Comment on Krause and Van Etten, page 4236
Separating graft-versus-host disease (GvHD) from the graft-versus-leukemia (GvL) effect is central to the success of adoptive immunotherapy. In this issue, Krause and Van Etten describe how a murine model of CML can be used to test strategies to dissect GvL from GvHD.
The year 1990 marks 2 major advances in the study and therapy of chronic myeloid leukemia (CML). Lethally irradiated mice that received transplants of BCR-ABL-transduced bone marrow from syngeneic donors were shown to develop a CML-like myeloproliferative disease.1 Almost simultaneously, it was demonstrated that relapse of CML after allografting could be effectively treated with donor leukocyte infusions (DLIs), providing proof of concept for adoptive immunotherapy.2 Surprisingly, it is only now that the transduction/transplantation model is being used to study adoptive immunotherapy.
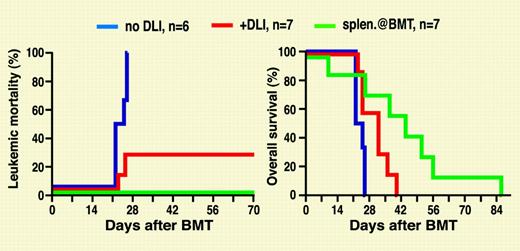
In the Krause and Van Etten study, lethally irradiated mice received transplants of T-cell-depleted BCR-ABL-transduced syngeneic bone marrow cells together with allogeneic cells from a different strain. With major histocompatibility complex (MHC)-mismatched allogeneic donors, mixed chimerism and CML developed, mimicking relapse after allografting. Addition of donor splenocytes to the graft prevented leukemia but at the price of death from graft-versus-host disease (GvHD). Administration of splenocytes to mixed chimeras with established leukemia, in analogy to DLI for relapsed CML, induced remissions, indicating a graft-versus-leukemia (GvL) effect. Additional experiments showed that GvL could be induced by CD4+ but also CD4- cells. Similar results were seen in a murine model of BCR-ABL-positive acute lymphoblastic leukemia. Unfortunately, all mice eventually succumbed to GvHD. In additional experiments, MHC-matched mice were used as donors to see whether minor histocompatibility antigens (miHAs) may induce GvL, while avoiding GvHD. No GvHD occurred, but the antileukemic activity of DLI in this setting was only moderate, and the mice died from leukemia.FIG1
Leukemia mortality (left) and overall survival (right) in mice that received transplants of BCR-ABL-transduced syngeneic bone marrow and T-cell-depleted allogeneic bone marrow. Allogeneic donor splenocytes were injected at the time of the transplantation or as DLIs starting on day 14.
Leukemia mortality (left) and overall survival (right) in mice that received transplants of BCR-ABL-transduced syngeneic bone marrow and T-cell-depleted allogeneic bone marrow. Allogeneic donor splenocytes were injected at the time of the transplantation or as DLIs starting on day 14.
Although DLI for CML has been much refined,3 the conundrum remains the difficulty of avoiding GvHD, while at the same time maintaining GvL. Thus, animal models are needed where DLI can be studied in a physiologic context. One major advantage of the Krause-Van Etten model is that the recipient's normal hematopoiesis and the leukemia have the same genetic background, unlike models using leukemia cell lines with multiple genetic lesions. Nonetheless, refinements will be necessary to generate a model that truly reflects adoptive immunotherapy of relapsed human CML. One problem is that the murine disease, although morphologically “chronic,” is much more aggressive than its human equivalent, killing the mice before an effective miHA-directed immune response can be launched, whereas DLI in MHC-mismatched recipientskills the mice along with the leukemia. Thus, attenuation of the leukemia may be instrumental for establishing a more physiologic system. Further, the degree of miHA disparity and the dose and type of donor lymphocytes are important. A recent study with different miHA-disparate donor/recipient combinations showed that simultaneous transplantation of unselected donor lymphocytes (from lymph nodes) together with BCR-ABL-transduced syngeneic cells prevented leukemia, without inducing GvHD. Leukemia developed in mice that received CD8+ donor lymphocytes, but was subsequently cleared, if the T-cell dose was sufficiently high; this came, however, at the cost of GvHD.4 An additional caveat is that the immune response to leukemia-specific antigens, such as myeloperoxidase, plays a major role in HLA-matched allografts.5 Nonetheless, the work of Krause and Van Etten is an important step toward developing a physiologic model for the study of adoptive immunotherapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal