The development of multiple myeloma (MM) bone disease is mediated by increased number and activity of osteoclasts (OCs). Using an in vitro osteoclastogenesis model consisting of unstimulated and unfractionated peripheral blood mononuclear cells (PBMCs) from patients with MM, we showed that T cells support the formation of OCs with longer survival. Different from T-cell–depleted MM PBMC cultures, exogenous macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) were necessary for the formation of OCs; however, they did not exhibit longer survival. We found up-regulated production of RANKL, osteoprotegerin (OPG), and TNF-related apoptosis-inducing ligand (TRAIL) by fresh MM T cells. Despite high OPG levels, the persistence of osteoclastogenesis can be related to the formation of the OPG/TRAIL complex demonstrated by immunoprecipitation experiments and the addition of anti-TRAIL antibody which decreases OC formation. OCs overexpressed TRAIL decoy receptor DcR2 in the presence of MM T cells and death receptor DR4 in T-cell–depleted cultures. In addition, increased Bcl-2/Bax (B-cell lymphoma-2/Bcl2-associated protein X) ratio, following Bcl-2 up-regulation, was detected in OCs generated in the presence of T cells. Our results highlight that MM T cells support OC formation and survival, possibly involving OPG/TRAIL interaction and unbalanced OC expression of TRAIL death and decoy receptors.
Introduction
Multiple myeloma (MM) is a B-cell neoplasm characterized by clonal expansion of malignant plasma cells in the bone marrow (BM) with frequent occurrence of lytic bone disease; in about one third of cases, MM follows the monoclonal gammapathy of undetermined significance (MGUS).1 MM lytic bone disease, causing a high morbidity, is observed in most patients. It results from an unbalanced bone turnover with enhanced resorption related to increased osteoclast (OC) recruitment and activity and low bone formation. The interaction between MM cells and BM microenvironment is essential for maintenance and progression of the disease process. In particular, the reciprocal relationship between MM cells and OCs is known to be critical for the induction of osteoclastogenesis and the activation of bone resorption1,2 as well as for the inhibition of osteoblasts, thus preventing lesion repair.3 The linkage between immunoregulation by T cells and bone loss is becoming more evident in MM and other bone loss–associated diseases.4-11
Osteoclastogenesis is positively or negatively regulated by a complex signaling system that involves the receptor activator of nuclear factor (NF)–κB (RANK), osteoprotegerin (OPG), and receptor activator of NF-κB ligand (RANKL), all belonging to the tumor necrosis factor (TNF) family.12 The osteoclastogenic factor RANKL is expressed by osteoblasts and stromal cells as a membrane-bound protein, cleaved into a soluble molecule (sRANKL) by metalloproteinases13-15 ; it is also secreted by activated T cells in a primary soluble form, being a crucial paracrine link between bone metabolism and the immune system.16,17 RANKL promotes differentiation and fusion of OC precursor cells (OCPs) and activates mature OCs to bone resorption by binding to its specific receptor RANK.17 Early stage OCPs express c-fms, that is the cellular receptor for macrophage colony-stimulating factor (M-CSF) necessary for OC development.18 Further, TNF-α synergizes with RANKL and potentiates osteoclastogenesis.19
OPG, a soluble decoy receptor secreted by osteoblasts and BM stromal cells, competes with RANK for binding to RANKL, preventing its osteoclastogenic effect.13-15,20-24 In addition, OPG can act as a decoy receptor for TNF-related apoptosis-inducing ligand (TRAIL), exerting an antiapoptotic effect.25-28
TRAIL is a cytotoxic protein inducing apoptosis mostly in tumor cells, upon binding to death-domain–containing receptors DR4 and DR5. It can activate the apoptotic pathway of MM cells by modulating the Bcl-2 family proteins29 ; among these, the ratio between Bcl-2 and Bax, antiapoptotic and proapoptotic molecules, respectively, is of major relevance for cell survival.30,31 TRAIL activity can be modulated by association with 2 membrane-bound decoy receptors, namely DcR1 and DcR2, which lack functional death domains and conferring TRAIL resistance on expressing cells. Furthermore, it has been shown that TRAIL may regulate OPG activity through the OPG/TRAIL interaction, which can induce cross-regulatory mechanisms between the 2 molecules.25
OC biology appears to be dominantly regulated by the RANK/RANKL/OPG axis. In particular, the RANK/RANKL interaction is implicated in induction of osteoclastogenesis and activation of bone resorption. The binding of soluble OPG to RANKL, by interference with the RANK/RANKL interaction, inhibits OC differentiation and activation neutralizing the bone resorption effect. However, OPG binding to TRAIL can be followed by both inhibition of OPG anti-osteoclastogenic activity and blocking of TRAIL-induced apoptosis.25,32,33 A disruption of the RANKL/OPG ratio, following up-regulation of RANKL and down-regulation of OPG expression, has been reported in MM.34,35 More recently, Giuliani et al have suggested that T cells can contribute to MM-induced osteoclastogenesis through up-regulated RANKL and down-regulated interferon γ (IFN-γ) secretion.11
On the basis of the novel paradigm for T cells as regulators of bone turnover,5 the primary aim of our study was to investigate the potential involvement of MM T cells in osteoclastogenesis using in vitro models consisting of unfractionated peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BM MNCs) derived from patients with MM and control subjects, respectively; parallel T-cell–depleted PBMC and BM MNC cultures derived from patients with MM were also established. Our secondary aim was to assess the expression of the major mediators of osteoclastogenesis in our culture system. The results showed that MM T cells support the formation of OCs and their longer survival via RANKL, OPG, and TRAIL.
Patients, materials, and methods
Patients
The samples included peripheral blood (PB) and BM aspirate from 32 patients diagnosed as having MM. The controls included PB and BM aspirate from 32 subjects with non-neoplastic disease without any skeletal involvement, matched for age and sex. The study was approved by the Institutional Review Board of the Department of Internal Medicine and Public Medicine of University of Bari. The patients gave written informed consent. The diagnosis and the stage of MM were established according to the Southwest Oncology Study Group (SWOG) criteria.36,37 The main clinical characteristics of the patients (15 men and 17 women), aged from 50 to 82 years (median, 65.5 ± 8.7 years), are listed in Table 1. Three of them had stage IA, 7 stage IIA, and 21 stage III (A in 19 and B in the other 2). The M-component was immunoglobulin A (IgA) in 7, IgG in 20, and only light chains in 5 patients. The light chain type was K in 20 and λ in 12 patients. Sixteen patients were included in the study at diagnosis, and the other 16 were included at relapse or at progression of their disease. The latter had already undergone treatment for MM, but they did not receive chemotherapy in the 6 months prior their study entry.
Main clinical characteristics of 32 patients with multiple myeloma
Patient no. . | Sex . | Age, y . | HC . | LC . | Disease status . | Stage . | Lytic bone lesions . |
|---|---|---|---|---|---|---|---|
| 1 | F | 58 | λ | D | IIIA | + | |
| 2 | F | 50 | K | D | IIIA | + | |
| 3 | F | 51 | G | λ | D | IA | + |
| 4 | M | 70 | A | λ | D | IIIA | + |
| 5 | F | 82 | λ | D | IIIB | + | |
| 6 | M | 82 | G | λ | D | IIIA | + |
| 7 | F | 65 | λ | D | IIIA | + | |
| 8 | F | 53 | G | K | D | IIIA | + |
| 9 | M | 64 | A | K | PR | IIA | + |
| 10 | F | 72 | G | K | PR | IA | + |
| 11 | M | 74 | G | K | PR | IIIA | + |
| 12 | F | 62 | A | λ | PR | IIIB | + |
| 13 | F | 65 | G | K | OR | IIIA | + |
| 14 | F | 58 | A | λ | Relapse | IIIA | + |
| 15 | F | 74 | G | K | Relapse | IIIA | + |
| 16 | F | 62 | K | NR-PD | IIIB | + | |
| 17 | M | 76 | G | K | NR-PD | IIIA | + |
| 18 | M | 74 | G | K | NR-PD | IIIA | + |
| 19 | M | 69 | G | λ | NR-SD | IIIA | + |
| 20 | M | 73 | G | K | D | IA | - |
| 21 | F | 74 | G | K | Relapse | IIIA | - |
| 22 | F | 55 | G | K | D | IIA | - |
| 23 | M | 55 | G | K | Relapse | IIA | - |
| 24 | M | 59 | A | λ | D | IIA | - |
| 25 | M | 71 | A | K | D | IIA | - |
| 26 | M | 63 | G | K | Relapse | IIA | - |
| 27 | M | 57 | A | K | Relapse | IIA | - |
| 28 | F | 66 | G | λ | D | IIIA | + |
| 29 | M | 62 | G | K | Relapse | IIIA | + |
| 30 | M | 73 | G | λ | D | IIIA | + |
| 31 | F | 75 | G | K | D | IIIA | + |
| 32 | F | 67 | G | K | D | IIIA | + |
Patient no. . | Sex . | Age, y . | HC . | LC . | Disease status . | Stage . | Lytic bone lesions . |
|---|---|---|---|---|---|---|---|
| 1 | F | 58 | λ | D | IIIA | + | |
| 2 | F | 50 | K | D | IIIA | + | |
| 3 | F | 51 | G | λ | D | IA | + |
| 4 | M | 70 | A | λ | D | IIIA | + |
| 5 | F | 82 | λ | D | IIIB | + | |
| 6 | M | 82 | G | λ | D | IIIA | + |
| 7 | F | 65 | λ | D | IIIA | + | |
| 8 | F | 53 | G | K | D | IIIA | + |
| 9 | M | 64 | A | K | PR | IIA | + |
| 10 | F | 72 | G | K | PR | IA | + |
| 11 | M | 74 | G | K | PR | IIIA | + |
| 12 | F | 62 | A | λ | PR | IIIB | + |
| 13 | F | 65 | G | K | OR | IIIA | + |
| 14 | F | 58 | A | λ | Relapse | IIIA | + |
| 15 | F | 74 | G | K | Relapse | IIIA | + |
| 16 | F | 62 | K | NR-PD | IIIB | + | |
| 17 | M | 76 | G | K | NR-PD | IIIA | + |
| 18 | M | 74 | G | K | NR-PD | IIIA | + |
| 19 | M | 69 | G | λ | NR-SD | IIIA | + |
| 20 | M | 73 | G | K | D | IA | - |
| 21 | F | 74 | G | K | Relapse | IIIA | - |
| 22 | F | 55 | G | K | D | IIA | - |
| 23 | M | 55 | G | K | Relapse | IIA | - |
| 24 | M | 59 | A | λ | D | IIA | - |
| 25 | M | 71 | A | K | D | IIA | - |
| 26 | M | 63 | G | K | Relapse | IIA | - |
| 27 | M | 57 | A | K | Relapse | IIA | - |
| 28 | F | 66 | G | λ | D | IIIA | + |
| 29 | M | 62 | G | K | Relapse | IIIA | + |
| 30 | M | 73 | G | λ | D | IIIA | + |
| 31 | F | 75 | G | K | D | IIIA | + |
| 32 | F | 67 | G | K | D | IIIA | + |
HC indicates heavy chain; LC, light chain; D, at diagnosis; PR, partial remission; OR, objective response; NR, no response; PD, progressive disease; SD, stable disease; + presence of lytic bone lesions; and -, absence of lytic bone lesions.
The patients were divided in 2 subgroups according to the presence (group a) or the absence (group b) of osteolysis. This was documented by skeleton standard radiography and in some cases also by nuclear magnetic resonance (NMR).
Cell cultures
OCs were obtained from unfractionated PBMCs and BM MNCs of the patients with MM and the controls as well as from T-cell–depleted PBMCs and BM MNCs of the patients with MM. PBMCs and BM MNCs were isolated by centrifugation over Histopaque 1077 density gradient (Sigma Chemical, St Louis, MO), diluted at 1 × 106 cells/mL in α-Minimal Essential Medium (α-MEM) and supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco Limited, Uxbridge, United Kingdom). To obtain fully differentiated human OCs, the PBMCs and BM MNCs were then cultured for about 30 days in the presence or absence of 25 ng/mL recombinant human macrophage colony-stimulating factor (rh-MCSF) and increasing amounts of RANKL, ranging from 30 to 100 ng/mL (R&D Systems, Minneapolis, MN). At the end of the culture period, mature OCs were identified as tartrate-resistant acid phosphatase–positive (TRAP+) multinucleated cells (Sigma Aldrich, Milan, Italy) containing 3 or more nuclei. Their resorbing activity was demonstrated by plating the cells on Millennium multiwell slides (Millennium Biologix, Kingston, ON, Canada). To visualize the pits formed by the OCs, the cells were removed by adding NaOCl to each well. The OCs were purified from their precursors and other cell types by trypsin treatment. The photomicrographs of Figures 1 and 2 were obtained using a Nikon Ellipse E400 microscope equipped with Nikon Plan Fluor 10×/0.30 dicl. The microscope was connected with a Nikon digital camera D×M 1200; the acquisition software was Lucia G version 4.61 (build 0.64) for Nikon Italy. The mature OCs, strongly adherent to the plastic, were further characterized by reverse transcriptase–polymerase chain reaction (RT-PCR) for the expression of specific markers such as β3 integrin, cathepsin K, matrix metalloproteinase-9 (MMP-9), calcitonin receptor (CTR), and c-fos, using the primers reported in Table 2. The T-cell depletion was performed by using anti-CD2 antibody (Ab)–coated immunomagnetic Dynabeads (Dynal, Lake Success, NY). Briefly, CD2+ cells were captured from the PB or BM buffy coats, incubating 2 × 107 beads/mL with 5 × 106 cells/mL for 30 minutes at 4°C on an apparatus which allows both gentle tilting and rotation. The T-cell–depleted MM PBMCs and BM MNCs were cultured with or without exogenous cytokines, in the same conditions of the unfractionated cultures.
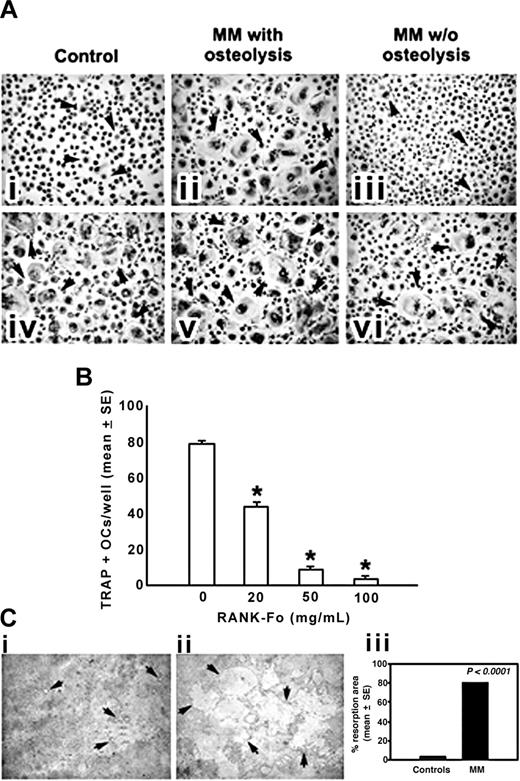
OCs generated from human MM PBMCs. (A) OCs were obtained from unfractionated PBMCs of patients with MM with or without osteolysis (ii,iii,v,vi) and controls (i,iv). Numerous and large-sized OCs (arrows) developed in the unstimulated cultures from patients with MM with osteolysis (ii), whereas rare and small-sized OCs were observed in the cultures from patients with MM without osteolysis (iii) and controls (i). No significant increase in OC formation was observed in MM PBMCs from the patients with osteolysis by exogenous M-CSF and RANKL (v), whereas these cytokines were essential to triggering the OC formation in patients with MM without osteolysis (vi) and controls (iv). Multinucleated (> 3 nuclei per cell) and TRAP+ cells were identified as OCs. The arrows point to the OCs (magnification, × 200). (B) The inhibition of RANKL by RANK-Fc prevented in a dose-dependent manner the OC formation in unstimulated and unfractionated PBMC cultures from patients with MM. *P < .001. (C) Photomicrographs of the pits formed on Millennium Osteologic slides by the OCs. Numerous and large resorption areas (arrows) were observed in the MM bone disease samples (ii) with respect to the few and small pits detected in the controls (i). The percentage of mineral surface resorbed by the OCs was quantified with an image analyzer. The data reported in the graph correspond to the mean ± SE (iii). See “Cell cultures” for figure acquisition info.
OCs generated from human MM PBMCs. (A) OCs were obtained from unfractionated PBMCs of patients with MM with or without osteolysis (ii,iii,v,vi) and controls (i,iv). Numerous and large-sized OCs (arrows) developed in the unstimulated cultures from patients with MM with osteolysis (ii), whereas rare and small-sized OCs were observed in the cultures from patients with MM without osteolysis (iii) and controls (i). No significant increase in OC formation was observed in MM PBMCs from the patients with osteolysis by exogenous M-CSF and RANKL (v), whereas these cytokines were essential to triggering the OC formation in patients with MM without osteolysis (vi) and controls (iv). Multinucleated (> 3 nuclei per cell) and TRAP+ cells were identified as OCs. The arrows point to the OCs (magnification, × 200). (B) The inhibition of RANKL by RANK-Fc prevented in a dose-dependent manner the OC formation in unstimulated and unfractionated PBMC cultures from patients with MM. *P < .001. (C) Photomicrographs of the pits formed on Millennium Osteologic slides by the OCs. Numerous and large resorption areas (arrows) were observed in the MM bone disease samples (ii) with respect to the few and small pits detected in the controls (i). The percentage of mineral surface resorbed by the OCs was quantified with an image analyzer. The data reported in the graph correspond to the mean ± SE (iii). See “Cell cultures” for figure acquisition info.
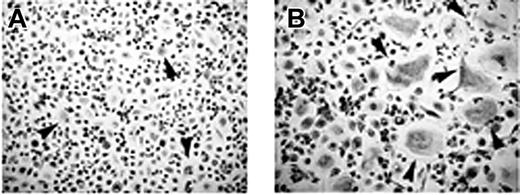
T cells mediate osteoclastogenesis in human MM PBMCs. Osteoclastogenesis occurred in T-cell–depleted MM PBMCs cultured in the absence (A) or in the presence (B) of M-CSF and RANKL. Small-sized OCs developed in the absence of M-CSF and RANKL (A), whereas exogenous cytokines triggered the formation of large-sized OCs (B). Multinucleated and TRAP+ cells were identified as OCs. The arrows point to the OCs (magnification × 200).
T cells mediate osteoclastogenesis in human MM PBMCs. Osteoclastogenesis occurred in T-cell–depleted MM PBMCs cultured in the absence (A) or in the presence (B) of M-CSF and RANKL. Small-sized OCs developed in the absence of M-CSF and RANKL (A), whereas exogenous cytokines triggered the formation of large-sized OCs (B). Multinucleated and TRAP+ cells were identified as OCs. The arrows point to the OCs (magnification × 200).
Primer sequences, annealing temperatures, and cycle numbers
Gene . | Sense primer . | Antisense primer . | Annealing temp, °C . | Cycles . | Product size, bp . |
|---|---|---|---|---|---|
| RANKL | 5′-gccagtgggagatgttag-3′ | 5′-ttagctgcaagttttccc-3′ | 63 | 30 | 486 |
| OPG | 5′-gctaacctcaccttcgag-3′ | 5′-tgattggacctggttacc-3′ | 54 | 30 | 324 |
| TNF-α | 5′-tcagcctcttctccttcctg-3′ | 5′-gatgcggctgatggtgtg-3′ | 54 | 25 | 384 |
| MCSF | 5′-cagatggagacctcgtgccaa-3′ | 5′-gcaggccttgtcatgctcttc-3′ | 54 | 30 | 241 |
| FAS | 5′-ccaagtgactgacatcaactc-3′ | 5′-ctctttgcacttggtgttgctgg-3′ | 66 | 30 | 427 |
| FAS-L | 5′-gaaggagctggcagaactccgag-3′ | 5′-gaccagagagagctcagatacgttgac-3′ | 66 | 30 | 478 |
| TRAIL | 5′-caatgacgaagagagtatga-3′ | 5′-cccccttgatagatggaata-3′ | 54 | 30 | 536 |
| DcR1 | 5′-gccggaagtgtagcaggtg-3′ | 5′-gtagagtggatggtcagtg-3′ | 58 | 25 | 546 |
| DcR2 | 5′-cccccggcaggacgaagtt-3′ | 5′-ctcctccgctgctggggtttt-3′ | 58 | 25 | 418 |
| DR4 | 5′-ccgcggccacacccagaaagt-3′ | 5′-gtacatgggaggcaagcaaacaaa-3′ | 58 | 25 | 415 |
| DR5 | 5′-gcgcccacaaaatacaccgacgat-3′ | 5′-gcagcgcaagcagaaaaggag-3′ | 58 | 25 | 437 |
| Bcl-2 | 5′-gatgtccagccagctgcacctg-3′ | 5′-cacaaaggcatcccagcctcc-3′ | 63 | 25 | 255 |
| Bax | 5′-ggacccggtgcctcagga-3′ | 5′-caaagatggtcacggtctgc-3′ | 63 | 25 | 495 |
| Cathepsin K | 5′-tccatccataaccttgaggctt-3′ | 5′-ccacagccatcattctcagacaca-3′ | 65 | 20 | 360 |
| Integrin β 3 | 5′-gtgacctgaaggagaatctgc-3′ | 5′-ttcttcgaatcatctggcc-3′ | 65 | 35 | 183 |
| CTR | 5′-gtattgtcctatcagttctgcc-3′ | 5′-agagataataccaccgcaagcg-3′ | 65 | 35 | 577 |
| MMP-9 | 5′-ccatttcgacgatgacgagttg-3′ | 5′-cttgtcgctgtcaaagttcgag-3′ | 65 | 25 | 544 |
| c-fos | 5′-aaggagaatccgaagggaaaggaataagatggct-3′ | 5′-agacgaaggaagacgtgtaagcagtgcagct-3′ | 65 | 25 | 612 |
| GAPDH | 5′-ggagtcaacggatttggt-3′ | 5′-gtgatgggatttccattgat-3′ | 63 | - | 209 |
Gene . | Sense primer . | Antisense primer . | Annealing temp, °C . | Cycles . | Product size, bp . |
|---|---|---|---|---|---|
| RANKL | 5′-gccagtgggagatgttag-3′ | 5′-ttagctgcaagttttccc-3′ | 63 | 30 | 486 |
| OPG | 5′-gctaacctcaccttcgag-3′ | 5′-tgattggacctggttacc-3′ | 54 | 30 | 324 |
| TNF-α | 5′-tcagcctcttctccttcctg-3′ | 5′-gatgcggctgatggtgtg-3′ | 54 | 25 | 384 |
| MCSF | 5′-cagatggagacctcgtgccaa-3′ | 5′-gcaggccttgtcatgctcttc-3′ | 54 | 30 | 241 |
| FAS | 5′-ccaagtgactgacatcaactc-3′ | 5′-ctctttgcacttggtgttgctgg-3′ | 66 | 30 | 427 |
| FAS-L | 5′-gaaggagctggcagaactccgag-3′ | 5′-gaccagagagagctcagatacgttgac-3′ | 66 | 30 | 478 |
| TRAIL | 5′-caatgacgaagagagtatga-3′ | 5′-cccccttgatagatggaata-3′ | 54 | 30 | 536 |
| DcR1 | 5′-gccggaagtgtagcaggtg-3′ | 5′-gtagagtggatggtcagtg-3′ | 58 | 25 | 546 |
| DcR2 | 5′-cccccggcaggacgaagtt-3′ | 5′-ctcctccgctgctggggtttt-3′ | 58 | 25 | 418 |
| DR4 | 5′-ccgcggccacacccagaaagt-3′ | 5′-gtacatgggaggcaagcaaacaaa-3′ | 58 | 25 | 415 |
| DR5 | 5′-gcgcccacaaaatacaccgacgat-3′ | 5′-gcagcgcaagcagaaaaggag-3′ | 58 | 25 | 437 |
| Bcl-2 | 5′-gatgtccagccagctgcacctg-3′ | 5′-cacaaaggcatcccagcctcc-3′ | 63 | 25 | 255 |
| Bax | 5′-ggacccggtgcctcagga-3′ | 5′-caaagatggtcacggtctgc-3′ | 63 | 25 | 495 |
| Cathepsin K | 5′-tccatccataaccttgaggctt-3′ | 5′-ccacagccatcattctcagacaca-3′ | 65 | 20 | 360 |
| Integrin β 3 | 5′-gtgacctgaaggagaatctgc-3′ | 5′-ttcttcgaatcatctggcc-3′ | 65 | 35 | 183 |
| CTR | 5′-gtattgtcctatcagttctgcc-3′ | 5′-agagataataccaccgcaagcg-3′ | 65 | 35 | 577 |
| MMP-9 | 5′-ccatttcgacgatgacgagttg-3′ | 5′-cttgtcgctgtcaaagttcgag-3′ | 65 | 25 | 544 |
| c-fos | 5′-aaggagaatccgaagggaaaggaataagatggct-3′ | 5′-agacgaaggaagacgtgtaagcagtgcagct-3′ | 65 | 25 | 612 |
| GAPDH | 5′-ggagtcaacggatttggt-3′ | 5′-gtgatgggatttccattgat-3′ | 63 | - | 209 |
FAS indicates fatty acid synthase; FASL, Fas ligand; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
For some experiments, PBMCs were cultured in the presence of different concentrations of RANK-Fc (20-100 ng/mL) or anti-TRAIL monoclonal antibodies (mAbs; 10-500 ng/mL; R&D Systems) with or without RANKL at the concentration of 30 ng/mL.
Cell viability assay
Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. PBMCs were cultured in 96-well tissue-culture plates in the presence or absence of T cells. M-CSF (25 ng/mL) and RANKL (30 ng/mL) were added to the T-cell–depleted cultures. At day 25, 30, and 35, 200 μL/well MTT 0.5 mg/mL were added, followed by 4 hours of incubation at 37°C in a humidified 5% CO2 atmosphere. The reaction was stopped by the addition of 150 μL 0.04 N HCl in absolute isopropanol. The optical density was read at 570 nm using an automatic plate reader (550 Microplate Reader; Bio-Rad Laboratories, Hercules, CA). The results were normalized to cells incubated under control conditions.
RNA isolation and RT–PCR amplification
The OCs obtained from PBMC cultures of the patients with MM and the controls were subjected to mRNA extraction using spin columns (RNeasy, QIAGEN, Hilden, Germany), according to the manufacturer's instructions, to detect the expression of TRAIL and its death and decoy receptors (ie, DR4 and DR5, DcR1 and DcR2, respectively) as well as of Bcl-2 family members (ie, Bcl-2 and Bax). Before RNA extraction, cell cultures were trypsinized to remove early or late OC precursors. The cells persisting in the culture following trypsin treatment were fully differentiated OCs. RNA was also extracted from freshly prepared T cells from PBMCs and BM MNCs of patients with MM and controls to evaluate the expression of M-CSF, TNF-α, RANKL, OPG, and TRAIL. Furthermore, mRNA levels of RANKL were detected in CD138+ fresh MM cells.11 Briefly for the first-strand cDNA synthesis (SuperScript First-Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA), an RT mixture containing 1 μg total RNA, dNTPs (deoxyribonucleoside triphosphates), Oligo(dT), RT buffer, MgCl2, DTT (dithiothreitol), RNaseOUT, SuperScript II RT, DEPC (diethyl pyrocarbonate)–treated water to final volume 100 μL was prepared, according to the manufacturer's instructions. Diluted cDNA (2 μL) was transferred into a 50-μL PCR reaction mixture containing dNTPs, MgCl2, primers, autoclaved distilled water, and Platinum Taq DNA polymerase (Invitrogen).
Amplification reactions specific for the cDNAs of RANK-L, M-CSF, OPG, TNF-α, TRAIL, DcR1, DcR2, DR4, DR5, Bcl-2, Bax, and the housekeeping genes glyceraldehyde phosphate dehydrogenase (GAPDH) were carried out using taq/polymerase (Invitrogen). The primers and RT-PCR conditions are reported in Table 2. PCR products were analyzed by 1.5% agarose gel electrophoresis containing 0.01% ethidium bromide, and the resulting bands were detected by a light sensitive CCD (charge-coupled device) video system (BioDocAnalyze; Whatman Biometra, Goettingen, Germany).
Western blot analysis
Proteins from OCs, developed in PBMC cultures of patients with MM and controls, were solubilized with lysis buffer [50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 8), 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1% NP40, and 1 mM phenylmethyl sulfonyl fluoride]. Protein determination was performed by BCA (bicinchoninic acid) Protein assay Reagent Kit (Pierce Biotechnology, Rockford, MN). Cell proteins (50 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and subsequently transferred to nitrocellulose membranes (Hybond; Amersham Pharmacia, London, United Kingdom). The blots were probed overnight at 4°C with mouse anti-OPG, anti–β-actin (Chemicon International, Temecula, CA), anti-TRAIL, anti-RANKL mAb (R&D Systems); rabbit anti-DR4, anti-DR5, anti-DcR1, anti-DcR2 polyclonal Ab (Oncogene Research Products, Cambridge, MA); mouse anti-Bcl-2 and Bax mAb (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with appropriate peroxidase-conjugated secondary Ab, specific reactions were revealed with the electrochemiluminescence (ECL) detection kit and visualized on Hyperfilm (Amersham Pharmacia, Buckinghamshire, United Kingdom). To detect the TRAIL-OPG complex, immunoprecipitation of PBMC fresh T-cell lysates and the media collected from PBMC cultures was performed using anti-TRAIL mAb or IgG. Briefly, 150 μg T-cell lysate and 1 mg supernatant were incubated with 1 μg/mL anti-TRAIL or IgG for 1 hour at 4°C; then, 20 μL protein-G PLUS-agarose (Santa Cruz Biotechnology) was added and incubated with mixing overnight at 4°C. Pellets were collected by centrifugation and washed 3 times with lysis buffer. After the final wash, the immunoprecipitated material was recovered by boiling in sample loading buffer and separated on 10% SDS-PAGE gel.
ELISA assay
RANKL, OPG, and TRAIL were detected in the media of unfractionated and T-cell–depleted PBMC cultures from the patients with MM as well as from the controls by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions. The ELISA kit for sRANKL (Biomedica, GmbH, Wien, Austria) is designed to determine soluble uncomplexed human RANKL, whereas those for OPG (Biomedica) and for TRAIL (Biomol Research Laboratories, Plymouth Meeting, PA), respectively, are designed to detect uncomplexed and complexed molecules. The samples were diluted to the concentrations within the standard curve range. The absorption was determined with an ELISA reader at 450 nm (550 Microplate Reader; Bio-Rad), and the results were expressed as mean ± SE.
Statistical analyses
Statistical analyses were performed by Student t test with the Statistical Package for the Social Sciences (spssx/pc) software (SPSS, Chicago, IL). The results were considered statistically significant for P values less than .05.
Results
Spontaneous osteoclastogenesis in unstimulated cultures of unfractionated PBMCs from patients with MM
Numerous large TRAP+ OCs were identified in the unstimulated PBMC cultures from patients with MM with osteolysis (OC average number/well = 62 ± 4; Figure 1Aii), whereas smaller and fewer OCs appeared in the PBMC cultures from the patients with MM without osteolysis (Figure 1Aiii) and the controls (Figure 1Ai) (OC average number/well = 6 ± 2 and 4 ± 1, respectively). Moreover, the addition of M-CSF and RANKL to the PBMC cultures from patients with MM with osteolysis did not significantly change the OC number (OC average number/well = 70 ± 6) (Figure 1Av) compared with the parallel unstimulated cultures (Figure 1Aii). In particular, the addition of increasing amounts of RANKL did not further enhance the osteoclastogenesis, thereby already maximal (data not shown). However, the above cytokines were essential to trigger and sustain osteoclastogenesis of PBMCs from patients with MM without osteolysis (Figure 1Avi) and from the controls (Figure 1Aiv) (OC average number/well = 62 ± 3 and 58 ± 7, respectively). These results suggest the presence of osteoclastogenic factors in the PBMC cultures derived from patients with MM with osteolysis. The experiment performed in the presence of RANK-Fc indicates RANKL as the major cytokine involved in the spontaneous osteoclastogenesis from PBMCs of patients with MM bone disease (Figure 1B). Moreover, the resorbing activity of the OCs obtained in unstimulated PBMC cultures from patients with MM with osteolysis was also demonstrated (Figure 1C), resulting significantly higher than that detected in the unstimulated PBMC cultures from the controls (Figure 1Cii and i, respectively).
T-cell–mediated osteoclastogenesis and OC survival in patients with MM
The unstimulated cultures of T-cell–depleted PBMCs from patients with MM with osteolysis resulted in the development of few small-sized OCs (OC average number/well = 10 ± 2) (Figure 2A). By contrast, the addition of M-CSF and RANKL to these cultures induced the formation of numerous large TRAP+ OCs (OC average number/well = 68 ± 5) (Figure 2B), like those observed in the presence of T cells (Figure 1Aii). In the T-cell–depleted and stromal cell-free BM cultures, OCs did not form (data not shown).
By MTT assay, we found that the OCs generated in the presence of MM T cells exhibited a significantly longer survival than those formed in MM T-cell–depleted PBMCs and in the controls. In particular, on the 30th day of culture, the viability of the OCs generated in the absence of T cells was significantly lower than that displayed by the OCs from unfractionated MM PBMC cultures. This result was even more evident by the 35th day of culture (Figure 3), being also confirmed by the presence of a lower number of immunostained multinucleated TRAP+ OCs (data not shown). Therefore, the longer survival displayed by the OCs derived from the unstimulated cultures of MM PBMCs clearly appeared to be T cell dependent.
T cells increase cell viability of the OCs from human MM PBMCs. Cell viability was measured by MTT assay. MM PBMCs from patients with osteolysis were cultured in the presence (OCs + T cells) or in the absence (OCs – T cells) of T cells for 25, 30, and 35 days. M-CSF and RANKL were added to the T-cell–depleted cultures to allow OC formation. The viability of the OCs generated in the presence of T cells without addition of exogenous cytokines was significantly higher than that of the OCs formed in the absence of T cells.
T cells increase cell viability of the OCs from human MM PBMCs. Cell viability was measured by MTT assay. MM PBMCs from patients with osteolysis were cultured in the presence (OCs + T cells) or in the absence (OCs – T cells) of T cells for 25, 30, and 35 days. M-CSF and RANKL were added to the T-cell–depleted cultures to allow OC formation. The viability of the OCs generated in the presence of T cells without addition of exogenous cytokines was significantly higher than that of the OCs formed in the absence of T cells.
Cytokine expression by fresh T cells, MM cells, and culture media
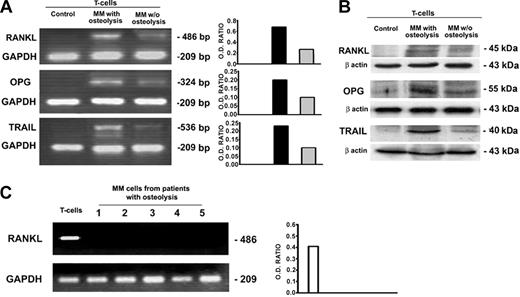
In patients with MM with osteolysis, fresh T cells purified from PBMCs as well as from BM MNCs overexpressed RANKL at mRNA and protein levels; in the fresh T cells from patients with MM without osteolysis, a very low expression of RANKL was observed, whereas no expression was found in the T cells from the controls (Figure 4A-B). Furthermore, MM cells were found not expressing RANKL at mRNA level (Figure 4C), indicating that T cells were the only source of RANKL in our system. No difference was detected in the expression of M-CSF among patients with MM with or without osteolysis and in the controls (data not shown). Despite the enhanced osteoclastogenesis, we found that OPG resulted in overexpression in the fresh T cells isolated from patients with MM with osteolysis; however, it was barely detectable in those without osteolysis and undetectable in the controls (Figure 4A-B). This finding could be explained by the higher levels of ambient TRAIL, demonstrated by RT-PCR and Western blotting in the fresh T cells from patients with MM with osteolysis compared with the lower levels detected in those from the patients without osteolysis and in the controls (Figure 4A-B).
Cytokine expression by fresh T and MM cells. Fresh T cells purified from PBMCs of patients with MM with or without osteolysis as well as from controls were analyzed for RANKL, OPG, and TRAIL expression, respectively, by RT-PCR (A) and Western blotting (B). MM cells purified from BM of patients with MM with osteolysis were analyzed for RANKL expression by RT-PCR (C). The results show the overexpression of RANKL, OPG, and TRAIL by fresh T cells from patients with MM with osteolysis compared with those without osteolysis and controls (A-B). MM cells were found not expressing RANKL (C). The intensity of the bands obtained by RT-PCR was quantified by densitometry (histograms) and normalized to GAPDH.
Cytokine expression by fresh T and MM cells. Fresh T cells purified from PBMCs of patients with MM with or without osteolysis as well as from controls were analyzed for RANKL, OPG, and TRAIL expression, respectively, by RT-PCR (A) and Western blotting (B). MM cells purified from BM of patients with MM with osteolysis were analyzed for RANKL expression by RT-PCR (C). The results show the overexpression of RANKL, OPG, and TRAIL by fresh T cells from patients with MM with osteolysis compared with those without osteolysis and controls (A-B). MM cells were found not expressing RANKL (C). The intensity of the bands obtained by RT-PCR was quantified by densitometry (histograms) and normalized to GAPDH.
On the basis of the spontaneous osteoclastogenesis occurring in the cultures from patients with MM with osteolysis, the expression of RANKL, OPG, and TRAIL was evaluated by ELISA assay in the media collected from unfractionated as well as T-cell–depleted PBMCs of patients with MM with osteolysis and from the controls. Detectable levels of RANKL and OPG were found only in the media from unfractionated MM PBMC cultures, whereas these cytokines were undetectable in the media from T-cell–depleted MM PBMCs and the controls (Table 3). TRAIL levels demonstrated in the media from unfractionated MM PBMCs were significantly higher than those found in the media from T-cell–depleted MM PBMC cultures or controls, suggesting that T cells could be the major but not the exclusive source of this cytokine (Table 3).
Concentration of RANKL, OPG, and TRAIL in the media collected from osteoclastic cultures of patients with MM with osteolysis (MM) and controls (Ctr)
. | Ctr . | MM- T cells . | MM+ T cells . |
|---|---|---|---|
| RANKL, pmol/L | ND | 0.3 ± 0.1 | 5.5 ± 0.5* |
| OPG, pmol/L | ND | ND | 2.5 ± 0.1* |
| TRAIL, pmol/L | 21 ± 0.9 | 21.54 ± 1 | 42.86 ± 0.57* |
. | Ctr . | MM- T cells . | MM+ T cells . |
|---|---|---|---|
| RANKL, pmol/L | ND | 0.3 ± 0.1 | 5.5 ± 0.5* |
| OPG, pmol/L | ND | ND | 2.5 ± 0.1* |
| TRAIL, pmol/L | 21 ± 0.9 | 21.54 ± 1 | 42.86 ± 0.57* |
RANKL indicates receptor activator of nuclear factor κB; OPG, osteoprotegerin; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; ND not detectable; absence (-) and presence (+) of T cells in cultures.
P < .001 versus Ctr
OPG/TRAIL complex in fresh T cells and culture media from PBMCs of patients with MM
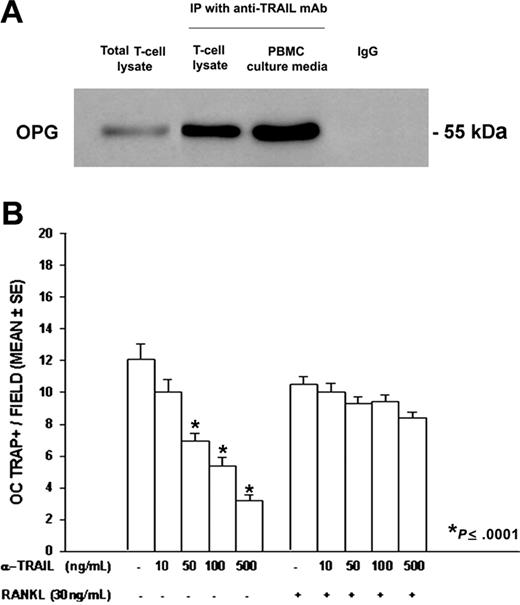
To assess whether TRAIL bound OPG inhibiting the antiosteoclastogenic OPG effect, we performed an immunoprecipitation with an anti-TRAIL mAb on PBMC fresh T-cell lysates and media collected from the PBMC cultures of patients with MM with osteolysis. Our findings indicated that TRAIL and OPG formed a complex in both T-cell lysates and culture media. OPG was identified as a band of about 55 kDa, more evident in the culture media than in the T-cell lysates (Figure 5A).
OPG/TRAIL complex in fresh T cells and culture media from PBMCs of patients with MM, and anti-TRAIL mAb effect on osteoclastogenesis. TRAIL and OPG were coimmunoprecititated by a mAb against TRAIL in PBMC fresh T-cell lysates and PBMC culture media. Mouse IgG was used as control, and a total T-cell lysate was also loaded. OPG was identified as a 55 kDa molecular weight band (A). PBMCs from patients with MM were cultured in the presence of an anti-TRAIL mAb at different concentrations ± RANKL. The anti-TRAIL mAb, added to the culture media in the absence of exogenous RANKL, significantly reduced osteoclastogenesis (B). Further, the addition of RANKL rescued the anti-TRAIL mAb inhibitory effect. The graph represents the mean values ± SE of a representative experiment.
OPG/TRAIL complex in fresh T cells and culture media from PBMCs of patients with MM, and anti-TRAIL mAb effect on osteoclastogenesis. TRAIL and OPG were coimmunoprecititated by a mAb against TRAIL in PBMC fresh T-cell lysates and PBMC culture media. Mouse IgG was used as control, and a total T-cell lysate was also loaded. OPG was identified as a 55 kDa molecular weight band (A). PBMCs from patients with MM were cultured in the presence of an anti-TRAIL mAb at different concentrations ± RANKL. The anti-TRAIL mAb, added to the culture media in the absence of exogenous RANKL, significantly reduced osteoclastogenesis (B). Further, the addition of RANKL rescued the anti-TRAIL mAb inhibitory effect. The graph represents the mean values ± SE of a representative experiment.
In addition, the involvement of TRAIL in T-cell–mediated osteoclastogenesis was further demonstrated by the addition of different concentrations of a neutralizing anti-TRAIL mAb to the media of PBMCs from patients with MM with osteolysis. Our results showed a dose-dependent inhibition of spontaneous osteoclastogenesis; this effect was completely abolished by the addition of RANKL (Figure 5B).
Expression of OC TRAIL receptors is under T-cell control
In the assessment of the mechanisms involved in the longer survival displayed by the OCs from unfractionated PBMCs of patients with MM with osteolysis, we evaluated the expression of death and decoy TRAIL receptors. Despite the fact that Fas/FasL system seems to be the dominant mechanism in OC apoptosis, we did not detect any difference in Fas receptor expression among the OCs developed in the presence of T cells, those from T-cell– depleted cultures, and the controls. However, consistent with the literature data, the fresh T cells isolated from MM PBMCs were found to express FasL at higher levels than those from the controls (data not shown).
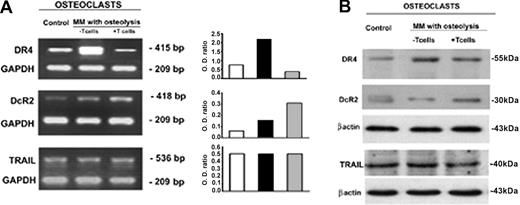
Consequently, we evaluated whether T cells could regulate the expression of TRAIL receptors on the OCs displaying longer survival. In the OCs generated from unfractionated MM PBMCs, the analysis of TRAIL receptors demonstrated down-regulation of death receptor DR4 and up-regulation of decoy receptor DcR2, at both mRNA and protein levels (Figure 6). On the contrary, DR4 resulted in overexpression and DcR2 reduced in the OCs developed from T-cell–depleted cultures. In addition, the expression of death DR5 and decoy DcR1 receptors was not modulated by T cells (Figure 6). Thus, in MM bone disease, OCs seem to be protected by TRAIL-induced apoptosis via up-regulation of its decoy receptor DcR2 and down-regulation of death receptor DR4, possibly explaining the effect of T cells on OC survival.
OC expression of TRAIL and its receptors. OC expression of TRAIL and its death (DR4) and decoy (DcR2) receptors was analyzed by RT-PCR (A) and Western blotting (B). Fully differentiated OCs were obtained in unfractionated as well as T-cell–depleted MM PBMC cultures (the latter following the addition of M-CSF and RANKL). The OCs generated in the absence of T cells overexpressed TRAIL DR4 receptor at both mRNA (A) and protein levels (B). On the contrary, the OCs from T-cell–depleted cultures overexpressed DcR2 at both mRNA (A) and protein levels (B). No significant difference was found in TRAIL expression. The intensity of the bands was quantified by densitometry (histograms) and normalized to GAPDH.
OC expression of TRAIL and its receptors. OC expression of TRAIL and its death (DR4) and decoy (DcR2) receptors was analyzed by RT-PCR (A) and Western blotting (B). Fully differentiated OCs were obtained in unfractionated as well as T-cell–depleted MM PBMC cultures (the latter following the addition of M-CSF and RANKL). The OCs generated in the absence of T cells overexpressed TRAIL DR4 receptor at both mRNA (A) and protein levels (B). On the contrary, the OCs from T-cell–depleted cultures overexpressed DcR2 at both mRNA (A) and protein levels (B). No significant difference was found in TRAIL expression. The intensity of the bands was quantified by densitometry (histograms) and normalized to GAPDH.
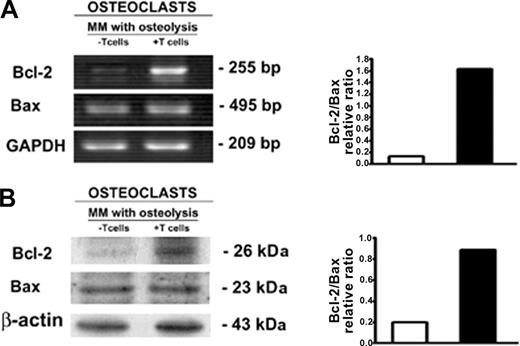
To support the potential regulatory role of T cells in OC survival, we evaluated the OC expression of antiapoptotic and proapoptotic Bcl-2 family proteins by RT-PCR and Western blotting in unfractionated as well as T-cell–depleted PBMC cultures from patients with MM with osteolysis. The antiapoptotic protein Bcl-2 resulted up-regulated at mRNA and protein levels in the OCs generated in the presence of T cells (Figure 7), whereas no significant change of the proapoptotic protein Bax was found (Figure 7). Our data show that the T cells induce the positive modulation of Bcl-2/Bax ratio, indicating a possible mechanism by which T cells promote the OC longer survival.
OC expression of Bcl-2 and Bax. The analysis of Bcl-2 and Bax expression by OCs, developed in unfractionated as well as T-cell–depleted PBMC cultures derived from patients with MM with osteolysis, was performed by RT-PCR (A) and Western blotting (B). T-cell–depleted cultures were stimulated with exogenous M-CSF and RANKL. The intensity of the bands was quantified by densitometry. The graphs represent the mean optical density (OD) of Bcl-2/Bax ratio normalized to the OD of GAPDH or β-actin of a representative experiment for RT-PCR and Western blotting, respectively. Bcl-2 is highly expressed in the OCs obtained in the presence of T cells.
OC expression of Bcl-2 and Bax. The analysis of Bcl-2 and Bax expression by OCs, developed in unfractionated as well as T-cell–depleted PBMC cultures derived from patients with MM with osteolysis, was performed by RT-PCR (A) and Western blotting (B). T-cell–depleted cultures were stimulated with exogenous M-CSF and RANKL. The intensity of the bands was quantified by densitometry. The graphs represent the mean optical density (OD) of Bcl-2/Bax ratio normalized to the OD of GAPDH or β-actin of a representative experiment for RT-PCR and Western blotting, respectively. Bcl-2 is highly expressed in the OCs obtained in the presence of T cells.
Discussion
A regulatory role of bone turnover played by T cells in physiologic and pathologic conditions is emerging from the literature. In particular, T-cell involvement in OC activation through RANKL overexpression has been reported in osteoporosis,38 periodontal disease,39 adjuvant arthritis,5 rheumatoid arthritis,40 adult T-cell leukemia with hypercalcemia,10 and MM bone disease.11
In the assessment of biologic mechanisms involved in the pathogenesis of MM bone disease, Giuliani et al35 had shown that myeloma cells induce an OPG/RANKL imbalance, playing a critical role in this condition. Later, the same investigators suggested a possible involvement of T cells in the OC activation through the cross talk between RANKL and IFN-γ; in particular, they demonstrated the MM cell–induced up-regulation of RANKL and down-regulation of IFN-γ, which is known to be a strong suppressor of osteoclastogenesis.11 Moreover, Giuliani et al11 indicated the possible implication of interleukin 7 (IL-7) in osteoclastogenesis through its stimulatory effect on the RANKL production by T cells and suggested a vicious loop between IL-6 and IL-7 involving the MM cells. They detected high levels of IL-7 in the supernatants of human MM cell lines (HMLC) as well as in BM plasma cells and sera of patients with MM bone disease patients.
In line with the primary aim of our study, we used a culture system for OC development as paradigm of in vivo bone resorption to assess the mechanism by which T cells could support the increase in number and activity of OCs, occurring in MM bone disease. In particular, our in vitro PBMC osteoclastogenesis model represents a good system to study the effect of T cells on osteoclastogenesis because of the absence of stromal cells, a possible source of osteoclastogenic cytokines. In unfractionated PBMC cultures from patients with MM bone disease, we observed the formation of numerous, mature, multinucleated, and bone resorbing OCs with a longer survival that were not evident in the same type of cultures derived from the patients with MM without lytic bone lesions and the controls. Major evidences supporting a T-cell regulation of osteoclastogenesis came from the parallel MM T-cell–depleted PBMC cultures, in which the addition of exogenous M-CSF and RANKL was necessary to the OC formation, suggesting that T cells alone could provide the above cytokines in our system. Moreover, OCs obtained in T-cell–depleted PBMC cultures were not protected from apoptosis, indicating that T cells could also have a role in promoting the longer OC survival. Thus, for the first time, our findings demonstrate T-cell–dependent osteoclastogenesis from human MM PBMCs, and OCs exhibiting a longer survival only in the presence of MM T cells. On the basis of the knowledge that OCs form normally in BM, we demonstrated a T-cell regulation of osteoclastogenesis in the BM cultures from patients with MM, whereas in the T-cell–depleted and stromal cell–free system OCs did not form. These results are consistent with the recent literature data, showing the contribution of MM BM T cells to MM-induced osteolysis through RANKL overexpression.11
According to our secondary aim, we evaluated the cytokines possibly involved in OC formation and survival, demonstrating that RANKL, OPG, and TRAIL were overexpressed by MM PB fresh T cells at both mRNA and protein levels and were also detectable in the media collected from unfractionated PBMC cultures. Interestingly, the levels of these cytokines were higher in PB fresh T cells from patients with MM bone disease compared with those from patients without clinical evidence of lytic bone lesions and were undetectable in the controls. The SE findings show that MM T cells could be the only source of these molecules in our system except for TRAIL, whose significantly higher release in the media from MM PBMC cultures shows that it is produced by both T cells and OCs, as we demonstrated by RT-PCR and Western blot analysis of OC lysates. A confirmation of RANKL production by MM T cells came from the exogenous RANKL necessary to obtain mature OCs not displaying, however, longer survival in the absence of T cells. Furthermore in our system, the involvement of RANKL as major osteoclastogenic cytokine is confirmed by the inhibitory effect exerted by RANK-Fc on spontaneous osteoclastogenesis in unstimulated PBMC cultures from the patients with MM bone disease.
The OPG overexpression we detected by human MM T cells had been previously reported only in murine T cells.7 On the basis of RANKL and OPG effects on bone remodeling, consisting of RANKL stimulation and OPG inhibition of osteoclastogenesis, we can argue that the persistence of osteoclastogenesis despite the high levels of OPG in our system could be explained by the OPG binding to TRAIL, blocking OPG anti-osteoclastogenic activity. Although the affinity of OPG for RANKL is higher than that for TRAIL, the OPG/TRAIL interaction could be favored by the elevated TRAIL levels detected in our culture media. This possibility is supported by the detection of OPG/TRAIL complex in T-cell lysates as well as the culture media of PBMCs from patients with MM bone disease that we demonstrated by immunoprecipitation with an anti-TRAIL mAb. Moreover, the neutralizing anti-TRAIL Ab caused a dose-dependent inhibition of spontaneous osteoclastogenesis that recurred after the addition of exogenous RANKL. These findings are consistent with the literature data, showing that TRAIL blocks the anti-osteoclastogenic effect of OPG in mice.25 We cannot exclude that the OPG/TRAIL interaction also blocks the apoptotosis-inducing TRAIL activity. Moreover, the overexpression of OPG, resulting in a protective cell strategy against apoptosis in the presence of high TRAIL levels, is of major relevance as indicated by in vitro studies prospecting OPG as a survival factor for human prostate cancer cells32 and human MM cells.33 The high levels of OPG detected in our system were consistent with some literature reports on bone loss–associated diseases such as postmenopausal osteoporosis,41 seropositive rheumatoid arthritis,42 and prostate cancer bone metastases.32 Otherwise, low OPG levels in BM microenvironment as well as in BM plasma and sera were detected in patients with MM bone disease.34,35,43-45 These findings were explained with MM cell–induced impairment of OPG production by osteoblast/stromal cells.34,35 Later on, a further mechanism consisting of binding, internalization, and/or degradation of OPG by MM cells was provided, as supported by both in vitro uptake of I-OPG and demonstration of plasma cells containing OPG in BM biopsies from patients with MM.44 The absence of MM cells in our system could explain the OPG overexpression that we detected in the fresh T cells purified from patients with MM bone disease. In addition, the high OPG levels that we demonstrated in the culture media of PBMCs from the same patients could be related to the ELISA kit recognizing both free and bound forms of OPG.
The longer survival, exhibited exclusively by the OCs generated in the presence of MM T cells, prompted a T-cell control of OC survival. In these OCs, we demonstrated up-regulated decoy receptor DcR2 and down-regulated death receptor DR4 expression, whereas DR4 was found overexpressed and DcR2 downregulated on the OCs from T-cell–depleted cultures. Our data indicate that T cells can regulate OC survival by modulating their surface expression of TRAIL death and decoy receptors, whose ratio in turn results critical for OC sensitivity to TRAIL-mediated apoptosis. This is consistent with the evidence of OC apoptosis in the T-cell–depleted cultures, in which the OC DNA fragmentation following the TRAIL addition was observed (S.C., G.B., A. Oranger, R.R., G. Mori, A.Z., and M.G., manuscript in preparation); however, the mechanism by which T cells control OC survival is not yet defined. Furthermore, we detected comparable Fas receptor levels in the OCs from both unfractionated and T-cell–depleted MM PBMC cultures, whereas FasL was overexpressed by MM fresh T cells, suggesting that OC Fas receptor expression was not regulated by T cells in our system. Therefore, we cannot exclude the possibility that Fas/FasL pathway is activated in our system leading to the normal life-span of OCs generated from T-cell–depleted cultures. Finally, the mechanism by which T cells control OC survival seems to involve the positive modulation of Bcl-2/Bax ratio through the up-regulation of Bcl-2, detected in the OCs generated in the presence of MM T cells. In other systems, it has been demonstrated that the Bcl-2/Bax ratio is important to determine the fate of the cells toward apoptosis or survival.30,31 Several mechanisms could be involved in the modification of this Bcl-2/Bax ratio, but they are still unknown in the OCs. A possible modulator could be TRAIL, described in a variety of normal cells32,46-48 and currently being investigated in our laboratory in relation to its effect on OC apoptosis in normal conditions.
In conclusion, our results highlight the T-cell regulation of OC formation and survival through RANKL, OPG, and TRAIL overexpression and provide the basis for a potential role of the novel OPG/TRAIL interaction in the biology of MM bone disease.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-02-0474.
Supported by Ministero dell'Istruzione Università e Ricerca Progetto di Ricerca di Interesse Nazionale Cofinanziato (COFIN PRIN) 2001, AIRC (Italian Foundation for Cancer Research) and Italian Space Agency (ASI), Ministero Dell'Istruzione Università e Ricerca-Consiglio Nazionale Delle Ricerche (MIUR-CNR) and Associazione Italiana Leucemielinfomi e Mieloni (AIL-Bari).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof D. Ribatti for helpful discussions and A. Grano for expert technical support.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal