Abstract
Platelet-restricted β1 tubulin is required for optimal thrombopoiesis and discoid cell shape. To identify interacting factors, we used the divergent β1-tubulin C-terminus as the bait in a yeast 2-hybrid screen of megakaryocyte (MK) cDNAs. We isolated secretory leukocyte protease inhibitor (SLPI), a serine protease antagonist characterized principally as a secreted factor with multiple roles in inflammation. SLPI is expressed in MKs and platelets in 2 discrete compartments. One pool resides in punctate cytoplasmic structures, whereas a significant fraction localizes along peripheral microtubules (MTs) and is lost with cold-induced MT disruption or in β1 tubulin-/- platelets. These findings reveal unexpected interaction between a prominent cytoskeletal protein and an inhibitor of proteolysis. SLPI-/- mice show intact proplatelet formation, platelet numbers and shape, and marginal MT bands; thus, SLPI is not essential for thrombopoiesis. However, SLPI is released upon platelet activation, which also reverses its association with the resting marginal band. Platelet SLPI inhibits neutrophil elastase, an activity that is reduced when β1 tubulin is absent. We conclude that SLPI localizes in part along the MK and platelet MT cytoskeleton by virtue of specific interactions with β1 tubulin. SLPI may thus have unanticipated roles in MK and platelet functions, including regulated proteolysis after activation. (Blood. 2004;104:3949-3957)
Introduction
Mature megakaryocytes (MKs) release platelets through intermediate structures known as proplatelets.1,2 Elaboration of proplatelets is driven by microtubules (MTs), which line the proplatelet shaft and coil at the ends where nascent blood platelets are assembled.3-5 Circulating platelets carry a single marginal MT coil that is wound in 8 to 12 turns and is responsible for the cell's discoid shape.6,7 β1 tubulin, the major β-tubulin isoform expressed in proplatelet and platelet MTs, is the product of a MK- and platelet-specific gene8,9 and is required for optimal platelet assembly; in its absence, mice are thrombocytopenic, and circulating platelets are spherical instead of the typical elliptical shape.7 In contrast to the 4 other mammalian β-tubulin genes, which share more than 95% amino acid identity, β1 tubulin shows less than 80% sequence conservation, with the highest divergence concentrated in the C-terminus.8 This region encodes 2 α-helices that are exposed on the surface of polymerized MTs10,11 and believed to interact with MT-associated proteins (MAPs) that help mediate assorted MT functions.12
To identify additional factors that may be important for platelet biogenesis or function, we sought to isolate proteins that bind specifically to the β1-tubulin C-terminus. In a yeast 2-hybrid screen using this domain as the bait, we identified the secretory leukocyte protease inhibitor SLPI as a protein that interacts selectively with the β1 isoform. SLPI has been characterized extensively as a secreted protein with potent inhibitory activity against serine proteases such as elastase, cathepsin G, trypsin, and chymase.13,14 SLPI mRNA expression is highest in the lung and spleen,15 and besides a well-characterized 12-kDa secreted form, an intracellular full-length protein of 14.3-kDa mass is reported in leukocytes.16,17 SLPI 3-dimensional structure reveals a boomerang shape formed by 2 homologous 53-residue domains.18 An N-terminal defensin-like domain can bind proteoglycans and diplays antimicrobial properties,14 whereas the C-terminal half harbors protease inhibitory activity. Additionally, SLPI has a plethora of independent functions, and mice with targeted gene disruption reveal essential roles in wound healing and in balanced innate immunity.19,20
Here we characterize SLPI as a factor that interacts selectively with the β1 tubulin isoform and is expressed in 2 discrete compartments in MKs and platelets, including in association with the platelet marginal MT band. Despite these associations, which depend on the presence of β1 tubulin, SLPI-deficient mice produce platelets in normal numbers and lack the abnormalities characteristic of β1 tubulin-/- platelets. However, SLPI's association with the marginal MT coil is lost upon platelet activation, and activated platelets display a dose-dependent elastase inhibitory activity that is reduced or lost in mice lacking either SLPI or β1 tubulin. Taken together, our findings reveal an intracellular MT-associated SLPI form in blood platelets and suggest that platelet MTs may play a role in regulating aspects of proteolysis.
Materials and methods
Yeast 2-hybrid screen
A cDNA corresponding to the C-terminal 131 amino acids of mouse β1 tubulin (XP_283812.2) or 124 C-terminal amino acids of β5 tubulin (NP_035785) was cloned into the EcoRI and SalI sites in pGBKT7 (Matchmaker System, Clontech, Palo Alto, CA). Plasmids were introduced into the parental AH109 yeast strain, positive clones were selected by growth on Trp- medium, and expression of GAL4 DNA-binding domain fusion proteins was verified by immunoblotting. A cDNA library from cultured mouse MKs was generated separately by MK enrichment over 2 consecutive bovine serum albumin (BSA; 1.5%-3%) gradients to deplete contaminating cells below 5%. An enriched polyA+ RNA fraction was reverse transcribed using superscript Choice System (Gibco BRL, Bethesda, MD) and size fractionated. Adapter-ligated double-stranded cDNA was cloned into pGADT7 prey vector (Clontech), yielding 5 × 105 independent clones with most inserts between 0.5 kilobase (kb) and 3 kb. This library was introduced into the AH109/β1-tubulin yeast strain, followed by selection on Trp-Leu-His- medium. Screening of 5 × 106 transformants yielded 110 clones that activated 3 independent reporter genes (histidine, adenine, and β-galactosidase) and were subjected to PCR-based sequencing.21
Animals and megakaryocyte culture
p45 NF-E2, β1 tubulin, and SLPI mutant mice were maintained as described.7,19,22 Fetal livers were recovered from mouse embryos between the thirteenth and fifteenth gestation days. A single-cell suspension was prepared by successive passages through 18- and 21-gauge needles and cultured in Dulbecco modified Eagle medium (DMEM, Gibco) supplemented with 10% fetal bovine serum, 2 mL L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and Tpo. On culture day 4 or 5 cells were separated into populations enriched or depleted for mature MKs over a 1.5% to 3% one-step BSA gradient over 30 minutes, washed, concentrated and processed for isolation of RNA, as described in the next paragraph.
Semiquantitative RT-PCR
Total RNA was isolated from enriched MK fractions using Trizol (Carlsbad, CA), reverse-transcribed with oligo-(dT) primers, and analyzed by polymerase chain reaction (PCR) in the linear range of amplification as described previously.23 Tracer α[32P]dCTP was included in the reaction mix, and products were analyzed by polyacrylamide gel electrophoresis. The following primers (5′ to 3′) were used at an annealing temperature of 62°C: SLPI, CCTGCGGCCTTTTACCTTTC and GCTTCCTCCACACTGGTTTG; hypoxanthine phosphoribosyl transferase (HPRT), CACAGGACTAGAACACCTGC and GCTGGTGAAAAGGACCTCT; and p45 NF-E2, AACCTTGCCGGTAGATGACTTTAAT and CACCAAATACTCCCAGGTGATATG.
Determination of platelet counts and shape
Blood for all studies was withdrawn from anesthesized mice by retro-orbital venipuncture. Platelet counts were determined in whole blood diluted 1:2 in isotonic saline solution (Hematall, Fisher Scientific, Pittsburgh, PA) and analyzed with an automated whole blood counter (Technicon H-1, Technicon Instruments, Tarrytown, NY), as described previously.22
Platelets were enriched by sequential centrifugation at 100g and 250g, incubated with 1 μM prostaglandin E1 (Sigma, St Louis, MO), pelleted gently, resuspended in platelet buffer, and fixed for 20 minutes in 1% glutaraldehyde. Platelet samples were transferred onto a coverslip chamber for differential interference contrast (DIC) microscopy (Zeiss Axiovert 200M) with a 100 × objective lens (DIC 1.4 N.A). Pictures were acquired at room temperature with an Orca II camera (Hamamatsu, Bridgewater, NJ) using Metamorph Imaging Software (Universal Imaging, Downingtown, PA). For scanning electron microscopy (SEM), fresh platelets were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3, dehydrated in a graded series of ethanol, and treated with hexamethyldisilazane (Electron Microscopy Sciences, Fort Washington, PA) for 1 hour, followed by cytocentrifugation onto poly-L-lysine-coated coverslips. Cells were coated with a Au-Pd target for 6 minutes using a Hummer V sputter-coater, mounted on microscopy platforms, and inspected in a Leo 1450VP scanning electron microscope with an Inca 3000 microanalyzer at 15 kV.
Whole blood and plasma clotting time
Whole blood and plasma clotting time were determined as described previously,24 using a Sienco Sonoclot Coagulation and Platelet Function Analyzer (Sienco, Wheat Ridge, CO). Onset time and rate of coagulation were determined using Firmware software. 280 μL of whole blood was recalcified by adding 20 μL 150 mM CaCl2. Plasma was generated by additional centrifugation at 15 000g to remove contaminating cells. Clotting time was induced under stirring conditions (800 rpm) in an aggregometer (Sienco) at 37°C by adding an equal volume of prewarmed 20 mM CaCl2 to the plasma in a siliconized tube.
Platelet stimulation
Thrombin was added to platelet suspensions for 5 minutes at room temperature. The suspension was centrifuged for 3 minutes at 1000g, the supernatant transferred in a new vial, and the pellet resuspended in sample buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 6.8, 10% glycerol, 1% sodium dodecyl sulfate, 5% 2-mercapto-ethanol, 10 mM EGTA (ethylene glycol tetraacetic acid), 0.002% bromphenol blue). The supernatant was mixed with 4 × sample buffer, yielding similar salt concentrations. Samples were analyzed on a 13.5% polyacrylamide gel.
Immunoblot analysis
Platelet pellets were lysed in prechilled lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 5 mM EDTA [ethylene diamine tetraacetic acid], 150 mM NaCl, 1% Triton X-100) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL each of aprotinin, leupeptin, and pepstatin A (Sigma) for 30 minutes on ice and centrifuged at 15 000g at 4°C for 20 minutes. Protein concentration in the supernatant was determined using a protein kit (BioRad, Hercules, CA). Ten micrograms of protein was used per lane after boiling in Laemmli sample buffer for 3 minutes and resolved by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto polyvinylidenefluoride (PVDF) membranes in transfer buffer (25 mM Tris-HCl, pH 7.5, 192 mM glycine, 30% methanol) at 100 V for 1 hour. The membrane was blocked in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Tween-20 (TBS-T) supplemented with 4% (w/v) BSA for 1 hour prior to addition of SLPI (1:2000, Oncogene, San Diego, CA), PF4 (Peprotech, Rocky Hill, NJ), or RAMPS (a kind gift from T. Macdonald and C. Jackson, St Jude Hospital, Memphis, TN) antibody. Membranes were washed 3 times with TBS-T and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz, CA) for 1 hour in TBS-T, and washed again. Signals were developed with an enhanced chemoluminescence kit (Amersham, Piscataway, NJ).
Retrovirus production and MK transduction
Native or GFP-fused cDNAs were cloned into pWZL plasmids kindly provided by C. Furman and T. Roberts (Boston, MA). Retrovirus production followed published protocols.25 Briefly, Epstein-Barr nuclear antigen (EBNA)-episomal transfected 293 packaging cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), initially in the presence of 400 μg/mL G418 (Gibco), to 30% to 50% confluency. Triple-transfection was performed by calcium phosphate precipitation with 5 μg each of plasmids encoding gag/pol, vsvG, and the β1-tubulin fused with EGFP in the pWZL vector. After medium exchange the following day, cells were incubated for 48 to 60 hours for virus production. The supernatant was filtered through a 0.45-μm filter, and aliquots were stored at -80°C. MKs isolated from fetal liver cultures as described above were resuspended in DMEM containing FBS, 5 μg/mL polybrene (Sigma), and the retroviral supernatant. 24 hours later, this medium was replaced, and MKs were cultured for another 2 days prior to analysis.
Immunofluorescence and deconvolution microscopy
MKs were cytocentrifuged onto poly-l-lysine-coated coverslips, air-dried, fixed in 4% formaldehyde for 15 minutes, permeabilized with 0.5% Triton X-100 in PBS for 3 minutes, and blocked with 1% goat serum (Gibco, Grand Island, NY) in PBS for 30 minutes. Slides were stained by incubating with rabbit SLPI antiserum (Oncogene Research Products, San Diego, CA) for 20 minutes, washed 3 times with PBS, and stained with Texas rRed-conjugated goat-anti-rabbit IgG (Jackson Laboratories, West Grove, PA). Nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI) (final concentration 0.5 μg/mL) (Sigma) in the final wash step prior to mounting the cells with Fluoromount-G (Southern Biotech, Birmingham, AL). Resting platelet suspensions were fixed with an equal volume of 8% formaldehyde for 15 minutes and deposited on poly-l-lysine-coated coverslips, either without or after stimulation with thrombin as described.26 Immunostaining was performed using antibodies against SLPI, von Willebrand factor (Diagnostica Stago, Asnieres, France), serotonin (Abcam, Cambridge, United Kingdom), LAMP2 (CD107b, BD Biosciences, San Diego, CA), β tubulins (Sigma, clone 2-28-33), or GP IIb/IIIa and corresponding fluorophore-conjugated goat secondary antibodies (Jackson Laboratories). Coverslips were analyzed on an Olympus IX70 inverted fluorescence microscope at room temperature. Pictures were acquired using a CM350 CCD camera (Applied Precision, Issaquah, WA) using 60 × (Olympus PLAN-APO 1.40NA, 0.10 mm WD) or 100 × (PLAN-APO 1.40NA, 0.10 mm WD) oil objectives. Five to 50 cross-sections were taken at 0.2-μm spacing, and images were deconvolved using 10 cycles with the Ratio method, as provided by DeltaVision software (Applied Precision, Issaquah, WA) and as described recently.27 For some images the contrast was enhanced equally in test and control samples using the same software. Images were captured using Adobe Photoshop 7.0 software.
Neutrophil elastase assay
Platelets were adjusted to the same cell number and stimulated with thrombin as described in “Platelet stimulation.” Purified human neutrophil elastase (Calbiochem, EMD Biosciences, La Jolla, CA) was incubated in 0.1 M HEPES pH 8.0, 500 mM NaCl, and 10% dimethyl sulfoxide (DMSO) containing 2.5 mM elastase substrate (MeO-Suc-Ala-Ala-Pro-Val-pNA; Sigma) and 40 μL of platelet suspension. Elastase-mediated release of a proteolytic product28 increases absorbance at 410 nm, which was measured in a Beckman (Fullerton, CA) DU-640 spectrophotometer. The slope was determined and depicted as the change in A410 per minute. Inhibition by platelet suspensions is expressed in terms of the change in the slope (dA/min) reflecting elastase-dependent substrate accumulation.
Results
SLPI is an intracellular MK protein that associates specifically with β1 tubulin
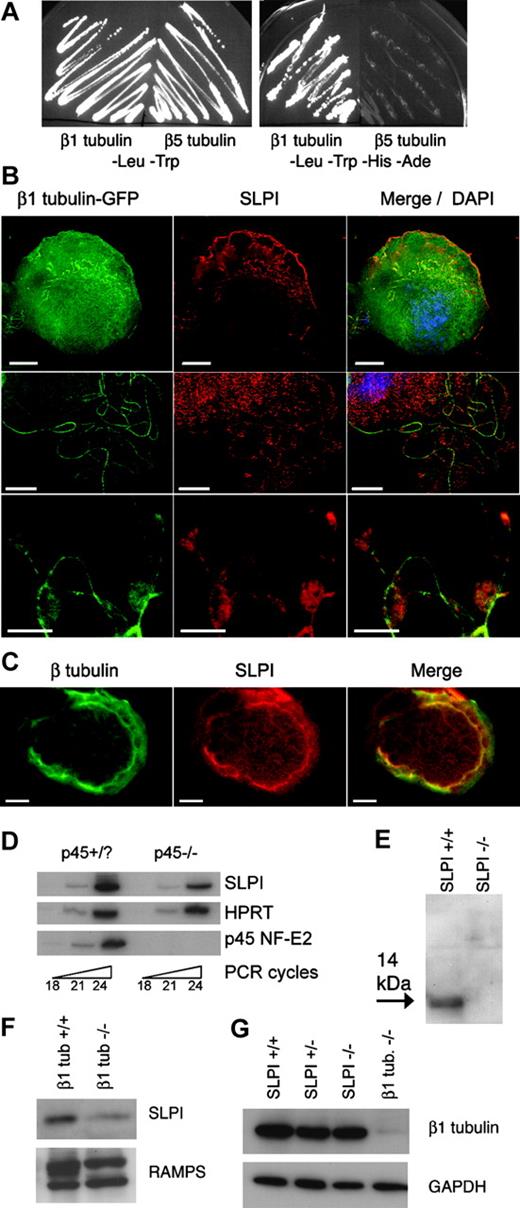
The requirement for β1 tubulin in MKs and platelets7,9 probably reflects in part the activities of selective MAPs. To identify proteins that interact specifically with β1 tubulin, we conducted a yeast 2-hybrid screen with the 134 C-terminal residues as the bait, including helices H11 and H12.11 This region encompasses the highest sequence diversity compared to other β-tubulins, including domains likely to mediate protein interactions. Nearly one third of clones from an MK-derived cDNA library that activated 3 independent reporter genes (histidine [His] and adenine [Ade] auxotrophy and β-galactosidase) correspond to the elongation factor eEF1α, which is known to bind tubulins29-31 ; others contain characterized tubulin-interacting domains such as the coiled-coil motif, and 11 genes were represented by 2 or more clones. Many genes isolated in the screen thus encode bona fide MAPs, and 2 clones encode full-length SLPI protein. Yeast cells cotransformed with SLPI and β1-tubulin C-terminal bait constructs activated reporter genes that depend on protein-protein interactions, as revealed by their growth on His-Ade- medium (Figure 1A). In contrast, cotransfection with the β5-tubulin C-terminus did not support growth in His-Ade- medium, which indicates that the association between SLPI and β1 tubulin is isotype-specific. Most other isolates in the screen displayed the same specificity, which suggests selective binding to the bait protein.
SLPI is expressed in megakaryocytes, binds specifically to β1 tubulin, and is reduced in platelets lacking β1 tubulin. (A) SLPI binds to β1 tubulin but not to the homologous β5 tubulin isotype in yeast 2-hybrid assays. Cells transformed with β5 tubulin fail to grow on His-Ade- selection medium, whereas growth of β1 tubulin-transformed cells indicates that SLPI and β1 tubulin interact and activate the reporter genes. Other transformants (not shown) with β5 tubulin grew on His-Ade- media and served as positive controls. (B) Immunofluorescence (IF) analysis of primary MKs expressing a β1 tubulin-GFP fusion protein. Cellular SLPI is detected by a specific antibody and Texas red-labeled secondary antibody. Merger of IF signals, including nuclear DAPI staining, is displayed in the third column, and each row represents different cells. Scale bars, 10 μm. (C) Double-label IF of primary MKs with β-tubulin (green) and SLPI (red) Ab, showing significant colocalization in the merged image. (D) SLPI mRNA is expressed in normal (p45+/?) primary MKs and reduced in p45 NF-E2-/- cells, as judged by semiquantitative RT-PCR over the indicated number of PCR cycles. (E) SLPI appears as a 14-kDa protein in platelet lysates, as shown by immunoblotting using a specific antibody. No signal is detected in SLPI-/- platelet lysates. (F-G) SLPI expression is reduced in β1 tubulin-/- platelets, whereas absence of SLPI does not affect β1-tubulin levels. Equal loading of wild-type and mutant platelets was verified by staining with antisera against whole mouse platelets (RAMPS) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
SLPI is expressed in megakaryocytes, binds specifically to β1 tubulin, and is reduced in platelets lacking β1 tubulin. (A) SLPI binds to β1 tubulin but not to the homologous β5 tubulin isotype in yeast 2-hybrid assays. Cells transformed with β5 tubulin fail to grow on His-Ade- selection medium, whereas growth of β1 tubulin-transformed cells indicates that SLPI and β1 tubulin interact and activate the reporter genes. Other transformants (not shown) with β5 tubulin grew on His-Ade- media and served as positive controls. (B) Immunofluorescence (IF) analysis of primary MKs expressing a β1 tubulin-GFP fusion protein. Cellular SLPI is detected by a specific antibody and Texas red-labeled secondary antibody. Merger of IF signals, including nuclear DAPI staining, is displayed in the third column, and each row represents different cells. Scale bars, 10 μm. (C) Double-label IF of primary MKs with β-tubulin (green) and SLPI (red) Ab, showing significant colocalization in the merged image. (D) SLPI mRNA is expressed in normal (p45+/?) primary MKs and reduced in p45 NF-E2-/- cells, as judged by semiquantitative RT-PCR over the indicated number of PCR cycles. (E) SLPI appears as a 14-kDa protein in platelet lysates, as shown by immunoblotting using a specific antibody. No signal is detected in SLPI-/- platelet lysates. (F-G) SLPI expression is reduced in β1 tubulin-/- platelets, whereas absence of SLPI does not affect β1-tubulin levels. Equal loading of wild-type and mutant platelets was verified by staining with antisera against whole mouse platelets (RAMPS) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
To confirm this interaction in a native context, we determined SLPI expression and subcellular localization in primary MKs by immunofluorescence (IF). We transduced MKs with β1 tubulin fused to the green fluorescent protein (GFP) and examined colocalization. SLPI expression was observed through much of the cytoplasm but also concentrated at the periphery of the most mature cells, where it colocalized with GFP-β1 tubulin, as judged by the individual and merged fluorescent signals (Figure 1B). As a control for nonspecific staining, we detected no SLPI signal in MKs derived from SLPI-/- mice (data not shown). One class of MK MTs is best visualized in relative isolation when terminally differentiated cells elaborate MT-based proplatelets.3-5,32 The proximity of SLPI with β1 tubulin was again observed along MK proplatelet extensions (Figure 1B). The association between SLPI and native MT structures also was evident with double immunostaining in lieu of overexpressing GFP-tagged protein (Figure 1C). Although SLPI has been studied principally as a secreted protein,33-36 it has a recognized intracellular form.16,17 Our results demonstrate that SLPI is present within MKs and indicate that its association with β1 tubulin, originally detected in yeast cells, also occurs in MKs. SLPI transcript levels are moderately reduced in MKs lacking p45 NF-E2 (Figure 1D), which are arrested in differentiation22 and slightly enriched in mature MKs (data not shown).
Because these findings imply that the SLPI gene product is endogenous to MKs and a late marker of MK maturation, the protein is likely to be expressed in blood platelets. We immunoblotted washed platelet lysates from wild-type mice and, as controls, platelets from SLPI-/- mice.19 The full-length SLPI protein is unglycosylated and has a molecular mass of 14 kDa, whereas the more common, secreted form has a 12-kDa mass after cleavage of the leader signal peptide.15 We detected a single 14-kDa band in wild-type platelets that is absent from SLPI-/- samples (Figure 1E). Thus, SLPI is present in mouse platelets in its native, uncleaved form, similar to its expression in human neutrophils.16
As SLPI binds to β1 tubulin selectively, interaction between the 2 proteins might affect their mutual expression in platelets. Indeed, SLPI expression is notably reduced in β1-tubulin-/- platelets compared to the wild type (Figure 1F). This result indicates that β1 tubulin is required for normal SLPI expression in blood platelets and provides genetic corroboration of an interaction between the 2 proteins. In contrast, β1 tubulin levels are normal in platelets from mice with reduced levels or absence of SLPI (Figure 1G).
SLPI expression in blood platelets
To define SLPI localization in platelets, we used double-label IF on fixed and permeabilized resting platelets and analyzed the results by deconvolution microscopy. As expected, β tubulin is concentrated in the marginal MT band in wild-type platelets, whereas absence of β1 tubulin results in disruption of this structure,7 with weak and diffuse residual β-tubulin staining (Figure 2A, left panels). SLPI is present in 2 distinct compartments in wild-type platelets. First, there is nonuniform distribution along the platelet periphery, in juxtaposition to the marginal MT band. This is reflected in the merger of individual fluorescent signals (Figure 2A) and points independently to the proximity between SLPI and β1 tubulin. Second, SLPI resides in a punctate distribution in the platelet body. Absence of β1 tubulin, which significantly reduces MT abundance in the marginal band but preserves this structure in some form,7 also eliminates SLPI from its peripheral distribution in resting platelets (Figure 2A, lower panels). Here, SLPI is confined to the platelet body, with preserved punctate distribution (Figure 2A) or along the few identifiable MT filaments (data not shown). Thus, SLPI shows bicompartmental distribution in resting blood platelets: a cortical, β1 tubulin-dependent localization along the marginal band and a central, punctate distribution that is β1 tubulin independent. The genetic evidence further supports our claim that SLPI interacts physically with β1 tubulin and argues strongly against nonspecific binding to the MT compartment.
SLPI is present in 2 distinct platelet compartments, including a β1 tubulin-dependent association with intact marginal band microtubules. (A) SLPI is found in 2 distinct compartments in normal resting platelets: a punctate distribution in the platelet body that suggests expression in granular stores, and a peripheral distribution wherein SLPI (red) colocalizes with β tubulin (green), as shown by the yellow color in the merged image. Only the latter distribution depends on β1 tubulin, as revealed by its loss in β1 tubulin (β1 tub)-null platelets. (B) The peripheral SLPI distribution depends on the presence of polymerized MTs: in cooled platelets (4°C) MTs depolymerize, losing both the marginal band together and peripheral SLPI staining (middle panels). Rewarmed platelets reconstitute the peripheral MT coil and restore native SLPI distribution (lower panels).
SLPI is present in 2 distinct platelet compartments, including a β1 tubulin-dependent association with intact marginal band microtubules. (A) SLPI is found in 2 distinct compartments in normal resting platelets: a punctate distribution in the platelet body that suggests expression in granular stores, and a peripheral distribution wherein SLPI (red) colocalizes with β tubulin (green), as shown by the yellow color in the merged image. Only the latter distribution depends on β1 tubulin, as revealed by its loss in β1 tubulin (β1 tub)-null platelets. (B) The peripheral SLPI distribution depends on the presence of polymerized MTs: in cooled platelets (4°C) MTs depolymerize, losing both the marginal band together and peripheral SLPI staining (middle panels). Rewarmed platelets reconstitute the peripheral MT coil and restore native SLPI distribution (lower panels).
To examine the basis of peripheral SLPI localization independently, we exploited the fact that MT polymers are sensitive to cold temperature.26 Cooling wild-type platelets to 4°C disrupted the marginal MT band and restricted tubulin staining to the cell center, as expected (Figure 2B, left). If SLPI concentration at the platelet periphery is MT coil-dependent, SLPI staining should follow the same pattern, which it did (Figure 2B, right). Rewarming the cells to 37°C induced reassembly of the marginal band and also restored peripheral SLPI localization (Figure 2B). These findings together reveal that cortical SLPI distribution in platelets requires both presence of β1 tubulin and an intact marginal MT band and extend the significance of SLPI-β1 tubulin interactions detected in yeast cells.
Platelet SLPI is released after thrombin stimulation
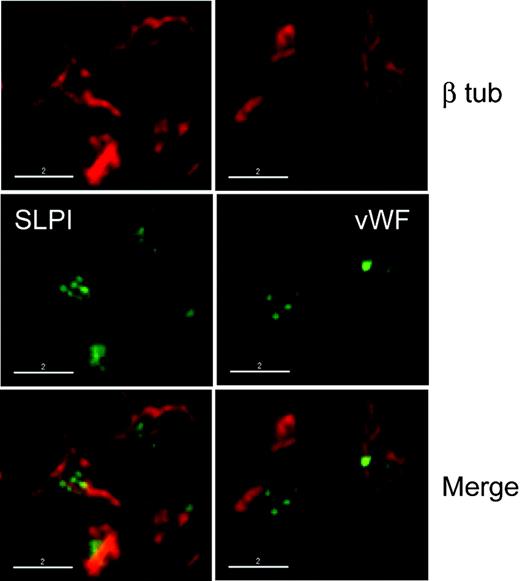
To determine if the punctate SLPI staining in blood platelets reflects its association with a known class of platelet granules, we double-stained platelets with antibodies against SLPI and one of the following markers: vonWillebrand factor (VWF), specific for α-granules; serotonin, a dense-granule component; and LAMP-2, a lysosomal marker that is also present on dense-granule membranes. In IF studies, we failed to colocalize SLPI with any of these 3 markers (Figure 3A), each of which stained in the predicted pattern. Nevertheless, we asked whether SLPI may be secreted when platelets are activated. We treated washed platelet suspensions from wild-type or β1 tubulin-/- mice with thrombin for 5 minutes, centrifuged the samples, separated the supernatant and pellet fractions, and performed immunoblot analysis using antibodies against SLPI or, as a positive control for a released α-granule protein, against platelet factor 4 (PF4). Like PF4, SLPI was released partially into the supernatant fraction after thrombin stimulation (Figure 3B), with an efficiency proportional to the thrombin concentration (data not shown). An antimouse platelet serum (RAMPS) revealed most proteins remaining in the pellet fraction (Figure 3C), which argues against extensive platelet lysis in this assay. Also, the number of SLPI-stained particles in the platelet body is reduced approximately 50% after activation (data not shown). Thrombin stimulation of equal numbers of platelets from β1 tubulin-/- mice resulted in markedly diminished SLPI release (Figure 3B). Concomitantly impaired release of the α-granule marker PF4 is consistent with our previous observation that β1 tubulin-/- platelets show reduced surface CD62P staining after thrombin stimulation.7 Thus, although we cannot definitively assign SLPI localization to a specific class of platelet granules, thrombin stimulation releases a fraction of total cellular SLPI, and this release is impaired in the absence of β1 tubulin.
SLPI does not colocalize with known platelet granule markers but is partially released after thrombin stimulation. (A) Platelets were stained with SLPI antibody and with antibodies specific for markers of α-granules (von Willebrand factor [vWF]), dense bodies (serotonin), or lysosomes (LAMP-2) and examined by deconvolution IF microscopy. The SLPI antibody again identifies bicompartmental expression, but the central punctate distribution failed to colocalize reliably with any of the tested granule markers. Scale bars, 2 μm. (B) Washed platelet suspensions were stimulated with thrombin (Thr, +) or phosphate-buffered saline (-) and centrifuged, and the pellet (P) and supernatant (S) fractions were analyzed by immunoblotting. Activation achieves partial release of SLPI and the α-granule protein platelet factor 4 (PF4). Thrombin-stimulated SLPI and PF4 release is impaired in β1-tubulin-/- platelets (right). (C) Control immunoblotting of platelet fractions with rabbit antimouse platelet serum (RAMPS) confirms that thrombin stimulation did not induce platelet lysis: only a few proteins, indicated by dots, transfer to the S fraction.
SLPI does not colocalize with known platelet granule markers but is partially released after thrombin stimulation. (A) Platelets were stained with SLPI antibody and with antibodies specific for markers of α-granules (von Willebrand factor [vWF]), dense bodies (serotonin), or lysosomes (LAMP-2) and examined by deconvolution IF microscopy. The SLPI antibody again identifies bicompartmental expression, but the central punctate distribution failed to colocalize reliably with any of the tested granule markers. Scale bars, 2 μm. (B) Washed platelet suspensions were stimulated with thrombin (Thr, +) or phosphate-buffered saline (-) and centrifuged, and the pellet (P) and supernatant (S) fractions were analyzed by immunoblotting. Activation achieves partial release of SLPI and the α-granule protein platelet factor 4 (PF4). Thrombin-stimulated SLPI and PF4 release is impaired in β1-tubulin-/- platelets (right). (C) Control immunoblotting of platelet fractions with rabbit antimouse platelet serum (RAMPS) confirms that thrombin stimulation did not induce platelet lysis: only a few proteins, indicated by dots, transfer to the S fraction.
SLPI-null mice show normal thrombopoiesis and platelet parameters
Knockout mice reveal 2 related and essential functions for β1 tubulin: efficient thrombopoiesis and generation or maintenance of platelet discoid shape.7,26 In light of SLPI's interaction with β1 tubulin in MKs and platelets, we evaluated its independent requirements and functions in this lineage. Circulating platelet counts in SLPI-/- mice (1047/μL, Table 1) were similar to those in wild-type controls (1025/μL) and SLPI-/- MKs produced proplatelets normally in vitro (data not shown). Together, these results exclude a significant thrombopoietic defect. In contrast to the atypical spherocytosis observed for β1 tubulin-/- platelets under differential interference contrast (DIC) or scanning electron (SEM) microscopy, SLPI-/- platelets resembled wild-type cells in their discoid resting shape (Figure 4A). These platelets also revealed a grossly intact marginal MT band in IF microscopy, similar to that seen in resting control platelets (Figure 4B). Moreover, SLPI-/- platelets showed normal expression of surface glycoproteins (GP) Ibα, IX, and IIb, and the activation markers CD62P and activated CD41 were not aberrantly expressed on resting SLPI-/- platelets (data not shown).
Platelet counts and clotting times of mutant and control mice
. | C57BL/6 strain . | . | . | C57-129/SV (mixed) strain . | . | |||
|---|---|---|---|---|---|---|---|---|
| SLPI genotype | −/− | +/− | +/+ | +/− | +/+ | |||
| β1 tub genotype | +/+ | +/+ | +/+ | +/− | +/− | |||
| Platelet count, per μL (no. tested) | 1047 ± 255 (12) | 1130 ± 245 (19) | 1025 ± 160 (14) | 1128 ± 109* (8) | 1173 ± 131* (6) | |||
| Blood clotting time, s (no. tested) | 221 ± 43 (9) | ND | 226 ± 56 (10) | 240 ± 65 (8) | 275 ± 23 (6) | |||
| Plasma clotting time, s (no. tested) | 111 ± 12† (6) | ND | 77 ± 13† (6) | 107 ± 24 (8) | 110 ± 10 (6) | |||
. | C57BL/6 strain . | . | . | C57-129/SV (mixed) strain . | . | |||
|---|---|---|---|---|---|---|---|---|
| SLPI genotype | −/− | +/− | +/+ | +/− | +/+ | |||
| β1 tub genotype | +/+ | +/+ | +/+ | +/− | +/− | |||
| Platelet count, per μL (no. tested) | 1047 ± 255 (12) | 1130 ± 245 (19) | 1025 ± 160 (14) | 1128 ± 109* (8) | 1173 ± 131* (6) | |||
| Blood clotting time, s (no. tested) | 221 ± 43 (9) | ND | 226 ± 56 (10) | 240 ± 65 (8) | 275 ± 23 (6) | |||
| Plasma clotting time, s (no. tested) | 111 ± 12† (6) | ND | 77 ± 13† (6) | 107 ± 24 (8) | 110 ± 10 (6) | |||
ND indicates not determined.
The platelet count for β1 tubulin+/− mice in the mixed genetic background is higher than we reported previously,7 most likely owing to strain differences. Similarly, plasma clotting time is highly sensitive to mouse strain differences, and comparisons are valid only across the same genetic background.
P < .025 in an unpaired Mann-Whitney-Wilcoxon rank test.
SLPI is dispensable for platelet discoid shape and resting integrity or activation-induced reorganization of the marginal MT band. (A) Differential interference contrast (DIC) and scanning electron (SEM, insets) microscopy reveal the elliptical shape of resting wild-type and SLPI-/- platelets, whereas β1 tubulin-/- platelets are spherical. (B) IF microscopy after staining with β-tubulin antibody reveals a normal marginal MT band in SLPI-/- platelets, in contrast to β1 tubulin-/- platelets, which have an indistinct marginal band. (C) Washed platelets were deposited on coverslips and stimulated with thrombin for 5 minutes prior to fixation. SLPI-/- platelets show normal contraction of the marginal band and short MT protrusions, similar to wild-type platelets, whereas β1 tubulin-/- platelets have a diminished resting marginal band that is defective in reorganization. (D-E) Platelets from compound SLPI+/- β1 tubulin+/- mice and control SLPI+/+ β1 tubulin+/- mice show normal discoid cell shape by DIC microscopy (D), intact marginal MT bands in β-tubulin IF (insets), and the normal MT morphology after thrombin stimulation. Scale bars: B-C, 10 μm; D-E, 2 μm.
SLPI is dispensable for platelet discoid shape and resting integrity or activation-induced reorganization of the marginal MT band. (A) Differential interference contrast (DIC) and scanning electron (SEM, insets) microscopy reveal the elliptical shape of resting wild-type and SLPI-/- platelets, whereas β1 tubulin-/- platelets are spherical. (B) IF microscopy after staining with β-tubulin antibody reveals a normal marginal MT band in SLPI-/- platelets, in contrast to β1 tubulin-/- platelets, which have an indistinct marginal band. (C) Washed platelets were deposited on coverslips and stimulated with thrombin for 5 minutes prior to fixation. SLPI-/- platelets show normal contraction of the marginal band and short MT protrusions, similar to wild-type platelets, whereas β1 tubulin-/- platelets have a diminished resting marginal band that is defective in reorganization. (D-E) Platelets from compound SLPI+/- β1 tubulin+/- mice and control SLPI+/+ β1 tubulin+/- mice show normal discoid cell shape by DIC microscopy (D), intact marginal MT bands in β-tubulin IF (insets), and the normal MT morphology after thrombin stimulation. Scale bars: B-C, 10 μm; D-E, 2 μm.
β1 tubulin-/- platelets are defective in selected thrombin responses, including surface expression of CD62P and contraction of the peripheral MT coil.7,26 As a β1 tubulin-specific binding protein, SLPI could participate in such events. However, CD62P expression on thrombin-stimulated SLPI-/- platelets did not show the shifted dose-response curve we previously reported7 for β1 tubulin-/- platelets (data not shown). MT rearrangements following thrombin stimulation of SLPI-/- platelets also were similar to the response in wild-type platelets: the peripheral MT coil condensed and formed filamentous protrusions (Figure 4C) and disruptions characteristic of β1 tubulin-/- platelets (poorly coiled MTs with few extensions26 ) were not observed. SLPI-/- platelets thus fail to phenocopy the overt MK and platelet defects seen in the absence of β1 tubulin.
Although SLPI appears to be dispensable or redundant for normal thrombopoiesis, a requirement conceivably could be masked when cellular levels of its interaction partner β1 tubulin are normal. Platelet parameters are normal or modestly affected in β1 tubulin+/- mice,7 which permits testing a dosage requirement for β1 tubulin in SLPI functions. However, murine β1 tubulin and SLPI genes are tightly linked (< 10 cM) on chromosome 2, which makes it difficult to generate mutant SLPI-/- β1 tubulin+/- mice by interbreeding knockout strains; accordingly, we studied only the compound heterozygotes. Platelet counts, bleeding time, and whole blood clotting time in SLPI+/- β1 tubulin+/- mice were the same as in β1 tubulin+/- controls (Table 1), and resting SLPI+/- β1 tubulin+/- platelets are elliptical in shape with intact marginal MT bands (Figure 4D). MT reorganization also was unaffected following activation of SLPI+/- β1 tubulin+/- platelets (Figure 4E).
Platelet activation disrupts colocalization between SLPI and β1-tubulin
As SLPI is partially released from platelets after thrombin stimulation, we asked how the 2 distinct SLPI pools respond to a platelet agonist. We treated wild-type platelets with thrombin and stained them with antibodies against β tubulin and either VWF to detect residual α-granules or SLPI. Platelet activation normally results in central accumulation of platelet granules37,38 ; indeed, we detected VWF-containing α-granules in the platelet center, surrounded by the condensed MT coil (Figure 5). SLPI was found in the same distribution, and MT-associated SLPI staining, which we readily appreciated in resting platelets (Figure 2A), was completely lost (Figure 5). This finding raises one of the following possibilities. Thrombin stimulation might selectively alter the MT-associated SLPI fraction so that it escapes antibody detection or translocates to the cell body. Alternatively, thrombin promotes SLPI release from the MT-associated pool. Because the SLPI antibody was generated against full-length protein and the total amount of SLPI is reduced in the pellet fraction of activated platelets (Figure 3B), we favor the latter possibility. In any event, our findings demonstrate that the physical interaction between SLPI and β1 tubulin is rapidly sensitive to events that follow platelet activation.
SLPI loses microtubular association after thrombin stimulation. Thrombin-stimulated wild-type platelets were stained with antibodies against β tubulin and either SLPI or the α-granule marker VWF. Following activation, SLPI is restricted to a single, central platelet compartment, similar to VWF, and, in contrast to resting platelets (see Figure 2A), no longer colocalizes with the rearranged MT filaments. Scale bars, 2 μm.
SLPI loses microtubular association after thrombin stimulation. Thrombin-stimulated wild-type platelets were stained with antibodies against β tubulin and either SLPI or the α-granule marker VWF. Following activation, SLPI is restricted to a single, central platelet compartment, similar to VWF, and, in contrast to resting platelets (see Figure 2A), no longer colocalizes with the rearranged MT filaments. Scale bars, 2 μm.
Activation-dependent SLPI release from platelets inhibits neutrophil elastase
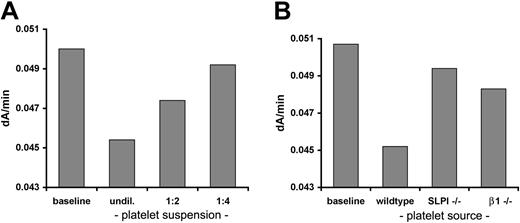
Finally, we asked whether platelet-expressed SLPI has a physiologic function. Murine and human SLPI are conserved and both can inhibit human and murine neutrophil elastase, albeit with different affinities39 ; accordingly, we adapted an in vitro elastase assay using MeO-Suc-Ala-Ala-Pro-Val-pNA as the substrate.28 Site-specific cleavage of this synthetic peptide by elastase releases a product that absorbs light at 410 nm. The rate of increase in A410 over time thus provides a reliable measure of enzyme and inhibitory activities, and we confirmed linearity of the temporal change in A410 (data not shown). Incubation of activated (Figure 6A) but not unstimulated (data not shown) wild-type platelet suspensions with the substrate prior to adding purified human neutrophil elastase inhibited enzyme activity in proportion to the platelet concentration. In contrast, SLPI-/- mice inhibited elastase activity poorly, suggesting that most of the inhibition derives from SLPI; β1 tubulin-/- platelets also showed reduced inhibitory activity (Figure 6B). Because we used a saturating thrombin dose, we attribute the reduced inhibitory activity of β1 tubulin-/- platelets to their reduced SLPI expression (Figure 1F) rather than to impaired thrombin response. These results provide genetic evidence that activated platelets inhibit neutrophil elastase mainly through SLPI and that β1 tubulin is required not only for normal SLPI expression in platelets but also for full expression of protease inhibitory activity.
Activated platelets inhibit neutrophil elastase mainly through SLPI. (A) Thrombin-stimulated platelets were incubated with a synthetic elastase substrate and purified human neutrophil elastase. Enzyme activity, measured as the linear rate of increase in A410, was measured spectrophotometrically after inclusion of serial 2-fold dilutions of suspensions of activated wild-type platelets. Elastase activity was inhibited in proportion to the platelet concentration, whereas resting platelets lacked this activity (data not shown). (B) Platelets from SLPI-/- or β1 tubulin-/- mice consistently showed reduced inhibition of in vitro elastase activity. The data shown are representative of 5 independent experiments with platelets pooled from variable numbers of mice to ensure equal input numbers.
Activated platelets inhibit neutrophil elastase mainly through SLPI. (A) Thrombin-stimulated platelets were incubated with a synthetic elastase substrate and purified human neutrophil elastase. Enzyme activity, measured as the linear rate of increase in A410, was measured spectrophotometrically after inclusion of serial 2-fold dilutions of suspensions of activated wild-type platelets. Elastase activity was inhibited in proportion to the platelet concentration, whereas resting platelets lacked this activity (data not shown). (B) Platelets from SLPI-/- or β1 tubulin-/- mice consistently showed reduced inhibition of in vitro elastase activity. The data shown are representative of 5 independent experiments with platelets pooled from variable numbers of mice to ensure equal input numbers.
Discussion
Mammalian genomes encode 5 to 6 distinct but highly homologous genes for α- and β-tubulin.8,40 Each potential α-β pair can interact and form MTs, though not all isotypes are fully interchangeable (reviewed in Luduena41 ) or expressed simultaneously. β1 tubulin, in particular, is restricted to MKs and platelets,8,9,40 where it operates downstream of NF-E2, a transcription factor that is required for thrombopoiesis. β1 tubulin also is the most divergent mammalian isoform, sharing less than 80% sequence identity with other β tubulins. Most of the divergence is concentrated in the C-terminus, a region rich in charged amino acids that probably mediate interactions with assorted regulatory and structural proteins (reviewed in Nogales12 ). As β1 tubulin is both restricted in cellular distribution and unusually divergent in sequence, we sought to identify proteins that bind it selectively and have characterized SLPI as a novel MT-associated protein. SLPI, which is most commonly regarded as a secreted inhibitor of serine proteases and extensively studied for anti-inflammatory roles,19,36,42 appears in 2 distinct intracellular pools in MKs and platelets. Although there is a punctate distribution in the cell center, a significant fraction of SLPI is associated with the marginal band. In MKs releasing platelets, SLPI decorates the proplatelet shaft, contiguous with MTs in the cell body, whereas absence of β1 tubulin or MT disruption in cooled platelets removes cortical SLPI localization. These findings indicate that SLPI associates with polymerized MK/platelet-specific MTs and also suggest a putative translocation mechanism. SLPI is present early in MK ontogeny, with diffuse cytosolic distribution, whereas β1 tubulin appears only at advanced differentiation stages. When β1 tubulin becomes available, SLPI may engage the cortical MTs that represent the origins of proplatelets and travel along these structures before resting in persistent association with the platelet marginal band.
SLPI expression has not previously been reported in MKs and platelets, even through 2 recent proteomic approaches.43,44 Although SLPI has a peptide leader sequence and is normally secreted as a 12-kDa protein, the full-length, 14-kDa protein can be retained in some cells.16,17 Our immunoblot data suggest it is this uncleaved SLPI form that is present in MKs and platelets. In combination with our mRNA expression data in MKs and the localization of SLPI in MKs and along the proplatelet shafts, these observations argue against its uptake from the plasma pool, as is described for fibrinogen or factor V.45 Moreover, our hematopoietic culture conditions generate a fairly enriched MK pool (> 95%) that lacks contaminating cells sufficient to serve as a significant donor population. SLPI expression in mice is restricted to a few tissues, including the spleen and type II alveolar cells in the lung15,42 ; splenic expression probably derives in part from MKs, as lymphocyte-enriched splenocyte fractions express little SLPI.42 Circulating SLPI levels are typically low and increase with inflammation,46 but the tissue origin has been unclear. The substantial and releasable SLPI we report suggests that activated platelets could represent one source of SLPI that is deposited locally at sites of vascular injury.
Interacting proteins identified in yeast 2-hybrid screens using SLPI as the bait include scramblase and proepithelin (PEPI).47,48 The latter association is thought to rely on electrostatic interactions, as SLPI bears 12 conserved positively charged residues and PEPI carries 5 net negative charges.48 The negatively charged C-termini of β tubulins could confer similar properties. Although the C-terminus of β5 tubulin also is negative in charge, SLPI interacts selectively with β1 tubulin in yeast cells and does not associate with β1 tubulin-/- MTs, in which β5 is the predominant β isotype; these findings exclude nonspecific electrostatic affinity as the basis for SLPI-MT interactions. The SLPI N-terminus binds glycosaminoglycans,49,50 which allows it to bridge PEPI to epithelial surfaces. Heparan sulfate proteoglycans also are present in platelets, where SLPI may help bridge other protein interactions.
The obvious challenge is to define SLPI function in MKs and/or platelets. SLPI-/- mice show normal thrombopoiesis, platelet shape, and activation response to thrombin, though it is possible that other gene products substitute for essential SLPI functions. SLPI is most extensively characterized as a secreted inhibitor of serine proteases, especially elastase and cathepsin G but also chymotrypsin and trypsin.13,14,36 Accordingly, a natural consideration for SLPI function in MKs or platelets is as a protease inhibitor. Cytoplasmic, full-length SLPI is thought to protect neutrophils from proteases spilled by azurophilic granules.16 As proteases also are present in MK and platelet lysosomes, SLPI could have a parallel function in these cells, and its concentration along the platelet marginal band may localize SLPI to protect selected targets from intracellular proteases. We also detected platelet SLPI in a punctate distribution distinct from known granule markers; similar staining recently was described for caspase-3a in MKs,51 where the authors suggest that sequestration might prevent premature activation of caspase pathways implicated in thrombopoiesis.
Useful clues about SLPI's cellular functions likely lie in our finding that thrombin stimulation results in partial release of platelet SLPI and disrupts its association with the marginal MT band. Thus, SLPI's principal role may follow platelet activation, to modulate pro-inflammatory or prothrombotic processes that initiate concomitantly. β-thromboglobulin and other platelet chemokines recruit leukocytes to sites of platelet activation,52 followed by neutrophil release of elastase and cathepsin G,53 which synergize to activate platelets further. SLPI release in this context may serve to limit the extent of local platelet stimulation and pro-inflammatory activity. Previous studies indicate that an unknown component of activated platelets inhibits neutrophil elastase54 ; our findings imply that platelet-derived SLPI, which inhibits elastase in vitro, could account for a significant fraction of this activity. The predicted effects are consistent with the prolonged wound healing observed in SLPI-/- mice, which occurs in part from enhanced elastase activity.19 Alternatively, SLPI may operate mainly to regulate hemostasis by restricting proteolysis at sites of platelet activation. Elastase and cathepsin G help cleave and activate factor V,55 and such events could represent targets of regulation by SLPI. We do observe the plasma clotting time of SLPI-/- mice to be significantly prolonged (Table 1, P < .025), but this measure of circulating plasma components is independent of platelets per se. Finally, SLPI may harbor functions unrelated to regulation of proteolysis, just as another protease inhibitor, plasminogen activator inhibitor-1, blocks cell migration by displacing integrins from their attachment sites.56
The complement of platelet proteins that is released after thrombin stimulation has been characterized in part43 and includes many unexpected factors. Published data point to a release of 2 other protease inhibitors, α2-macroglobulin and α1-antitrypsin, the latter in some abundance. Besides identifying SLPI as a new platelet-expressed protease inhibitor, our study reveals that a portion of the intracellular pool is sequestered along the marginal MT band in resting platelets. This association requires the presence of β1 tubulin and represents an original example of a protein's selective interaction with MK- and platelet-specific MTs. Besides diminishing MK- and platelet-intrinsic features of cell shape and thrombopoiesis, loss of β1 tubulin also affects interacting proteins like SLPI. In turn, platelet-derived SLPI is capable of modulating physiologic functions, possibly including platelet-leukocyte interactions, as reflected in inhibition of neutrophil elastase.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-03-1179.
Supported by grants R01HL63143, R01HL68130, and P01HL56949 from the National Institutes of Health, Bethesda, MD. H.S. was supported in part by the Deutsche Forschungsgemeinschaft (Schu1421/2-1). R.A.S. is a Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marian Walhout for advice on yeast 2-hybrid screening; Craig Furman and Romesh Subramanian for plasmid constructs; Nick Cowan, Sally Lewis, Bernhard Nieswandt, and Mortimer Poncz for antibodies; John Alberta for expert assistance with deconvolution fluorescence microscopy; Denisa Wagner for helpful discussions; and Beatrice Cambien for help with plasma clotting studies.


![Figure 3. SLPI does not colocalize with known platelet granule markers but is partially released after thrombin stimulation. (A) Platelets were stained with SLPI antibody and with antibodies specific for markers of α-granules (von Willebrand factor [vWF]), dense bodies (serotonin), or lysosomes (LAMP-2) and examined by deconvolution IF microscopy. The SLPI antibody again identifies bicompartmental expression, but the central punctate distribution failed to colocalize reliably with any of the tested granule markers. Scale bars, 2 μm. (B) Washed platelet suspensions were stimulated with thrombin (Thr, +) or phosphate-buffered saline (-) and centrifuged, and the pellet (P) and supernatant (S) fractions were analyzed by immunoblotting. Activation achieves partial release of SLPI and the α-granule protein platelet factor 4 (PF4). Thrombin-stimulated SLPI and PF4 release is impaired in β1-tubulin-/- platelets (right). (C) Control immunoblotting of platelet fractions with rabbit antimouse platelet serum (RAMPS) confirms that thrombin stimulation did not induce platelet lysis: only a few proteins, indicated by dots, transfer to the S fraction.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-03-1179/6/m_zh80240470810003.jpeg?Expires=1763474300&Signature=sSCHzXwBbJn6KQ79w0M9bBSJDPCELkK3knRJBZUYWMmxuzhLSDwuaEp2VWZdxVJE8Jx9ftLOsOHJo~o6kUGHHEIJrW6CheU27UpjjTxfMsGOAXik9e4Szh3TCybPRz796iHLG2VwdToCDMRxL24pi790v~gD1-2V7b8j8Vs89UgqNoiqSn-xIOZT2k7B8A1D6bLIl0Qf3AMOU7j8zAg-yxtPs9lAmG~JzCy3kjtQA3VO65BlIlTlcihZbL1bX8EVAZHN00FVtld7DjO9xx2DFs~ZtzSSqvxsPfdfTTN7GDB4MGcP5enxalNPFBb8DQXNJKbm9Z-t4ql5pvhhcBuBzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal