Abstract
Common lymphatic endothelial and vascular endothelial receptor-1 (CLEVER-1; also known as stabilin-1 or FEEL-1) is a large multifunctional glycoprotein implicated in scavenging, angiogenesis, and cell adhesion. Here we studied the function of human CLEVER-1 in leukocyte trafficking. Lymphatic vessels expressed CLEVER-1 constitutively in skin in vivo, whereas on vascular endothelium it appeared only upon inflammation. On isolated vascular endothelial cells, CLEVER-1 supported rolling and transmigration of peripheral blood mononuclear cells (PBMCs) under physiologically relevant laminar shear stress. Intriguingly, CLEVER-1 also mediated transmigration of leukocytes through cultured lymphatic endothelium under static conditions. Thus, synthesis of CLEVER-1 is differentially regulated on the 2 anatomically distinct vascular beds, and CLEVER-1 mediates the transmigration step of the leukocyte traffic in both of them. Notably, CLEVER-1 is the first adhesion molecule shown to be involved in the PBMC transmigration through the lymphatic arm of the immune system. (Blood. 2004;104:3849-3857)
Introduction
Lymphocyte recirculation is essential for the immune defense. Lymphocytes exit from the blood through the vascular endothelium in small postcapillary veins. They then migrate in the tissue in search of foreign antigens. Lymphocytes are collected from the peripheral tissues back to systemic circulation via the lymphatic vasculature. In the periphery, lymphocytes penetrate into blind-ended lymphatic vessels present in virtually all organs and are then transported to lymph nodes and other organized lymphatic tissues via the afferent lymphatics. From the lymph nodes, lymphocytes continue their travel via the efferent lymphatic vessels back to the major veins.1,2
The multistep adhesion cascade controlling lymphocyte extravasation from the blood consists of relatively well-understood phases of specific interactions between the leukocyte and the vascular endothelium that take place under laminar shear stress.3 First, the blood-borne lymphocyte tethers and starts to roll on the endothelium. If it receives appropriate activation stimuli, it can firmly adhere to the endothelium and finally transmigrate through the vascular wall into the tissue. The adhesion and activation molecules involved in the early parts of the cascade have been studied for a long time (reviewed in Lowe4 ; Kunkel and Butcher5 ; and Shimizu et al6 ), but the equally important molecules mediating the transmigration step started to attract attention only later.7,8
In contrast to the vascular side, the mechanisms governing leukocyte traffic in and out from lymphatic vessels remain practically unknown. During the last few years, several molecular markers have been defined that allow the identification of these irregular and thin-walled vessels.9-14 In terms of cell migration, however, only the expression of a chemokine receptor CCR7 has been hypothesized to contribute to the chemotactic migration of dendritic cells into the afferent lymph,15 and sphingosine-1-phosphate receptors may control lymphocyte exit from the lymph node into the efferent lymphatics.16,17 Mannose receptor18 and common lymphatic endothelial and vascular endothelial receptor-1 (CLEVER-1)19 are, to our knowledge, the only molecules directly shown to mediate lymphocyte adhesion to lymphatic vessels.
CLEVER-1 is a large glycoprotein expressed in lymphatic vessels and on high endothelial venules (HEVs).19 In frozen section binding assays, anti-CLEVER-1 monoclonal antibodies (mAbs) inhibit lymphocyte adhesion to HEVs and lymphatic vessels. CLEVER-1 is translated from a large approximately 7800-nucleotide mRNA. It contains 7 fasciclin domains, a proteoglycan link protein-like sequence, 22 epidermal growth factor (EGF)-like repeats, and 2 RGD motifs.19 The same molecule has been independently characterized as stabilin-1 and FEEL-1. Stabilin-1 was found to be a fasciclin-like hyaluronan receptor homolog, but no function has been alluded to it.20 FEEL-1, on the other hand, was reported to be a scavenging receptor that binds bacteria and endocytoses advanced glycation end products21 and promotes capillary angiogenesis in vitro.22
In the frozen section adhesion assays, it has not been possible to elucidate the dynamic step of the extravasation cascade supported by CLEVER-1 or the role of the hyaluronan-binding domain of CLEVER-1 in this process. Moreover, it has remained unknown whether similar or different isoforms of CLEVER-1 are expressed on vascular and lymphatic endothelium. Most importantly, earlier it had not been possible to analyze the contribution of CLEVER-1 to the interactions between leukocytes and living lymphatic endothelial cells. Here we studied these issues using endothelial cells expressing CLEVER-1 in vivo and in vitro. The results showed that CLEVER-1 is the first identified molecule that mediates diapedesis of PBMCs at 2 separate arms of the recirculation route.
Materials and methods
Antibodies
mAbs against CLEVER-1 (3-372 and 3-266) have been described.19 The phenotype of endothelial cells was analyzed using Abs against CD31 (Dako, Carpinteria, CA), CD44,23 vascular endothelial growth factor receptor 3 (VEGFR-3) (R&D Systems, Minneapolis, MN), PAL-E (Abcam, Cambridge, United Kingdom), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), and podoplanin (both from Research Diagnostics, Flanders, NJ). Nonbinding mAbs 3G6 (immunoglobulin G1 [IgG1]) and 11-7-3 (IgG2a) against chicken T cells (kind gifts from O. Vainio, Turku University), normal rabbit serum, and a binding HB116 mAb against HLA class I were used as controls.24 Fluorescein isothiocyanate (FITC)-conjugated anti-mouse, anti-rabbit, and anti-goat Igs were from Sigma (St Louis, MO); FITC-conjugated anti-mouse IgG1 and phycoerythrin (PE)-conjugated anti-mouse IgG2a were from Southern Biotechnology (Birmingham, AL); and peroxidase-conjugated sheep anti-mouse Ig was from Dako.
Cells and immunomagnetic separations
PBMCs were isolated using Ficoll as described.25 Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords according to the method of Jaffe et al.26 Human dermal microvascular endothelial cells (HDMECs) were obtained from Promocell (Heidelberg, Germany). Both endothelial cell types were cultured in a defined HDMEC medium (Endothelial Growth Medium Mv; Promocell) in gelatin (HUVECs) or fibronectin (HDMECs)-coated flasks and capillaries. For certain stainings, HUVECs were also cultured in RPMI 1640 medium supplemented with 4 mM l-glutamine, 10% AB serum, 15 μg/mL heparin, and 20 μg/mL endothelial cell growth factor, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
HDMECs were quickly expanded for separation of lymphatic and vascular endothelial cells using magnetic cell sorting as described.27,28 Briefly, the cells were gently trypsinized and incubated with an anti-CD44 mAb. This antigen is present on microvascular endothelial cells, but absent from lymphatic cells.27,29,30 Thereafter, a magnetic activated cell sorter (MACS) column LS was used to bind the positive cells. The flow through (CD44- lymphatic cells) and the retained fraction (CD44+ vascular endothelial cells) were collected for further culturing. Alternatively, fluorescence-activated cell-sorter (FACS) sorting (FACS Calibur) of CD44+ and CD44- cells was used to separate the 2 populations.
Immunochemical stainings
Acetone-fixed frozen sections were stained using immunoperoxidase staining as described.31 Skin samples were from psoriasis (n = 9), lichen (n = 2), mycosis fungoides (n = 2), erytrodermia (n = 3), exanthema (n = 4), folliculitis (n = 3), and normal (n = 15) skin. For double staining, the sections were sequentially exposed to anti-CLEVER-1 mAb, FITC-conjugated anti-mouse IgG1, anti-PAL-E, and PE-conjugated anti-mouse IgG2a. In other stainings, anti-LYVE-1 antibody, FITC-conjugated anti-rabbit Ig, anti-CLEVER-1 mAb, and PE-conjugated anti-mouse IgG1 were used. The sections were evaluated using a laser scanning confocal microscope (LSM 510; Carl Zeiss, Heidelberg, Germany).
For stainings, HDMECs were grown on fibronectin-coated coverslips overnight. Cells were fixed with a 3.7% (vol/vol) formaldehyde solution in phosphate-buffered saline (PBS) and permeabilized with 0.2% saponin solution. For double staining, the coverslips were sequentially incubated with anti-CLEVER mAb, PE-labeled second step antibody, and FITC-conjugated anti-CD44 mAb (FITC-Hermes-3), and examined using a laser scanning confocal microscope.
For FACS analysis, endothelial cells in suspension were stained for the various endothelial cell markers using indirect immunofluorescence. The cells were analyzed using FACSscan flow cytometer and CellQuest software (Becton-Dickinson, San Jose, CA).
In all stainings, appropriate isotype-matched controls were run in parallel.
Adhesion assays
PBMC binding to vessels in skin sections was studied using modified Stamper-Woodruff assay as described.32 In these experiments skin from mycosis fungoides, erytrodermia, and lichen patients was used as a target tissue. Anti-CLEVER-1 mAbs 3-266 and 3-372 were used as a pool (50 μg/mL each). The number of cells bound to vessels in the presence of control mAb (100 μg/mL) was defined as 100%.
For in vitro flow chamber assays, the endothelial cells were grown to confluency in gelatin-coated glass capillaries.33 The endothelial cells were induced with 100 U/mL tumor necrosis factor α (TNF-α) for 4 hours, and 1 μg/mL lipopolysaccharide (LPS) was added for the last 2 hours. For the last 30 minutes, anti-CLEVER-1 mAbs (pool of 3-266 and 3-372, 25 μg/mL each) or a binding control mAb against HLA class I were added. The capillaries were then connected to a computer-controlled syringe pump and to a PBMC reservoir. The adhesion assays with vascular endothelial cells were performed with a defined laminar shear. For a 1-minute stabilization period, binding buffer (RPMI supplemented with 0.1% bovine serum albumin [BSA]) was drawn over the endothelial cells at a constant shear of 1.0 dyne/cm2. Thereafter the valve was switched and PBMCs suspended in the binding medium supplemented with 5 μM histamine were perfused over the cells at the same shear. The induction with LPS was included to induce maximal CLEVER-1 expression, and TNF-α and histamine were used to induce the PBMC extravasation cascade (nonstimulated HUVECs do not bind PBMCs under flow to any significant extent). All adhesion experiments were done under an Olympus IX70 inverted microscope (Olympus, Hamburg, Germany) at room temperature.
In the experiments measuring rolling and firm adhesion on HUVECs, the cells were allowed to interact for 5 minutes under the same shear. Thereafter, 10 fields were recorded (× 100 magnification, HMC10Plan UIS objective [NA 0.25] [Modulation Optics, Greenvale, NY] equipped with a Hoffman modulator and Model G3 [NA 0.6] condenser [Modulation Optics]) for 60 seconds each using a CCD camera (C5405-01, Hamamatsu Photonics, Hamamatsu, Japan) coupled to a digital video recorder (Panasonic, Matsushita Electrical Industrial, Osaka, Japan). For the transmigration assay, the cells were perfused for 6 minutes. At that point, 10 fields were scanned using × 10 phase contrast optics (UplanF1 [NA 0.3] objective and IX-LMUCD [NA 0.55] condenser, Olympus Optical) to visualize the baseline situation. Thereafter, the cells were allowed to transmigrate for an additional 20 minutes in the presence of continuous flow (binding medium without cells, 1.0 dyne/cm2), and the same fields were recorded again.
To study PBMC adhesion to lymphatic endothelium, a modification of the flow chamber assay was developed. The lymphatic endothelial cells and PBMCs were induced exactly as in the vascular endothelium model. Then, PBMCs were gently perfused onto cells (3 minutes, 0.1 dyne/cm2), after which the flow was stopped, and the 2 cell types were allowed to interact for 20 minutes under static conditions. Thereafter, the nonadherent cells were flushed away with the binding medium (without cells) by a brief reintroduction of flow (7 minutes, 0.1 dyne/cm2), and the surface adherent and transmigrated cells were visualized using the CCD camera.
The flow chamber assays were analyzed off line from the videotapes. A PBMC was defined as rolling when it slowly moved to the direction of flow in an intimate contact with the endothelial cells. If the cell remained stationary for the entire 30-second observation period, it was scored as firmly adherent. In the transmigration assays, the number of surface-bound cells (phase-bright) and transmigrated cells (phase-dark) were counted separately. The number of transmigrated cells was counted as the number of phase-dark cells/number of dark and bright cells (ie, all interacting cells). Student t test was used for comparison between groups and P values less than .05 were considered significant.
On average, in the control HUVEC capillaries 189 ± 26 (mean ± SEM) cells rolled (during the 2.5-minute observation time), and 554 ± 85 (mean ± SEM) firmly adhered in the total area of 3.072 mm2 (10 fields) analyzed in each individual experiment (n = 6). In the transmigration assays (n = 5), 105 ± 29 (mean ± SEM) cells (17 ± 3% [mean ± SEM] of all interacting cells) transmigrated in the control capillaries (total area, 3.072 mm2). In the experiments with lymphatic endothelium, the absolute numbers of firmly adherent and transmigrated cells in the control capillaries were 318 ± 56 and 79 ± 14 cells (mean ± SEM, n = 5) (20 ± 2% [mean ± SEM] of all interacting cells transmigrated), respectively, in the total area of 3.072 mm2 analyzed in each individual experiment.
Transwell assays were performed by plating 50 000 HUVECs/well into fibronectin-coated and air-dried nitrocellulose inserts (5-μm pore size; Costar, Cambridge, MA) in HDMEC medium. After growing to confluency (visually verified by parallel 0.4-μm pore size Clear View inserts; Corning, Corning, NY), the wells were pretreated with anti-CLEVER-1 mAbs or controls for 20 minutes. Thereafter, 8 × 104 PBMCs fluorescently labeled with carboxyfluorescein succinimidyl ester were added into the upper wells, and 300 ng/mL recombinant human chemokine stromal-derived factor 1α was introduced into the lower chamber to establish a chemotactic gradient. After a 3-hour incubation at 37°C, the number of transmigrated cells in the wells was measured using Tecan Ultra fluoropolarimeter (Tecan, Durham, NC).
Binding of hyaluronan to CLEVER-1
CLEVER-1 was immunoaffinity purified from human lymph node lysate, as described.19 The same lysate was passed through a control column armed with an irrelevant mAb 3G6 to produce control eluate. The purity of the proteins was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses followed by silver staining. CLEVER-1 (0.25 μg/well) and control eluate (the same amount) were bound to the bottom of 96-well plates in PBS overnight. As a control, CD4 receptor globulin, CD44 receptor globulin, and BSA were used at 1 μg/well. The wells were washed and blocked with 0.1% Tween in PBS. Then, FITC-conjugated hyaluronan (a generous gift from Paraskivi Heldin, Uppsala University, Finland; 10 μg/mL), or anti-CD44, anti-CLEVER, or control mAbs (10 μg/mL) were added into the wells for 2 hours. After washings, the binding of antibodies was visualized with FITC-conjugated second-stage reagents. After final washes, the plates were read using a fluoropolarimeter (Tecan Ultra).
Capillary tube formation
To study the ability of anti-CLEVER mAbs to modulate angiogenesis in vitro, HUVECs were plated on top of a thick Matrigel layer in 96-well plates without any pretreatments or in the presence of anti-CLEVER (pool of 3-266 and 3-372, each at 20 μg/mL) or control (40 μg/mL) mAbs. After overnight culturing at 37°C, digital images were taken from 5 low-magnification microscopic fields (area 2.1 mm2 each; taken from standard points within the well) in each well, and 3 parallel wells were used for each pretreatment (ie, 15 fields/treatment/experiment; 3 independent experiments). The surface area covered by the capillary tubes and the number of capillary branches (the number of closed areas encircled by capillary tubes) were measured using an image analysis program.
Analyses of alternative forms of CLEVER-1
The molecular weight of CLEVER-1 in cell and tissue lysates was analyzed using 5% to 12% gradient SDS-PAGE gels (run for 48 hours to efficiently separate proteins) and immunoblotting for visualization, as previously described.19 For the glycosidase treatments, lymphatic and vascular endothelial cells were lysed in PBS containing 1% nonidet P-40, and aliquots of the lysates were left untreated, digested with 20 mU Vibrio cholerae neuraminidase (Dade Behring, Deerfield, IL) for 2 hours at 37°C, or sequentially digested with the neuraminidase and then with O-glycanase (2 μL recombinant O-glycanase in the O-glycosidase reaction buffer; ProZyme, San Leandro, CA) overnight at 37°C, essentially as described.19 Lysates were also digested with N-glycanase (4 U N-glycanase F [Roche, Indianapolis, IN] overnight at 37°C), but this resulted in the loss of the epitopes recognized by our anti-CLEVER-1 mAbs. The presence of an alternatively spliced form (exon 27) of CLEVER-1 in the same cells was analyzed using reverse-transcription-polymerase chain reaction (RT-PCR), as described.19
Results
CLEVER-1 is differentially regulated on vascular and lymphatic endothelium
To study CLEVER-1 in vascular and lymphatic vessels of skin, normal and inflamed samples from humans were stained immunohistochemically. In normal skin specimens from 15 different individuals, all blood vessels were completely devoid of CLEVER-1. In contrast, the small narrow vessels presumably representing lymphatic vessels were clearly CLEVER-1-positive in all specimens (Figure 1A). The nature of CLEVER-1-positive vessels was ascertained using 2-color stainings with endothelial markers. Confocal microscopy of normal skin revealed coexpression of LYVE-1 (a lymphatic specific marker)12 and CLEVER-1 in lymphatic endothelial cells (Figure 1C-E). In contrast, PAL-E (a marker specific for blood vessels34 )-positive cells in normal skin were completely devoid of CLEVER-1 (Figure 1F-H).
Both lymphatic and vascular endothelial cells express CLEVER-1. Immunohistologic stainings of (A) normal and (B) inflamed skin (psoriasis). CLEVER-1 is constitutively expressed in the lymphatic endothelial cells (some pointed out by thin black arrows) but induced in vascular HEV-like vessels (thicker white arrows) only in inflammation. Bar, 50 μm. (C-E) Confocal microscopy shows that lymphatic vessels in normal skin are CLEVER-1 positive (C, CLEVER-1 in red; D, LYVE-1 in green; E, merged image), whereas CLEVER-1 is absent from blood vessels (F, CLEVER-1 in green; G, PAL-E in red; H, merged image, no double-positive vessels). In the inflamed psoriatic skin, lymphatic vessels retain CLEVER-1 expression (I, CLEVER-1 in red; J, LYVE-1 in green; K, merged image). In inflammation, CLEVER-1 is induced also in blood vessels (HEV-like venule coexpresses CLEVER-1 [green, L] and PAL-E [red, M] as seen by the yellow signal in the merged image [N]; 0.3-μm optical sections). Representative negative control stainings are shown in the inserts. Bars, 20 μm. (O) An immunofluorescent image of permeabilized HDMECs shows that they are a mixed population of endothelial cells consisting of islets of vascular (CD44+, green) and lymphatic (CD44-, no green fluorescence) endothelial cells. Both types of endothelial cells are CLEVER-1 positive (red, mainly intracellular staining of fine granules/vesicles). Bar, 20 μm. A5× higher magnification of the area highlighted in the schematic picture is shown on the right. BEC indicates blood endothelial cells; LEC, lymphatic endothelial cells. (P) Surface stainings of immunomagnetically separated vascular and lymphatic endothelial cells, spontaneously enriched lymphatic cells, and HUVECs are shown for the indicated antigens. In the bottom line, gray histograms show staining with the negative control mAb 3G6 and black histograms, with anti-CLEVER-1 mAb 3-372. The mean fluorescence intensities of negative control and CLEVER-1 histograms are depicted in the upper right corner (neg.co/CLEVER-1). (Q) Lymphatic endothelial cells isolated from HDMECs based on the absence of CD44 (see P), and HUVECs were immunofluorescently stained for the expression of an established lymphatic marker, podoplanin.
Both lymphatic and vascular endothelial cells express CLEVER-1. Immunohistologic stainings of (A) normal and (B) inflamed skin (psoriasis). CLEVER-1 is constitutively expressed in the lymphatic endothelial cells (some pointed out by thin black arrows) but induced in vascular HEV-like vessels (thicker white arrows) only in inflammation. Bar, 50 μm. (C-E) Confocal microscopy shows that lymphatic vessels in normal skin are CLEVER-1 positive (C, CLEVER-1 in red; D, LYVE-1 in green; E, merged image), whereas CLEVER-1 is absent from blood vessels (F, CLEVER-1 in green; G, PAL-E in red; H, merged image, no double-positive vessels). In the inflamed psoriatic skin, lymphatic vessels retain CLEVER-1 expression (I, CLEVER-1 in red; J, LYVE-1 in green; K, merged image). In inflammation, CLEVER-1 is induced also in blood vessels (HEV-like venule coexpresses CLEVER-1 [green, L] and PAL-E [red, M] as seen by the yellow signal in the merged image [N]; 0.3-μm optical sections). Representative negative control stainings are shown in the inserts. Bars, 20 μm. (O) An immunofluorescent image of permeabilized HDMECs shows that they are a mixed population of endothelial cells consisting of islets of vascular (CD44+, green) and lymphatic (CD44-, no green fluorescence) endothelial cells. Both types of endothelial cells are CLEVER-1 positive (red, mainly intracellular staining of fine granules/vesicles). Bar, 20 μm. A5× higher magnification of the area highlighted in the schematic picture is shown on the right. BEC indicates blood endothelial cells; LEC, lymphatic endothelial cells. (P) Surface stainings of immunomagnetically separated vascular and lymphatic endothelial cells, spontaneously enriched lymphatic cells, and HUVECs are shown for the indicated antigens. In the bottom line, gray histograms show staining with the negative control mAb 3G6 and black histograms, with anti-CLEVER-1 mAb 3-372. The mean fluorescence intensities of negative control and CLEVER-1 histograms are depicted in the upper right corner (neg.co/CLEVER-1). (Q) Lymphatic endothelial cells isolated from HDMECs based on the absence of CD44 (see P), and HUVECs were immunofluorescently stained for the expression of an established lymphatic marker, podoplanin.
Upon inflammation, marked differences in the expression became apparent. CLEVER-1 was induced in chronically inflamed blood vessels in psoriatic skin that displays the typical high-endothelial venule (HEV)-like appearance (Figure 1B). The lymphatic vessels in inflamed psoriatic skin retained their CLEVER-1 synthesis. The 2-color immunofluorescence stainings confirmed the expression of CLEVER-1 in LYVE-1-positive lymphatic vessels in psoriatic skin (Figure 1I-K). Coexpression of PAL-E, a vascular endothelium specific marker, and CLEVER-1 in HEV-like vessels in inflamed psoriatic skin was also confirmed by the 2-color confocal analyses (Figure 1L-N). The induction of vascular CLEVER-1 was apparent in all tested samples derived from different inflammatory skin disorders including psoriasis, lichen, mycosis fungoides, erythrodermia, and exanthema. There was also a striking positive correlation with the appearance of lymphocytic infiltrate and CLEVER-1 positivity in these clinical specimens. Thus, both the vascular and lymphatic endothelium can express CLEVER-1, although the synthesis of this molecule is differentially regulated upon inflammation on these 2 endothelial cell types.
Isolated human dermal microvascular endothelial cells (HDMECs) express CLEVER-1 intracellularly and on the surface
We next sought to isolate the vascular and lymphatic endothelial cells for functional analyses. In a mixed population of cultured HDMECs, in which lymphatic and vascular endothelial cells typically grow as differentiated islands,27,28 intracellular CLEVER-1 was found in both endothelial cell types. This is clearly evident in confocal stainings, in which both CD44+ (a marker expressed in vascular but not in lymphatic endothelial cells27,30,35 ) vascular endothelial cells and CD44- lymphatic endothelial cells express CLEVER-1 (Figure 1O).
Using immunomagnetic separation, it is possible to isolate the 2 endothelial cell populations from HDMECs.27,28 Labeling of the endothelial cells with anti-CD44 mAbs allowed positive selection of the vascular endothelium, whereas the negative fraction was composed of lymphatic endothelial cells. After expansion in vitro, the phenotype of the cells was verified using FACS stainings for CD44 (Figure 1P). The endothelial cell types that were used in functional assays were also analyzed for expression of podoplanin, a lymphatic endothelium specific marker.36 The CD44- lymphatic cells expressed podoplanin, whereas the CD44+ vascular endothelial cells were podoplanin negative (Figure 1Q). These data confirm the utility of CD44 expression in differentiating between the 2 endothelial cell types and the purity of our cell populations.
CLEVER-1 was expressed on the surface of the lymphatic endothelial cells, albeit at a low level (Figure 1P). It was also found on the surface of dermal vascular endothelial cells. The surface expression of CLEVER-1 on both these cell types was notably variable depending on the cell batch, passage number, and propagation time. Since the expression of stabilin-1 is known to be induced in polarized macrophages by cytokines and dexamethasone,20 we incubated HDMECs with 100 ng/mL LPS and 0.4 μg/mL dexamethasone for various times. LPS usually induced a modest increase in CLEVER-1 expression (although the response was quite variable), whereas the dexamethasone pretreatment showed no consistent induction (data not shown). Interestingly, the highest CLEVER-1 expression levels were found on lymphatic endothelial cells that were spontaneously enriched from some mixed HDMEC populations (Figure 1P). Thus, CLEVER-1 is always found intracellularly in the isolated lymphatic and vascular HDMECs, but its surface expression is variable (and therefore the surface expression of CLEVER-1 was always verified in subsequent experiments by FACS analyses).
FEEL-1 has been reported to be expressed in HUVECs based on immunoblotting results.22 We extended these findings by showing that HUVECs are surface positive for CLEVER-1 in FACS stainings (Figure 1P). The level of CLEVER-1 staining on HUVECs was always very low, and the maximal expression required growth to confluency in the presence of VEGF. Together these data for the first time show evidence for surface expression of CLEVER-1 on isolated vascular and lymphatic endothelial cells.
Multiple forms of CLEVER-1 are present both in vascular and lymphatic endothelial cells
CLEVER-1 is a large 270- to 300-kDa glycoprotein encoded by 69 exons, which creates multiple possibilities for posttranslational modifications and alternative splicing. We have shown that in lysates made from whole tissue containing both vascular and lymphatic vessels, several forms of CLEVER-1 exist.19 Isolation of dermal vascular and lymphatic endothelial cells to purity allowed us to ask whether certain isoforms are exclusively expressed by one or the other endothelial cell type. Immunoblotting for CLEVER-1 in cell lysates revealed a molecule that had the molecular weight in the same range as CLEVER-1 in intact tissue (Figure 2A). Similar to native CLEVER-1, multiple CLEVER-1 isoforms were observed in unselected HDMEC populations containing both types of endothelial cells. Analyses of the purified endothelial subpopulations showed that both vascular and lymphatic endothelial cells express multiple isoforms of CLEVER-1, although there were certain differences in the relative abundance of each form depending on the cell type (Figure 2A). Glycosidase digestions revealed that different CLEVER-1 isoforms in both lymphatic and vascular endothelial cells contained sialic acid and O-linked oligosaccharides (Figure 2B).
Vascular and lymphatic endothelial cells express multiple isoforms of CLEVER-1. (A) The indicated cell (2 different HDMEC populations containing both vascular and lymphatic endothelial cells [mixed], pure vascular and pure lymphatic fractions of HDMECs, and HUVECs) and tissue lysates (tonsil) were analyzed by immunoblotting for CLEVER-1 protein. Molecular weight (Mw) markers in kilodaltons are indicated on the left. (B) Different isoforms of CLEVER-1 contain posttranslational modifications in endothelial cells. Lysates from lymphatic (pure lymphatic fraction of HDMECs) and vascular (HUVECs) endothelial cells were left untreated (-), digested with sialidase (Sial), or digested with sialidase followed by O-glycanase (Sial + O-glyc) and analyzed by immunoblotting. (C) The presence of the variably spliced exon 27 in the samples of lymphatic and vascular HDMECs, and HUVECs was analyzed by RT-PCR. The upper band represents the exon 27-containing transcript of the CLEVER-1 gene, whereas the lower band indicates the presence of CLEVER-1 mRNA isoform in which the exon 27 has been spliced out. Representative results of 2 independent experiments are shown.
Vascular and lymphatic endothelial cells express multiple isoforms of CLEVER-1. (A) The indicated cell (2 different HDMEC populations containing both vascular and lymphatic endothelial cells [mixed], pure vascular and pure lymphatic fractions of HDMECs, and HUVECs) and tissue lysates (tonsil) were analyzed by immunoblotting for CLEVER-1 protein. Molecular weight (Mw) markers in kilodaltons are indicated on the left. (B) Different isoforms of CLEVER-1 contain posttranslational modifications in endothelial cells. Lysates from lymphatic (pure lymphatic fraction of HDMECs) and vascular (HUVECs) endothelial cells were left untreated (-), digested with sialidase (Sial), or digested with sialidase followed by O-glycanase (Sial + O-glyc) and analyzed by immunoblotting. (C) The presence of the variably spliced exon 27 in the samples of lymphatic and vascular HDMECs, and HUVECs was analyzed by RT-PCR. The upper band represents the exon 27-containing transcript of the CLEVER-1 gene, whereas the lower band indicates the presence of CLEVER-1 mRNA isoform in which the exon 27 has been spliced out. Representative results of 2 independent experiments are shown.
Exon 27 of the CLEVER-1 gene can be alternatively spliced in various tissues.19 RT-PCR analysis of the variable splicing of this exon in the 2 types of endothelial cells showed that both RNA variants of CLEVER-1 are transcribed in both populations (Figure 2C). This exon contains no putative N- or O-glycosylation sites and it encodes only for 32 amino acids. Thus, it alone cannot explain the size difference of CLEVER protein isoforms seen in immunoblots, and thus other posttranslational modifications and/or other splice variants must exist. In conclusion, these data show that both vascular and lymphatic endothelial cells can produce multiple isoforms of CLEVER-1, which contain posttranslational oligosaccharide modifications and have apparent molecular weights similar to those seen in lymphoid tissues in vivo.
Vascular CLEVER-1 mediates PBMC rolling and transmigration during the multistep adhesion cascade under flow
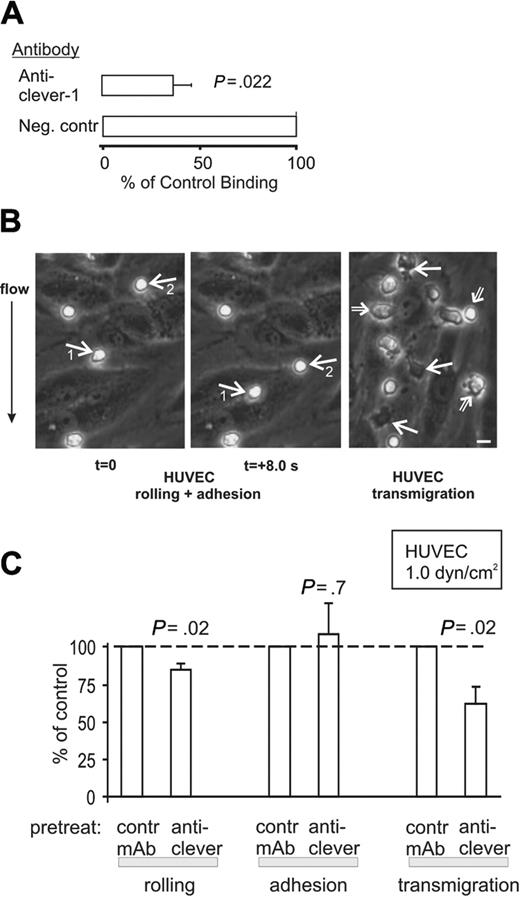
In vitro adhesion assays using frozen sections showed that anti-CLEVER-1 mAbs statistically significantly inhibit PBMC binding to blood vessels in inflamed skin (Figure 3A), but it was technically impossible to analyze the adherence to small and irregular lymphatic vessels in these specimens. Moreover, this assay does not permit any detailed analyses of the role of CLEVER-1 in the extravasation cascade. Therefore to study whether cell surface-expressed CLEVER-1 on living endothelial cells can support physiologically relevant leukocyte extravasation, in vitro laminar flow chamber assays were used. In this assay, PBMCs are perfused with a defined shear stress over a monolayer of endothelial cells under a videomicroscope. There are 3 types of interactions—rolling, firm adhesion, and transmigration—that can be easily distinguished and analyzed from these experiments (Figure 3B).
CLEVER-1 mediates transmigration through vascular endothelium under shear flow. (A) In the in vitro frozen section assay, anti-CLEVER-1 mAbs significantly inhibit PBMC binding to blood vessel endothelium in inflamed skin samples. Results are shown as mean ± SEM (compared with binding in the presence of a nonblocking negative control mAb). (B) PBMCs were perfused over a confluent monolayer of HUVECs at a laminar shear stress of 1.0 dyne/cm2. In 2 frames taken 8 seconds apart, the 2 rolling cells (white arrows) can be seen to travel downward (the direction of flow is from top to bottom). The 2 other PBMCs remain stably adherent. In the third panel, 3 PBMCs, which have transmigrated (and have become completely phase dark) through the HUVEC monolayer, are pointed out by white solid arrows. All other PBMCs (3 pointed out by open arrows) remain surface adherent (phase bright; the brightness of these cells varies depending on the extent of flattening during the firm adhesion). Bar, 10 μm. These 3 different forms of interactions between PBMCs and HUVECs can be much more readily seen in the video (Supplementary video 1 on the Blood website; see the Supplemental Video link at the top of the online article). (C) The numbers of rolling, adherent, and transmigrated cells were enumerated from video playbacks after different pretreatments. The results are expressed as percentage of binding when compared with control mAb (a mAb against HLA class I, which stains the HUVECs but does not block PBMC adhesion). The results are mean ± SEM from 5 to 6 independent experiments using different HUVEC and PBMC donors.
CLEVER-1 mediates transmigration through vascular endothelium under shear flow. (A) In the in vitro frozen section assay, anti-CLEVER-1 mAbs significantly inhibit PBMC binding to blood vessel endothelium in inflamed skin samples. Results are shown as mean ± SEM (compared with binding in the presence of a nonblocking negative control mAb). (B) PBMCs were perfused over a confluent monolayer of HUVECs at a laminar shear stress of 1.0 dyne/cm2. In 2 frames taken 8 seconds apart, the 2 rolling cells (white arrows) can be seen to travel downward (the direction of flow is from top to bottom). The 2 other PBMCs remain stably adherent. In the third panel, 3 PBMCs, which have transmigrated (and have become completely phase dark) through the HUVEC monolayer, are pointed out by white solid arrows. All other PBMCs (3 pointed out by open arrows) remain surface adherent (phase bright; the brightness of these cells varies depending on the extent of flattening during the firm adhesion). Bar, 10 μm. These 3 different forms of interactions between PBMCs and HUVECs can be much more readily seen in the video (Supplementary video 1 on the Blood website; see the Supplemental Video link at the top of the online article). (C) The numbers of rolling, adherent, and transmigrated cells were enumerated from video playbacks after different pretreatments. The results are expressed as percentage of binding when compared with control mAb (a mAb against HLA class I, which stains the HUVECs but does not block PBMC adhesion). The results are mean ± SEM from 5 to 6 independent experiments using different HUVEC and PBMC donors.
Since vascular cells isolated from HDMECs proved to be difficult to grow in glass capillaries and have not been routinely used in these types of assays, we used CLEVER-1-positive HUVECs in the flow assays. As shown in Figure 3C, anti-CLEVER-1 mAbs had a very small, although statistically significant, inhibitory effect on PBMC rolling on HUVECs at a laminar shear stress of 1.0 dyne/cm2. In contrast, blocking of CLEVER-1 had no effect on the number of firmly adherent cells. When the adherent cells were allowed to transmigrate for 20 minutes in the presence of constant shear, inhibition of CLEVER-1 with mAbs diminished the number of transmigrating PBMCs by almost 40% in comparison with control-treated cells (Figure 3C). It should be noted that the percent of transmigrating cells is defined as the number of transmigrated cells from all interacting cells (ie, surface adherent plus transmigrated). Thus, the possible slight increase in the absolute numbers of firmly adhered cells in the presence of anti-CLEVER mAbs does not bias these results. Anti-CLEVER mAbs also blocked 32 ± 2% (mean ± SEM, n = 6) of PBMC transmigration through HUVEC monolayers in 3-hour static Transwell assays when compared with control mAb-treated wells (P < .01). Thus CLEVER-1 on vascular endothelial cells plays a significant role in the extravasation cascade, and especially in transmigration, under physiologically relevant laminar shear stress.
CLEVER-1 mediates hyaluronan-independent interactions
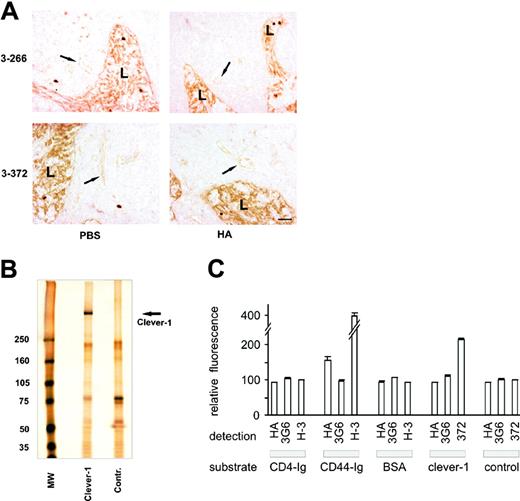
CLEVER-1 protein contains a cartilage link domain sequence,19,20 which is typically found in hyaluronan receptors.37 Since endothelial hyaluronan mediates leukocyte rolling,38 it was of great interest to determine whether CLEVER-1 binds hyaluronan. When tissue sections were pretreated with soluble hyaluronan, no effect on binding of anti-CLEVER-1 mAbs 3-266 or 3-372 to endothelial CLEVER-1 was observed (Figure 4A). We then measured binding of FITC-labeled hyaluronan to immunoaffinity-purified CLEVER-1 from human peripheral lymph nodes and to control proteins binding to control columns (Figure 4B). In these enzyme-linked immunosorbent assays (ELISAs), hyaluronan effectively interacted with CD44 receptor globulin, a known hyaluronan receptor,39 but not with nonspecific proteins in the eluates, CD4 receptor globulin or BSA (Figure 4C). Hyaluronan did not bind to CLEVER-1 either, whereas anti-CLEVER mAbs specifically reacted with the immobilized protein. These data show that CLEVER-1 is not a hyaluronan-binding protein, and hence its function in leukocyte-endothelial interactions is hyaluronan independent.
The link protein-like sequence of CLEVER-1 does not mediate hyaluronan binding. (A) Peripheral lymph node sections were pretreated with 200 μg/mL hyaluronan (HA) or left untreated (PBS). Thereafter the sections were stained for CLEVER-1 expression using mAbs 3-266 and 3-372. Arrows point to HEV; L indicates lymphatic endothelium. Bar, 50 μm. (B) CLEVER-1 and control proteins were immunoaffinity purified from human peripheral lymph node lysate. Silver staining shows the purity of the isolated endogenous CLEVER-1 protein (arrow). MW indicates molecular weight standards in kilodaltons. (C) CD4 receptor globulin (CD4-Ig), CD44 receptor globulin (CD44-Ig), BSA, CLEVER-1 protein, and proteins from a control eluate (control) were absorbed on the bottom of an ELISA plate. The immobilization of the relevant proteins was confirmed by sequential incubation of the wells with anti-CD44 mAb Hermes-3 (H-3), anti-CLEVER-1 mAb 3-372, or a negative control mAb. The hyaluronan binding capacity of the bound proteins was analyzed by their ability to bind FITC-labeled hyaluronan (HA). The results from a representative experiment (2 wells for each treatment) are shown as the absolute fluorescent absorbances (mean ± SEM). Similar results were obtained in 2 other independent experiments.
The link protein-like sequence of CLEVER-1 does not mediate hyaluronan binding. (A) Peripheral lymph node sections were pretreated with 200 μg/mL hyaluronan (HA) or left untreated (PBS). Thereafter the sections were stained for CLEVER-1 expression using mAbs 3-266 and 3-372. Arrows point to HEV; L indicates lymphatic endothelium. Bar, 50 μm. (B) CLEVER-1 and control proteins were immunoaffinity purified from human peripheral lymph node lysate. Silver staining shows the purity of the isolated endogenous CLEVER-1 protein (arrow). MW indicates molecular weight standards in kilodaltons. (C) CD4 receptor globulin (CD4-Ig), CD44 receptor globulin (CD44-Ig), BSA, CLEVER-1 protein, and proteins from a control eluate (control) were absorbed on the bottom of an ELISA plate. The immobilization of the relevant proteins was confirmed by sequential incubation of the wells with anti-CD44 mAb Hermes-3 (H-3), anti-CLEVER-1 mAb 3-372, or a negative control mAb. The hyaluronan binding capacity of the bound proteins was analyzed by their ability to bind FITC-labeled hyaluronan (HA). The results from a representative experiment (2 wells for each treatment) are shown as the absolute fluorescent absorbances (mean ± SEM). Similar results were obtained in 2 other independent experiments.
CLEVER-1 on lymph endothelium is involved in the transmigration of PBMCs
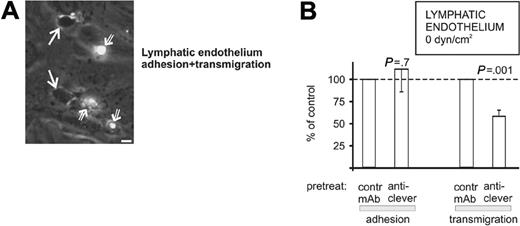
The flow in the lymphatic vasculature in vivo is weak in comparison with the venules in the circulation. To study the adhesive function of CLEVER-1 in the lymphatics, lymphatic endothelial cells (75% were surface positive for CLEVER-1) were seeded in the capillaries, and PBMCs were perfused on them. Thereafter, the flow was stopped and the cells were allowed to interact under static conditions. After 20 minutes, the noninteracting cells were gently washed away, and the numbers of surface adherent and transmigrated cells were determined as above.
This assay showed that PBMCs indeed bind firmly to lymphatic endothelial cells and transmigrate through the endothelial monolayer (Figure 5A). Enumeration of the adherent cells showed that the anti-CLEVER-1 mAb treatment has no significant effect. In contrast, when the function of CLEVER-1 was blocked, the number of transmigrating cells was decreased by approximately 40% (Figure 5B). In other experiments, lymphatic endothelial cells, which had become surface negative for CLEVER-1, showed no inhibition of transmigration in response to anti-CLEVER mAb treatment, further confirming the specificity of the mAb treatment in this assay (data not shown). Thus, CLEVER-1 on lymphatic endothelium is important in mediating PBMC transmigration through this type of endothelial cell.
CLEVER-1 is involved in PBMC transmigration through lymphatic endothelium. (A) PBMCs were allowed to adhere and transmigrate through a confluent monolayer of lymphatic endothelium under static conditions (see “Materials and methods”). In the micrograph, 2 transmigrated cells (solid white arrows; phase dark) and 3 surface adherent cells (white open arrows; phase bright; the level of brightness depending on the extent of flattening of the adherent cells) are shown. Bar, 10 μm. (B) The numbers of adherent and transmigrated cells were then enumerated from video playbacks after different mAb pretreatments. The results are expressed as percentage of binding when compared with control mAb (a binding mAb against HLA class I, which does not block PBMC adherence). The results are mean ± SEM from 5 independent experiments.
CLEVER-1 is involved in PBMC transmigration through lymphatic endothelium. (A) PBMCs were allowed to adhere and transmigrate through a confluent monolayer of lymphatic endothelium under static conditions (see “Materials and methods”). In the micrograph, 2 transmigrated cells (solid white arrows; phase dark) and 3 surface adherent cells (white open arrows; phase bright; the level of brightness depending on the extent of flattening of the adherent cells) are shown. Bar, 10 μm. (B) The numbers of adherent and transmigrated cells were then enumerated from video playbacks after different mAb pretreatments. The results are expressed as percentage of binding when compared with control mAb (a binding mAb against HLA class I, which does not block PBMC adherence). The results are mean ± SEM from 5 independent experiments.
Discussion
We show here that CLEVER-1 is expressed in lymphatic, but not vascular, endothelial cells in normal skin in vivo. In contrast, in many skin diseases chronically inflamed vascular vessels surrounded by lymphocytic infiltrate start to express CLEVER-1. CLEVER-1 is also present on cultured dermal lymphatic endothelial cells and to a lesser extent on vascular endothelial cells of dermal or umbilical vein origin. We report here a functional role for CLEVER-1 in PBMC-endothelial cell interactions during the multistep adhesion cascade under physiologically relevant shear stress. CLEVER-1 on both vascular and lymphatic endothelium mainly supports PBMC transmigration. These data add CLEVER-1 into the growing family of endothelial adhesion molecules supporting leukocyte egress from the blood vessels and are the first description of a lymphatic adhesion molecule functionally supporting PBMC traffic through this special kind of endothelium.
The unambiguous surface expression of native CLEVER-1 on our cultured lymphatic and vascular endothelial cells is the first direct evidence for the luminal location of CLEVER-1/FEEL-1/stabilin-1. In fact, stabilin-1 was not detectable in HUVECs either at mRNA level or using mAb MS-1 stainings,20,40 although the mAb stains HEVs and in continuous vascular endothelial cells in certain angiogenetic/inflammatory lesions in skin.40,41 Based on the functional results, the anti-FEEL-1 mAb must detect recombinant FEEL-1 on Chinese hamster ovary (CHO) transfectants (although no FACS stainings have been reported), whereas our mAbs detected only intracellular CLEVER-1 in more than 20 independent CHO transfections we have performed so far, and mAb MS-1 is also reported to react exclusively with a cytoplasmic protein in 293 cells transfected with stabilin-1.20 Hence, mAbs against CLEVER-1 and those against FEEL-1 and stabilin-1 most likely see different epitopes on this large glycoprotein, and this should be taken into account when interpreting the results on the multifunctional nature of CLEVER-1 obtained with different anti-CLEVER-1/FEEL-1/stabilin-1 mAbs.
On vascular endothelium, CLEVER-1 contributes mostly to the transmigration process. The 40% inhibition of PBMC transmigration seen after blockade of CLEVER-1 is quite remarkable, when taking into account the low expression level of this molecule on cultured HUVECs and the intactness of all other adhesion molecules in this system. Since leukocytes are generally believed to transmigrate through the endothelial junctions, it is interesting to note that ultrastructural studies on stabilin-1 (using mAb MS-1) have shown it to be concentrated at intercellular junctions in sinusoidal cells.40 So far only a few endothelial molecules have been shown to mediate lymphocyte transmigration through vascular endothelium. Of these, CD31, junctional adhesion molecules A, and C are best documented, but CD99, intercellular adhesion molecule, vascular cell adhesion molecule 1, and vascular adhesion protein-1 also appear to contribute to this process.42-47 Since anti-CLEVER-1 treatment, like inhibition of these other molecules, blocks the transmigration only partially, we believe that all these molecules act in parallel to support the complex process of diapedesis in vivo. It is intriguing that of all these molecules only CLEVER-1 is completely absent from normal vascular endothelial cells in nonlymphoid organs. Our data thus suggest that therapeutic blockade of CLEVER-1 might preferentially interfere with leukocyte accumulation at the desired site (inflammatory lesions) while leaving physiologic extravasation of lymphocytes in vessels of nonlymphoid peripheral tissues completely intact.
CLEVER-1 is constitutively expressed on lymphatic endothelium both in vitro and in vivo. We have shown earlier with the frozen section binding assay that anti-CLEVER-1 mAbs block PBMC adhesion to larger efferent lymphatic vessels in lymphoid tissues.19 Here we show that small afferent lymphatic endothelial cells (isolated from skin) also use CLEVER-1 for interactions with PBMCs. Moreover, we were able to demonstrate for the first time that it is the transmigration step of PBMC traffic in the lymphatic vasculature that is mediated via CLEVER-1. CLEVER-1 appears to be expressed both on the luminal and abluminal surface of lymphatic endothelium. Therefore, we envision that the few lymphocytes continuously recirculating through normal skin or those immigrating into inflamed skin in massive numbers may use CLEVER-1 when they penetrate into the blind-ended lymphatic vessels in the skin on their way to the lymph nodes. In organized lymphatic tissues, CLEVER-1 may again be active when the lymphocytes emigrate into the tissue from the subcapsular sinus or from ill-defined radial/cortical sinuses penetrating into the node. As CLEVER-1 also mediates lymphocyte binding to efferent lymphatics in the lymph node, it is likely to be important in the entry of blood-originated lymphocytes to the efferent arm of the lymphatic vasculature as well. Thus, CLEVER-1 is the first endothelial adhesion molecule shown to be involved in PBMC transmigration through living lymphatic endothelial cells and, in fact, through 2 fundamentally different (lymphatic and vascular) types of endothelial cells.
The functional role of CLEVER-1 in transmigration of PBMCs through both lymphatic and vascular endothelium makes it an attractive target for antiadhesive therapies. Thus, blockade of CLEVER-1 is likely to cause reduced emigration of blood-borne leukocytes to the sites of inflammation. If a CLEVER-1 blocking agent is delivered to the lymphatic vasculature, it should also diminish lymphocyte trafficking into and through lymph nodes and hence down-modulate the generation of unwanted immune responses. CLEVER-1 also mediates vascular and lymphatic binding of tumor cells.48 Thus, these same functional modalities of CLEVER-1 should also be useful for therapeutic interventions aiming at inhibition of metastatic processes through these 2 types of vascular systems.
The sequence of CLEVER-1 suggests that it might be a hyaluronan receptor.19,20 Here we found no experimental evidence for this interaction when we tested hyaluronan binding capacity of natural CLEVER-1 isolated from human peripheral lymph nodes. Since the cartilage link protein-like sequence is quite membrane proximal in the large CLEVER-1 protein (176 amino acids above the predicted transmembrane sequence), it may simply not extend long enough above the cell glycocalyx to be functional. However, the finding that purified CLEVER-1 protein does not bind hyaluronan strongly suggests that this motif is not functional at all in CLEVER-1.
It has been shown that an antibody against FEEL-1 results in an increase of the empty area surrounded by the capillary tube network in a Matrigel assay,22 suggesting that FEEL-1 facilitates in vitro angiogenesis. In contrast, our anti-CLEVER-1 mAbs had absolutely no discernable effect on the size of the areas surrounded by capillary tubes or in the number of capillary branches in the similar Matrigel assays (data not shown). Since our antibodies are function blocking in leukocyte binding, these data show that different epitopes of CLEVER-1 are used for PBMC-endothelial adhesion and endothelial-endothelial adhesion. Thus, in terms of potential antiadhesive applications of CLEVER-1 blockade, our mAbs should have no unwanted side effects on angiogenesis.
In conclusion, we have shown here that CLEVER-1 is expressed on both vascular and lymphatic endothelium both in vitro and in vivo. During the lymphocyte extravasation cascade, this molecule plays a significant role in transmigration of cells through vascular and lymphatic endothelium. Hence, blocking of the function of CLEVER-1 may be useful in diminishing inappropriate inflammatory reactions.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-01-0222.
Supported by the Finnish Academy, Technology Development Centre of Finland, the Juselius Foundation, and Cancer Union of Finland.
K.K. and T.H. contributed equally to this work.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Kirsti Kalimo for skin specimens, Dr Olli Vainio for control mAbs, and Dr Heikki Irjala for fruitful discussions. We are thankful to Mrs Mari Parsama, Riikka Sjöroos, Pirjo Heinilä, and Laila Reunanen for invaluable technical help and Mrs Anne-Sovikoski-Georgieva for secretarial assistance.

![Figure 1. Both lymphatic and vascular endothelial cells express CLEVER-1. Immunohistologic stainings of (A) normal and (B) inflamed skin (psoriasis). CLEVER-1 is constitutively expressed in the lymphatic endothelial cells (some pointed out by thin black arrows) but induced in vascular HEV-like vessels (thicker white arrows) only in inflammation. Bar, 50 μm. (C-E) Confocal microscopy shows that lymphatic vessels in normal skin are CLEVER-1 positive (C, CLEVER-1 in red; D, LYVE-1 in green; E, merged image), whereas CLEVER-1 is absent from blood vessels (F, CLEVER-1 in green; G, PAL-E in red; H, merged image, no double-positive vessels). In the inflamed psoriatic skin, lymphatic vessels retain CLEVER-1 expression (I, CLEVER-1 in red; J, LYVE-1 in green; K, merged image). In inflammation, CLEVER-1 is induced also in blood vessels (HEV-like venule coexpresses CLEVER-1 [green, L] and PAL-E [red, M] as seen by the yellow signal in the merged image [N]; 0.3-μm optical sections). Representative negative control stainings are shown in the inserts. Bars, 20 μm. (O) An immunofluorescent image of permeabilized HDMECs shows that they are a mixed population of endothelial cells consisting of islets of vascular (CD44+, green) and lymphatic (CD44-, no green fluorescence) endothelial cells. Both types of endothelial cells are CLEVER-1 positive (red, mainly intracellular staining of fine granules/vesicles). Bar, 20 μm. A5× higher magnification of the area highlighted in the schematic picture is shown on the right. BEC indicates blood endothelial cells; LEC, lymphatic endothelial cells. (P) Surface stainings of immunomagnetically separated vascular and lymphatic endothelial cells, spontaneously enriched lymphatic cells, and HUVECs are shown for the indicated antigens. In the bottom line, gray histograms show staining with the negative control mAb 3G6 and black histograms, with anti-CLEVER-1 mAb 3-372. The mean fluorescence intensities of negative control and CLEVER-1 histograms are depicted in the upper right corner (neg.co/CLEVER-1). (Q) Lymphatic endothelial cells isolated from HDMECs based on the absence of CD44 (see P), and HUVECs were immunofluorescently stained for the expression of an established lymphatic marker, podoplanin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-01-0222/6/m_zh80240471020001.jpeg?Expires=1769097460&Signature=sKC6faOdUUlZJh443kPqOLVEezjVgfIcIUDGrwGFS7PsRmKyQg~PJY3DvCkbOLOUJzcbJEav07UUDrs3M8H-J6494mH~z~VDblAtLgnlwxXuiiOAzofKysNo34y5SJndp9R2m3Utr5P2DIrSGrjFcZb1OiN5I2C9FiTU8CYvu9oLMHp-S7jCUGDK5tp9vcq0NgNvDfr8WiHa4552iYHS8ncnXvfnNH~NxHSB1d76juALcWAupc7EWsgZqM4r7dQxMhRtJ~NQTJHu8GjeFJCFcgk~mL3Rk7sBbmlZlLNQt~~BNxkWsyLvaXIQ8658dKQv1qWlm2CgtpnHTby1gLc5yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Vascular and lymphatic endothelial cells express multiple isoforms of CLEVER-1. (A) The indicated cell (2 different HDMEC populations containing both vascular and lymphatic endothelial cells [mixed], pure vascular and pure lymphatic fractions of HDMECs, and HUVECs) and tissue lysates (tonsil) were analyzed by immunoblotting for CLEVER-1 protein. Molecular weight (Mw) markers in kilodaltons are indicated on the left. (B) Different isoforms of CLEVER-1 contain posttranslational modifications in endothelial cells. Lysates from lymphatic (pure lymphatic fraction of HDMECs) and vascular (HUVECs) endothelial cells were left untreated (-), digested with sialidase (Sial), or digested with sialidase followed by O-glycanase (Sial + O-glyc) and analyzed by immunoblotting. (C) The presence of the variably spliced exon 27 in the samples of lymphatic and vascular HDMECs, and HUVECs was analyzed by RT-PCR. The upper band represents the exon 27-containing transcript of the CLEVER-1 gene, whereas the lower band indicates the presence of CLEVER-1 mRNA isoform in which the exon 27 has been spliced out. Representative results of 2 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-01-0222/6/m_zh80240471020002.jpeg?Expires=1769097460&Signature=sZe~NTTnAktiO2TibK-btqgido7dhUh-pRdbOMjp3bRFH6lTbLw3YO~KRpfqADO7XKpFyxeGgXZgcdqPKPGBo8E3mpXla78GUEHTK52wjU5zQ0y5Tvi49b-9FMf6UF4yKioPUZ8E9BAL8RpcwUbKz9Eug0OrfQQANN8GiP2cDF9cdGoUZFqoQEgCrnKmVa44jgy3Xvi88FKkbrDBut-xsJs8SKp4EMQA~wlLoSv9pxEhkEZbdA-eD46jhQ5OocY-SEDvqJpUPtU4lK3jyoNGhJYLXictjS4HJOleREg7Bq1r42dI78XFQ3vXp5FLWMF-vJEDVQkaOb73c4Vib9opGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal