhere,5 and were analyzed by FACSort (Becton Dickinson, Franklin Lakes, NJ). Two cell populations differing in forward-angle light scatter (FSC) were consistently observed. The cells in the lower FSC compartment were excluded from analysis on the basis of uniform staining with propidium iodide (PI), indicating nonviability. Results were analyzed by visual inspection of the overlaid histograms and by calculation of the percentage of live cells staining positively.
Expression of mRNA for LPARs by hMCs
Total RNA extraction, first-strand synthesis, and polymerase chain reaction (PCR) were performed as detailed elsewhere.5 Primer sequences were: LPA1, forward 5′-TGCAGACTTCTTTGCTGGGTTGG-3, reverse 5′-CAGCAAGGAGAAGGAAGAATTTCT-3′;LPA2, forward 5′-TCAGCGTGCTGGTGCTGCTG-3′, reverse 5′-CAGAGGATGTATAGTGGACAGAC-3′;LPA3, forward 5′-CTCTGGTCA TCGCGGCAGTGA-3′, reverse 5′-TGCGGAGACAAGACGTTGGTTTT-3′; and LPA4/GPR23, forward 5′-GGTACC TATGGGTGACAGAAGATTC-3′, reverse 5′-GAATTCCTAAAAGGTGGATTCTAGC-3′. Primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (Clontech, PaloAlto, CA) were used as positive control. PCR reactions were carried out with 4 U DNApolymerase (Advantage 2-HF; Clontech) in 300 mM Tris-HCl, 75 mM ammonium sulfate, 10 mM MgCl2, pH 9.0, except for LPAR3, where a pH of 9.5 and the inclusion of 10 mM MgCl2 were optimal. Bands of the expected sizes were detected by electrophoresis through ethidium bromide-stained 1.5% agarose gels after 35 cycles of amplification.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblot analysis
For immunoblotting to detect proteases, cells maintained for 3 weeks without and with LPA (1.25-10 μM), lysed in 2 × Tris-glycine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Novex, San Diego, CA) containing 5% 2-ME at 1 × 107 cells/mL, and boiled for 5 minutes. Samples of 1 × 105 hMCs were loaded onto precast 12% Tris-glycine-SDS gels, and the proteins were separated by electrophoresis at 30 mA and 150 mV. After overnight transfer to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA), the blots were blocked for 1 hour in 1 × Tris-buffered saline with 0.5% Tween-20 (Bio-Rad; TTBS) containing 3% nonfat dry milk and 0.25% normal goat serum and were incubated in the same buffer containing antibodies against human α- and β-tryptase (1:2000), chymase (1:500), and cathepsin G (1:500). After extensive washes in TTBS, the membranes were incubated for 1 hour in the presence of a 1:3000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and washed again, and the bands were detected by autoradiography using enhanced chemiluminescence (Amersham, Piscataway, NJ). Densities of the bands corresponding to tryptase were quantitated using a densitometer and Scion Image software version Beta 4.02. To assess phosphorylation of Akt as an index of phosphatidylinositol 3-kinase (PI3K) activity, 3-week-old hMCs derived in the presence of LPA were washed into fresh AIM V medium without LPA or cytokines and were incubated for 2 hours at 37°C. Samples of 2 × 105 hMCs were then stimulated with LPA (5 μM) or medium alone for 5 minutes. Reactions were stopped by incubating the samples on ice, and the cells were lysed in a buffer containing 1% SDS, 1% Triton X-100, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycotetraacetic acid), 0.2 mM (phenylmethylsulfonyl fluoride) PMSF, 5 μg/mL leupeptin, and 1 μg/mL pepstatin in 10 mM Tris (pH 8.0). Samples were loaded with an equal volume of Tris-glycine-SDS loading buffer (Novex) and were then electrophoresed and transblotted as detailed above. Membranes were probed with a 1:2000 dilution of a rabbit polyclonal antibody specific for phosphorylated Akt (Cell Signaling Technologies, Beverly, MA), then stripped and reprobed with the same dilution of a polyclonal antibody directed against total Akt (Cell Signaling, Bedford, MA). The same blots were sequentially probed with 1:2000 dilutions of antibodies against phosphorylated and total ERK, respectively (Cell Signaling).
Cell activation
hMCs derived in serum-free medium in the presence of LPA (5 μM) with SCF, IL-6, and IL-10 were incubated overnight in the presence of semipurified human myeloma IgE (Calbiochem, Tecaluma, CA) at 2 μg/mL. For some cell samples, recombinant human IL-4 (10 ng/mL; Endogen) had been added to the culture medium 4 days earlier to optimize expression of FcϵRI and to enhance exocytosis in response to FcϵRI cross-linkage.34 Cells were then washed extensively and stimulated in triplicate samples of 1 × 105 cells in 200 μL aliquots to the wells of 96-well, flat-bottom, tissue culture-treated microtiter plates (Falcon, Cowley, United Kingdom). Supernatants and pellets were collected at 30 minutes and separated by centrifugation, and histamine was measured in each by enzyme-linked immunosorbent assay (ELISA) (Immunotech, Westbrook, ME), as detailed previously.34 Data for each experiment were expressed as a percentage of release. The AIM V culture medium from the cells maintained in the presence of 5 μM LPA was assayed for the potential endogenously generated nerve growth factor (NGF)35 and vascular endothelial growth factor (VEGF) using commercial standard ELISAs according to the manufacturer's protocols (Promega, Madison, WI).
Proliferation and apoptosis
To assess basal proliferation, aliquots of cells (5 × 104) cultured for 3 to 4 weeks in serum-free medium with SCF, IL-6, and IL-10 and different concentrations of LPA (1.25-10 μM) were transferred into 96-well microtiter assay plates (Costar, Cambridge, MA), cultured for 5 additional days, and pulsed with 1 μCi (0.037 MBq) [3H]-thymidine overnight on day 5. Incorporated radioactivity was measured in triplicate samples for each concentration of LPA, and the results were expressed as mean ± SD. In some experiments, the effects of receptor-selective antagonists and signaling inhibitors on the LPA-dependent, comitogenic responses were assessed. For these studies, 3-week-old hMCs (more than 90% pure) developed in the presence of SCF, IL-6, IL-10, and LPA (5 μM) were washed into fresh AIM V medium containing SCF and LPA (5 μM) without IL-6 or IL-10, in the absence or presence of the LPAR1/LPAR3-selective antagonist VP-32179 (provided by Dr Kevin Lynch, University of Virginia, Charlottesville)31 at final concentrations of 5 and 10 nM. Some cell samples were treated overnight with PTX (100 ng/mL; Sigma) to interfere with Gi signaling, and some were treated for 1 hour with the selective inhibitor of PI3K, LY294002 (10 μM; Sigma), with the selective inhibitor of mitogen-activated extracellular signal-regulated protein kinase (MEK), UO126 (5 μg/mL), or with the PPAR-γ antagonist GW9662 (5 μM; Sigma). The cells were pulsed with thymidine overnight after 4 days of culture under these conditions in triplicate samples of 5 × 104 cells, and their incorporation was measured on the following day by scintillation. In these experiments, data were expressed as absolute net counts for each experimental condition after subtracting the basal incorporation (ie, incorporation by hMCs maintained in the presence of SCF but without LPA or inhibitors). Because LPA 18:1 may contain trace amounts of other constituents, such as di-oleoyl-phosphatidic acid that can act on LPAR4,20 comitogenic effects of LPA 18:1 at the optimal dose of 5 μM were compared with those of LPA 18:1 and 18:2 (both from Echelon Biosciences, Salt Lake City, UT). Rosiglitazone (10 μM) was used to assess the effect of PPAR-γ activation on the proliferation of hMCs. The effect of each compound on net proliferation over a 4-day period was expressed as a percentage of the control (ie, proliferation induced by LPA 18:1; Sigma). Comitogenic effects of TH2 cytokines were assessed by adding IL-3 (5 ng/mL), IL-4 (10 ng/mL), IL-5 (5 ng/mL), IL-6 (50 ng/mL), or IL-9 (50 ng/mL) to the 3-week-old hMCs maintained in AIM V medium with SCF (100 ng/mL). Cells were pulsed with [3H]-thymidine, as detailed above. on day 4. The trends for [3H]-thymidine incorporation were similar, but the absolute values varied considerably for cell cultures obtained from different donors. Thus, the results were normalized to the response to the control culture with SCF alone. Annexin V/PI staining was performed according to the manufacturer's protocol (Annexin V- FITC Apoptosis Kit; BD Pharmingen, San Diego, CA) at weeks 3 and 4.
Statistics
Differences between groups were assessed using the nonparametric analysis of variance (ANOVA). P less than .05 was considered significant.
Results
Effects of LPA on hMC development
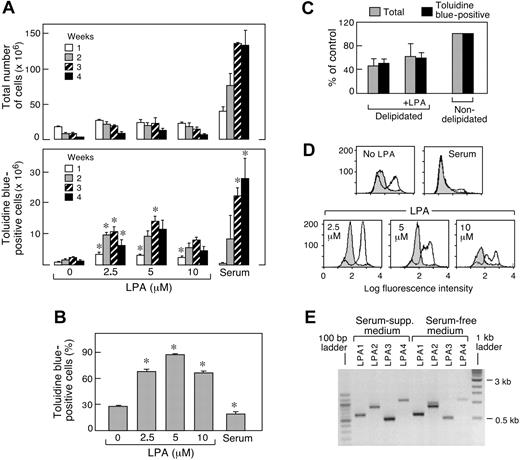
To study the potential effects of LPA on the development of hMCs, cultures of cord blood mononuclear cells were established in serum-free medium (to exclude endogenous serum-derived LPA), with the triad of recombinant SCF, IL-6, and IL-10.5 Cells were maintained in the absence or presence of LPA 18:1 (2.5-10 μM). Without LPA, the numbers of total cells decreased sharply, whereas the numbers of toluidine blue-positive cells increased by approximately 3-fold at 3 weeks before rapidly declining (Figure 1A). LPA 18:1 modestly increased the total numbers of cells in the cultures at each dose used but sharply increased the numbers of cells staining with toluidine blue at 2, 3, and 4 weeks (Figure 1A). The effect of LPA 18:1 on toluidine blue-positive cell numbers was statistically significant at doses of 2.5 μM and 5 μM, each producing approximately 10-fold increases, relative to cultures maintained in the absence of LPA at 3 weeks of culture. LPA 18:1 at 2.5, 5, and 10 μM significantly increased the proportion of cells staining positively for toluidine blue at 3 weeks (Figure 1B), with near homogeneity achieved at a concentration of 5 μM (averages, 13.9 ± 4 × 106 toluidine blue-positive cells and 16.9 ± 4 × 106 total cells; Figure 1A). Replicate cultures maintained with the same cytokine triad in conventional serum-supplemented medium contained higher total cell numbers than the serum-free cultures (Figure 1A) and greater total numbers of toluidine blue-positive cells but lower proportions of toluidine blue-positive cells compared with the LPA 18:1-supplemented samples at these early time points (Figure 1B). As reported previously,5 the conventional cultures required 6 to 9 weeks to reach homogeneity for toluidine blue staining (not shown). Medium supplemented with delipidated serum supported approximately 50% of the total and toluidine blue-positive cells resulting from replicate cultures maintained in normal serum for 3 weeks (Figure 1C). The addition of LPA 18:1 (5 μM) increased the numbers of total and toluidine blue-positive cells (each by approximately 30%), without altering purity in terms of the proportions staining with toluidine blue (Figure 1C). Supplementation with S1P (0.5-10 μM) slightly increased total cell numbers but did not increase toluidine blue-positive cells (n = 2; data not shown). The levels of potential endogenously generated growth factors NGF and VEGF in the culture medium obtained from 1-, 2-, and 3-week-old cultures were below the limits of detection (n = 2; data not shown).
Effect of LPA on the development of MCs from cord blood in serum-free medium in the presence of SCF, IL-6, and IL-10. (A) Cells maintained with the same cytokine triad and standard FCS-containing medium were included for comparison. (B) Data for week 3 in panel A are converted to the percentage of cells with toluidine blue granules in panel B and are the mean ± SEM from 6 separate experiments with cells from different donors. *P < .05. (C) Effect of serum delipidation and readdition of LPA on the numbers of total and toluidine blue-positive cells at week 3 of culture in serum-supplemented medium. Data are expressed as the percentage of control (nondelipidated serum supplementation) and are the mean ± half-range for 2 experiments. (D) Histograms depict cell surface Kit expression (unshaded tracings) and negative control antibody (shaded tracings). Data are representative of 4 experiments. (E) RT-PCR analysis of mRNA encoding LPA1R, LPA2R, LPA3R, and GPR23/LPA4R from cord blood-derived hMCs developed for 9 weeks in serum-supplemented medium or for 3 weeks in serum-free medium containing 5 μM LPA. Data are from 1 of 2 representative experiments.
Effect of LPA on the development of MCs from cord blood in serum-free medium in the presence of SCF, IL-6, and IL-10. (A) Cells maintained with the same cytokine triad and standard FCS-containing medium were included for comparison. (B) Data for week 3 in panel A are converted to the percentage of cells with toluidine blue granules in panel B and are the mean ± SEM from 6 separate experiments with cells from different donors. *P < .05. (C) Effect of serum delipidation and readdition of LPA on the numbers of total and toluidine blue-positive cells at week 3 of culture in serum-supplemented medium. Data are expressed as the percentage of control (nondelipidated serum supplementation) and are the mean ± half-range for 2 experiments. (D) Histograms depict cell surface Kit expression (unshaded tracings) and negative control antibody (shaded tracings). Data are representative of 4 experiments. (E) RT-PCR analysis of mRNA encoding LPA1R, LPA2R, LPA3R, and GPR23/LPA4R from cord blood-derived hMCs developed for 9 weeks in serum-supplemented medium or for 3 weeks in serum-free medium containing 5 μM LPA. Data are from 1 of 2 representative experiments.
Effects of LPA on cell surface expression of Kit
Cultured cells at 3 weeks were stained with mouse monoclonal antibodies against Kit, CD13, and CD14 and were analyzed cytofluorographically. The LPA 18:1-treated cultures contained a higher proportion of cells staining positively for Kit than did replicates maintained under serum-free conditions without LPA 18:1 (48% ± 7% Kit positive without LPA vs 76% ± 4% Kit positive in the presence of 5 μM; P equals .0002, as shown for 1 experiment; Figure 1D). Generally, the highest percentages of cells exhibiting surface Kit expression were observed at concentrations of 2.5 μM LPA 18:1 (Figure 1D), whereas some suppression of Kit was observed at higher concentrations of LPA 18:1. Negligible Kit expression was detected in the cells derived under serum-supplemented conditions (Figure 1D); this did not change with serum delipidation or adding back LPA (n = 2; not shown). The 3-week-old cells derived in the presence of 5 μM LPA 18:1 also showed high uniform staining for CD13 and no staining for CD14 (not shown). mRNA transcripts for all 4 LPARs were detected in hMCs derived in serum-free medium with 5.0 μM LPA 18:1 at 3 weeks and in hMCs derived with serum at 9 weeks (Figure 1E).
Effects of LPA on granule content
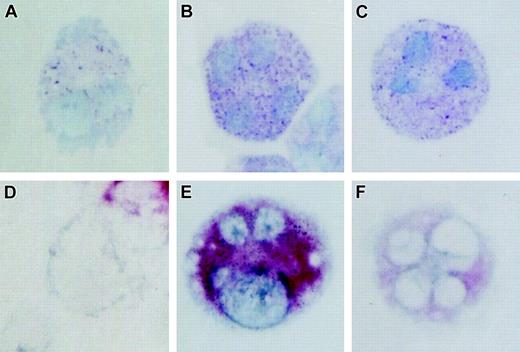
At 3 weeks, LPA 18:1 increased the proportion of tryptase-positive cells in a dose-dependent fashion (Figure 2), paralleling the increase for metachromasia (Figure 1B). Treatment with LPA 18:1 at 5 μM increased the proportion of tryptase-positive cells from approximately 30% to approximately 90% compared with LPA-free controls. LPA 18:1 dramatically up-regulated the cellular content of α/β tryptases, as demonstrated by immunoblotting (n = 3, as shown for 1 experiment; Figure 2). Quantitative densitometry revealed a 10- ± 1-fold increase in tryptase content at 2.5 and 5 μM LPA 18:1 (n = 2; data not shown). Immunostaining at 3 weeks revealed stronger staining for tryptase in individual cells in the cultures treated with LPA 18:1 (Figure 3D-F) compared with replicate cells that were maintained in its absence or with controls maintained in serum-supplemented conditions. The LPA 18:1- treated cells also contained granules that stained more strongly with toluidine blue than did the granules in the cells maintained in the absence of LPA. In contrast to tryptase, LPA 18:1 did not induce chymase or cathepsin G expression, as detected either by immunoblot or immunostaining (not shown). Furthermore, only approximately 10% of the cells grown under serum-free conditions were positive for chloroacetate esterase (an index of chymase activity), regardless of LPA 18:1 supplementation (n = 2; not shown).
Dose-dependent effect of LPA supplementation on cell tryptase expression. SDS-PAGE immunoblot (top) showing immunoreactivity for tryptase with an antibody that recognized α- and β-human tryptases in whole cell lysates prepared from 1 × 105 cells developed for 3 weeks in serum-free medium of SCF, IL-6, and IL-10, with or without the indicated concentrations of LPA. Data are from 1 of 3 representative experiments. Percentages of cells exhibiting tryptase immunoreactivity (bottom) with the same antibody are shown as the mean ± SEM from 3 experiments performed. *P < .05.
Dose-dependent effect of LPA supplementation on cell tryptase expression. SDS-PAGE immunoblot (top) showing immunoreactivity for tryptase with an antibody that recognized α- and β-human tryptases in whole cell lysates prepared from 1 × 105 cells developed for 3 weeks in serum-free medium of SCF, IL-6, and IL-10, with or without the indicated concentrations of LPA. Data are from 1 of 3 representative experiments. Percentages of cells exhibiting tryptase immunoreactivity (bottom) with the same antibody are shown as the mean ± SEM from 3 experiments performed. *P < .05.
Effect of LPA on histochemical characteristics of hMCs. (A-C) Toluidine blue staining and (D-F) immunocytochemistry for tryptase of representative hMCs developed in the presence of SCF, IL-6, and IL-10 in serum-free medium (A, D) without and (B, E) with 5.0 μm LPA, and (C, F) under standard serum-supplemented conditions. All cultures were 3 weeks old at the time of analysis. Data are from 1 of 2 representative experiments. Images depicted are 400 × magnifications.
Effect of LPA on histochemical characteristics of hMCs. (A-C) Toluidine blue staining and (D-F) immunocytochemistry for tryptase of representative hMCs developed in the presence of SCF, IL-6, and IL-10 in serum-free medium (A, D) without and (B, E) with 5.0 μm LPA, and (C, F) under standard serum-supplemented conditions. All cultures were 3 weeks old at the time of analysis. Data are from 1 of 2 representative experiments. Images depicted are 400 × magnifications.
Effects of LPA on proliferation and apoptosis
hMCs developed for 3 weeks in the presence of 5.0 μM LPA 18:1 proliferated at a rate approximately 4-fold higher than did replicates developed without LPA (Figure 4A). When these 3-week-old cultures maintained in the presence of LPA were transferred to fresh AIM V medium with SCF to maintain viability and basal proliferation, LPA 18:1 at 5 μM increased their proliferative rate by approximately 1000 cpm above that observed in replicates maintained in its absence for 4 days (Figure 4B). The comitogenic effect of LPA 18:1 was inhibited by the LPAR1/LPAR3-selective antagonist VP-32179 in a concentration-dependent manner, with virtually complete inhibition at 10 nM (Figure 4B). PTX pretreatment also inhibited the LPA effect (Figure 4B). The PPAR-γ antagonist GW9662 also decreased the LPA-mediated increment in proliferation, approaching statistical significance (P = .07; n = 5; Figure 4B). Neither VP-32179 nor PTX nor GW9662 altered the levels of thymidine incorporated by the cells maintained in SCF without LPA (n = 5; not shown). LY29004, a PI3K inhibitor, and UO126, a MEK/ERK inhibitor, markedly decreased proliferation, with or without LPA stimulation. When used at 5 μM, LPA 18:1 (Echelon Biosciences) induced 52% ± 30% of the proliferation rate observed with the Sigma LPA 18:1 at this dose. The proliferative responses to LPA 18:2 at 2.5 μM (82% ± 68%) and 5 μM (97% ± 91%) were indistinguishable from those occurring in response to Sigma LPA 18:1 (n = 5 for each condition; data not shown). Rosiglitazone also modestly increased proliferation (53% ± 20% of the response to LPA). LPA 18:1 was not cytoprotective because approximately 50% of the cells under all serumfree conditions were positive for Annexin V, PI, or both at weeks 3 and 4, irrespective of LPA concentration or purity (n = 3; not shown). Akt and ERK MAPK exhibited constitutive phosphorylation under these culture conditions, with minimal to negligible increments in response to LPA (n = 2; data not shown). LPA alone did not support proliferation in the absence of SCF (n = 2; not shown).
LPA-mediated proliferation of hMCs. (A) Effect of development with LPA at the indicated concentrations on basal proliferation of cells at 3 (•) and 4 (○) weeks of culture in serum-free medium with SCF, IL-6, and IL-10. Thymidine incorporation is expressed as the mean ± half-range for 2 experiments. (B) Comitogenic effects of LPA on 3-week-old hMCs developed in the presence of LPA and transferred to fresh AIM V medium in the presence of SCF with LPA (5 μM) in the absence or presence of the indicated inhibitors. Results depicted are the mean ± SEM from 5 experiments. *P < .05.
LPA-mediated proliferation of hMCs. (A) Effect of development with LPA at the indicated concentrations on basal proliferation of cells at 3 (•) and 4 (○) weeks of culture in serum-free medium with SCF, IL-6, and IL-10. Thymidine incorporation is expressed as the mean ± half-range for 2 experiments. (B) Comitogenic effects of LPA on 3-week-old hMCs developed in the presence of LPA and transferred to fresh AIM V medium in the presence of SCF with LPA (5 μM) in the absence or presence of the indicated inhibitors. Results depicted are the mean ± SEM from 5 experiments. *P < .05.
Functionality of hMCs derived in the presence of LPA
At 3 weeks, cells derived in serum-free medium supplemented with 5 μM LPA (consisting of nearly pure hMCs) were sensitized with IgE and challenged with anti-IgE to assess their capacity for activation and exocytosis of histamine. Additionally, these hMCs were studied for their characteristic responses to TH2 cytokines, including priming by IL-4 for IgE-dependent exocytosis. The hMCs derived in the presence of LPA in serum-free medium for 3 weeks contained 2.8 ± 0.3 μg histamine/106 cells and released small amounts (6% ± 3% net release; n = 3) when challenged with FcϵRI cross-linkage. Priming with IL-4 for 5 days increased IgE-dependent release of histamine (20% ± 4% release; P = .02; n = 3; data not shown), without altering histamine content. Background release was 5% or less. Cells grown for 3 weeks in serum-free medium without LPA released histamine only after priming with IL-4 (net release, 8% ± 4%). hMCs derived in the presence of LPA also proliferated above the baseline of cells cultured in SCF alone in response to IL-3, IL-9, IL-6, and IL-4 (Figure 5A). With the exception of IL-4, each cytokine also increased numbers of hMCs compared with SCF with or without LPA (Figure 5A). IL-5 caused no proliferation of hMCs derived in the absence of serum (Figure 5A) but did maintain increased cell numbers relative to replicates treated with SCF alone and was comitogenic for cells in 3-week-old cultures derived in serum from the same donors (data not shown). Under each condition, the hMCs retained their metachromasia and tryptase staining (not shown) and their Kit expression (Figure 5B), with the highest and most uniform staining in the IL-6 and IL-9 groups. IL-9, but not the other cytokines, induced strong CAE staining in a fraction of cells (51% ± 2% vs less than 25% in all other groups; n = 2; data not shown).
TH2 cytokine-mediated comitogenesis and phenotypic analysis. (A) hMCs developed for 3 weeks in the presence of SCF, IL-6, and IL-10 in serum-free medium containing 5 μM LPA were washed into new medium containing SCF and the indicated additives. [3H]-Thymidine incorporation (top) was measured after 3 days. Results are the mean ± half-range from 2 experiments and are expressed as a percentage increase over the baseline of SCF alone. Cell numbers (bottom) relative to SCF alone at 3 days from the same 2 experiments are displayed. (B) Cytofluorographic analysis for Kit at day 3 of comitogenic stimulation. Data are from 1 of 2 representative experiments. Histograms depict cell-surface kit expression (unshaded tracings) and negative control antibody (shaded tracings). Percentages of Kit-positive cells from this experiment are noted above each corresponding histogram.
TH2 cytokine-mediated comitogenesis and phenotypic analysis. (A) hMCs developed for 3 weeks in the presence of SCF, IL-6, and IL-10 in serum-free medium containing 5 μM LPA were washed into new medium containing SCF and the indicated additives. [3H]-Thymidine incorporation (top) was measured after 3 days. Results are the mean ± half-range from 2 experiments and are expressed as a percentage increase over the baseline of SCF alone. Cell numbers (bottom) relative to SCF alone at 3 days from the same 2 experiments are displayed. (B) Cytofluorographic analysis for Kit at day 3 of comitogenic stimulation. Data are from 1 of 2 representative experiments. Histograms depict cell-surface kit expression (unshaded tracings) and negative control antibody (shaded tracings). Percentages of Kit-positive cells from this experiment are noted above each corresponding histogram.
Discussion
Although TH2 cytokines orchestrate baseline and hyperplastic MC development at mucosal surfaces, reactive mastocytosis without an obvious TH2 component occurs in chronic inflammation,8,9 certain malignancies,36 and interstitial cystitis.37 Reactive mastocytosis was also a prominent pathologic feature in a T cell-independent model of autoantibody-induced arthritis and was required for the induction of synovitis.11 We thus sought a pathway for the costimulation of SCF-dependent MC growth that might be operative in such circumstances. Serum lysophospholipids were possible candidates because of their known effects on the proliferation of other cell types and their high levels in pathologic conditions.16
Serum-free culture conditions accelerate SCF-dependent MC development in limited numbers from human CD34+ progenitors from cord blood38 and are required for MC growth from peripheral blood-derived progenitors.39 Furthermore, fully differentiated human skin MCs proliferate in SCF-supplemented cultures only when serum is absent.30 Although MCs exhibit different responses to certain exogenous stimuli, depending on their source of origin and stage of development, all human MCs studied to date exhibit common features of SCF-dependent proliferation and comitogenic responses to certain cytokines. Therefore, we used the well-characterized cord blood-based culture system to study the effect of LPA. Cord blood mononuclear cells maintained in a serum-free medium with the triad of SCF, IL-6, and IL-10 increased in number more slowly than replicate cultures maintained in conventional serum-supplemented medium (Figure 1A) and showed modestly higher numbers of cells with MC granule markers (toluidine blue staining) and surface markers (Kit) at these early time points. By week 3, the numbers of toluidine blue-positive cells in these serum-free cultures without LPA had increased by approximately 3-fold relative to week 1 (despite a decrease in total cell number), declining thereafter because of cell death. Adding LPA at concentrations well within the range reported for normal serum15 and biologic fluids at inflammatory foci or malignancy selectively stimulated a 10-fold increase in toluidine blue-positive cells having high cytofluorographic levels of Kit and CD13 and lacking staining for CD14, a profile typical for MCs. In serum-supplemented cultures, delipidation resulted in decreased total and metachromatic cell numbers (Figure 1C). Adding LPA 18:1 at 5 μM did not completely compensate for the depleted factors, which likely included a complex mixture, including other factors that sustained MC viability, proliferation, or both. hMCs, whether derived in AIM V medium with LPA or under standard conditions, expressed mRNA transcripts for all the known LPARs (Figure 1E). The striking dose-dependent increments in hMCs observed in response to LPA, combined with the expression of LPAR transcripts, suggested that LPA could act through a receptor-mediated mechanism to stimulate MC development, especially when other constituents of serum were absent.
The effects of LPA on metachromatic granule development (Figure 1B) and Kit expression (Figure 1D) prompted us to examine its effect on the expression of MC-specific proteases. The proportion of cells staining for tryptase at 3 weeks was virtually superimposable with the proportion exhibiting toluidine blue staining (Figure 2). Even at concentrations as low as 1.25 μM, LPA increased the content of tryptase per cell, as determined by SDS-PAGE immunoblotting. Indeed, the 10-fold increase in signal intensity detected by densitometry likely exceeded the linear range of the assay used and, thus, underestimates the effect of LPA on cellular tryptase content. The comparative intensity of tryptase immunoreactivity in individual cells (Figure 3D-F) confirms that LPA increased the amount of tryptase per cell. This increase was paralleled by stronger metachromatic staining (Figure 3A-C), an index of proteoglycan content. Because proteoglycans regulate the proper processing of proteases in MC granules,40 LPA may regulate tryptase expression directly or through a posttranslational proteoglycan-dependent mechanism. In contrast to tryptase, LPA did not induce chymase or cathepsin G expression, as detected either by immunoblot or immunostaining (not shown), and did not alter the low numbers of cells exhibiting staining for chloroacetate esterase, an index of chymase activity. Hence, LPA selectively affects the packaging of tryptases and their cognate proteoglycans in secretory granules.
Soluble growth factors can increase cell numbers through proliferation, cytoprotection, or both. LPA is either proproliferative or antiproliferative for other cell types, depending on the concentration used and the receptor complex expressed by the cell.41,42 hMCs developed for 3 weeks in the presence of 5.0 μM LPA 18:1 proliferated at a rate approximately 4-fold higher than did replicates developed without LPA (Figure 4A). Although LPA 18:1 obtained from an alternative source was less potent for stimulating comitogenesis than the preparations of LPA 18:1 used for most of the experiments, LPA 18:2, the dominant LPA species associated with normal serum, was equipotent. Each of the LPARs, when cloned and expressed heterologously, binds LPA at concentrations well below the range required to increase hMC numbers in our study.17-20 Nonetheless, LPAR-mediated cellular responses in vitro often require doses in the micromolar range, as recently demonstrated for actin polymerization and calcium flux in human eosinophils.43 Because LPA also activates PPAR-γ when used at this concentration range,24 an alternative or additional contribution from this pathway was also possible. The comitogenic effect of LPA 18:1 was blocked by the potent LPAR1/LPAR3 dual-specific antagonist VP-32179 and was inhibited by PTX (Figure 4B), whereas basal SCF-driven proliferation was unaffected by these antagonists. These observations support the involvement of LPAR1, LPAR3, or both receptors acting through Gi proteins to initiate signaling essential for LPA to mediate the proliferation of hMCs. PI3K-dependent phosphorylation of Akt and of ERK is a downstream effect of this pathway in other cell types,44 though the high levels of basal Akt and ERK phosphorylation in this system confounded analysis. This is likely because of the actions of SCF, which is required to maintain MC viability and which elicits strong PI3K activation through Kit.45 In addition to the membrane receptor-mediated responses to LPA, a simultaneous contribution from the recently identified LPA/PPAR-γ pathway is suggested by the inhibitory effect of GW9662 and by the modest direct stimulatory effect of rosiglitazone. Indeed, the activity of PPAR-γ, which initiates apoptosis in other cell types,27 could be responsible for the fact that LPA was not cytoprotective. This lack of cytoprotection could have physiologic relevance because it may ensure limitation of the duration of any LPA-dependent MC hyperplasia that might occur in vivo. Although LPA can also act by inducing the production of endogenous growth factors such as VEGF in some cell types,46 we found no evidence that it elicited the generation of VEGF or NGF in our study. We cannot exclude other potential autocrine-acting factors. Whether LPA can induce the transactivation of Kit, as it does for other RTKs such as the receptors for epithelial growth factor (EGF)47 or platelet-derived growth factor,48 remains to be determined.
MCs characteristically undergo exocytosis in response to FcϵRI cross-linkage, releasing histamine and other bioactive mediators central to their effector function. Several TH2 cytokines support the proliferation and/or cytoprotection of MCs and their progenitors, an essential function in antihelminthic immunity.49 hMCs derived from cells cultured for 3 weeks in serum-free medium with LPA were fully functional, as revealed by the IgE-mediated release of histamine, a characteristic priming of this response by IL-4, and proliferation above the baseline of cells cultured in SCF alone in response to IL-3, IL-9, IL-6, and IL-4 (Figure 5B). With the exception of IL-4, which induces the apoptosis of mouse50 and human MCs,51 each cytokine also increased hMC numbers compared with SCF with or without LPA (Figure 5A). Surprisingly, IL-5, the most potent comitogen for mature (9 weeks) and progenitor (4 weeks) hMCs in our earlier studies,5 caused no proliferation of hMCs derived in the absence of serum (Figure 5A). Thus, the effect of IL-5 on the preservation of cell numbers likely reflects its known cytoprotective properties.6 Interestingly, IL-9 induced strong CAE staining in approximately 50% of the cells, suggesting that it up-regulates the expression or function of chymases, analogous to its actions on mouse MCs.52 Although hMCs developed with LPA retain comitogenic responses to TH2 cytokines, their contrasting responses to IL-5 and IL-4 imply that several microenvironmental variables (including additional serum-associated factors) likely determine the duration of local mastocytosis and suggest diversity of cytokine responsiveness among MC subpopulations.
LPA, the first lipid growth factor identified for MCs, could mediate direct and indirect effects on MC proliferation and differentiation. LPA-driven MC hyperplasia in vivo would likely be self-limited because LPA does not facilitate survival. The ability of LPA to simultaneously induce proliferation and maturation of hMCs is distinct from certain other nonprotein factors, such as plant flavinoids, that are reported to induce maturation in transformed MC lines while they suppress proliferation.53 This unique property could reflect the ability of LPA to concomitantly stimulate cell surface-associated LPARs and PPAR-γ, thus inducing multiple pathways of gene expression. We speculate that this mechanism may operate in MC hyperplasia at foci of subacute chronic inflammation and fibrosis,8-12 metastasizing tumors,36 and atherosclerotic plaques,54 where local LPAconcentrations are likely to be high.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-03-1166.
Supported by National Institutes of Health grants AI-48802, AI-52353, AI-31599, and HL-36110 and by grants from the Charles Dana Foundation, the Vinik Family Fund for Research in Allergic Diseases, and the Hyde and Watson Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Figure 5. TH2 cytokine-mediated comitogenesis and phenotypic analysis. (A) hMCs developed for 3 weeks in the presence of SCF, IL-6, and IL-10 in serum-free medium containing 5 μM LPA were washed into new medium containing SCF and the indicated additives. [3H]-Thymidine incorporation (top) was measured after 3 days. Results are the mean ± half-range from 2 experiments and are expressed as a percentage increase over the baseline of SCF alone. Cell numbers (bottom) relative to SCF alone at 3 days from the same 2 experiments are displayed. (B) Cytofluorographic analysis for Kit at day 3 of comitogenic stimulation. Data are from 1 of 2 representative experiments. Histograms depict cell-surface kit expression (unshaded tracings) and negative control antibody (shaded tracings). Percentages of Kit-positive cells from this experiment are noted above each corresponding histogram.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-03-1166/6/m_zh80240470620005.jpeg?Expires=1765928864&Signature=qwWYjCcl1xSMZgH7B01Aavg9zew21xrVWN6lpUiBdizT3hS5T0H8cwU3ogLCdt83w8aqLCtX4c6YcMtYNuVvvmZwePigjEsF~XDz5x8EN3I5l-zf0Cj0i1780hwvd0k~jWLDImKep5~ck4FTfKo1QWBtpXlic23unr0GmZOpxAl7-N4BeoJo8tCZo6iZvCNACIY-QlCkeAivLz2jcVOxFv5m3h8YtDpflTWO3ZvHxqD6tqKHmsmnYYD01v~DoECs0eJo9NbVd0VvKEGiOC~OtmQKFZCOJVf2zlfmnwdvW~CaHry-n25So2JKBAfxWvbS5eu9Gd9dT1ImEIcb5WtIBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal