The humanized anti-CD74 monoclonal antibody (mAb) hLL1 is under evaluation as a therapeutic agent. The effects of hLL1—at times in comparison with the CD20 mAb rituximab—were assessed on non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) cell lines and in tumor-bearing SCID mice. In vitro, hLL1 caused growth inhibition and induction of apoptosis in B-cell lines when cross-linked with an antihuman immunoglobulin G (IgG) second antibody. The sensitivity profile of the cell lines was different for hLL1 and rituximab, and antiproliferative activity was augmented when the 2 mAbs were combined. Unlike rituximab, hLL1 did not induce antibody-dependent cellular cytotoxicity or complement-mediated cytotoxicity. In xenograft models of NHL and MM, treatment with hLL1 yielded significant survival benefits without cross-linking agents. Efficacy was greater in the MM model, in which median survival time was increased more than 4.5-fold. Thus, hLL1 has therapeutic potential as a naked mAb for B-cell malignancies because of high antigen expression on malignant cells, specifically MM, with limited expression on normal tissue, and because of its antiproliferative activity. Further, hLL1 may be a therapeutic candidate for rituximab-resistant disease because the 2 antibodies apparently act through distinct mechanisms and exhibit different expression and sensitivity profiles, and activity can be augmented when the mAbs are combined.
Introduction
Monoclonal antibody (mAb) LL1 reacts with CD74, a cell surface–expressed epitope of the HLA class 2–associated invariant chain.1,2 Antigen-positive cell lines take up and catabolize nearly 107 molecules of LL1 per cell per day. The rapid internalization of LL1 is consistent with observations obtained with other anti-CD74 mAbs and were generalized to observations on diverse CD74-expressing cell lines, including a melanoma, a colon carcinoma, a T-cell lymphoma, and a B-lymphoblastoid cell line.1,3 This catabolic rate is approximately 100 times faster than that observed with other mAbs that are generally considered to be rapidly internalized, such as mAbs to CD19, CD22, and the transferrin receptor.1 Similar rapid uptake of surface-bound proteins occurs for ligand binding to receptors that are internalized through coated pits; thus, it seems likely that LL1 uptake is through coated pits.1
CD74 is expressed mainly in association with HLA-DR, where it plays a role in trafficking HLA-DR and antigen loading.4 The rapid internalization of LL1 is presumably attributed to the function of CD74 in antigen trafficking. In addition to its being a chaperone molecule, a role as an accessory signaling molecule has been attributed to CD74. CD74 was shown to be directly involved in the maturation of B cells through a pathway leading to the activation of transcription mediated by the NF-κB p65/RelA homodimer and its coactivator, TAFII105.5 An accessory role for CD74 also was identified during T-cell responses through interactions with CD44.6 Recently, CD74 was reported to be a high-affinity binding protein for the proinflammatory cytokine macrophage migration-inhibitory factor (MIF),7 providing further evidence for a role in signal transduction pathways. MIF binds to the extracellular domain of CD74, and CD74 is required for MIF-mediated phosphorylation of the extracellular signal-regulated kinase-1/2 (ERK-1/2), cell proliferation, and prostaglandin E2 (PGE2) production.
LL1 binds to the cell surface of all non-Hodgkin lymphoma (NHL) cell lines examined, and immunohistochemical analysis indicated strong binding to NHL biopsy specimens.2 We demonstrated recently that multiple myeloma (MM) biopsy specimens and cell lines also express CD74 by using immunohistochemical, flow cytometric, and polymerase chain reaction (PCR) analyses.8 Nineteen of 22 (86.4%) MM trephine bone marrow biopsy specimens exhibited positive immunohistochemical staining with the LL1 mAb (ie, greater than 50% of malignant plasma cells present), with 16 of the 19 specimens showing CD74 staining in more than 95% of MM plasma cells. This group of MM specimens was also assessed for HLA-DR expression. In contrast to most normal and malignant cell types, which coexpress DR and CD74, only 1 of the 22 specimens was DR+. Normal tissues are negative, except for plasma cells, dendritic cells, thymus, some endothelial cells, and lymphocytes (lymph node, tonsil, and spleen) (Immunomedics, data on file). Whether this spectrum of normal tissue reactivity will lead to any potential toxicity after clinical administration of the antibody remains to be established in clinical studies. However, it is encouraging that rituximab, an mAb that also reacts with normal B lymphocytes, is well tolerated.
In addition to its expression on normal and malignant B cells, CD74 has been shown to be expressed in other cancers, including gastric,9 renal,10 non–small-cell lung,11 and thymic epithelial neoplasms12 and certain types of sarcoma (specifically malignant fibrous histiocytoma).13 CD74 expression in many of these cancers has been suggested to be a prognostic factor, with higher relative rates of CD74 behaving as a marker of tumor progression. This correlation may be related to suppressive effects on host immune responses.
A humanized form of LL1, hLL1, has been generated by complementarity-determining region (CDR) grafting, and it exhibits antigen-binding and internalization properties comparable to those of the parental murine antibody.14 In this paper, the naked humanized anti-CD74 mAb hLL1 is shown to cause specific in vitro growth inhibition and induction of apoptosis in B-cell lines in the presence of a second cross-linking antibody. In addition, significant survival extensions were observed in NHL- and MM-bearing SCID mice treated with naked hLL1 without the need for an exogenous cross-linking agent. In comparisons between hLL1 and the chimeric anti-CD20 mAb rituximab, we observed that the 2 antibodies act through distinct mechanisms and exhibit different expression and sensitivity profiles on B-cell malignancies. Importantly, antiproliferative activity can be augmented when the anti-CD74 and anti-CD20 mAbs are combined.
Materials and methods
Cells
The Burkitt lymphoma lines Daudi, Raji, and Ramos were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Non-Burkitt lymphoma cell lines were obtained as follows: RL from Dr John Gribben (Dana-Farber Cancer Institute, Boston, MA), SU-DHL-6 from Dr Alan Epstein (University of Southern California, Los Angeles), DoHH2 and WSU-FCCL from Dr Mitchell Smith (Fox Chase Cancer Center, Philadelphia, PA), and SU-DHL-4 and Karpas422 from Dr Myron Czuczman (Roswell Park Cancer Institute, Buffalo, NY). MM cell lines were obtained as follows: ARH-77 and MC/CAR from the ATCC; KMS12-PE from Dr T. Otsuki (Kawasaki Medical School, Okayama, Japan); and ARK, ARD, and CAG from Dr Joshua Epstein (University of Arkansas, Fayetteville). Cells were grown as suspension cultures in Dulbecco modified Eagle medium (DMEM; Life Technologies, Gaithersburg, MD) and were supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100μg/mL), and l-glutamine (2 mM) (complete media).
Antibodies
The parental murine anti-CD74 mAb LL1 (formerly called EPB-1) was generated using the Raji cell line as the immunogen source.2 Development of hLL1, the humanized anti-CD74 mAb, was performed by methods similar to those described previously for other humanized mAbs, including epratuzumab (hLL2), hRS7, and IMMU-106.15-18 Briefly, the genes encoding Vκ and VH sequences of the parent murine LL1 were cloned by reverse transcription–polymerase chain reaction (RT-PCR) and 5′-RACE, respectively, and the sequences were determined by DNA sequencing. A chimeric LL1 (cLL1) IgG containing human light- and heavy-chain constant region domains was generated and was shown to have antigen-binding specificity and affinity comparable to those of the parent LL1, confirming the authenticity of the cloned V genes. The V genes of CDR-grafted (or humanized) LL1 were then designed and engineered by a combination of long DNA oligonucleotide synthesis and PCR.15,16 hLL1 was expressed in Sp2/0-Ag14 cells (ATCC) by transfection. A high-level hLL1-producing clone was developed using the procedures described previously.16 hLL1 was produced in a bioreactor and was purified by a combination of affinity chromatography on protein A columns and ion-exchange chromatography on Q-Sepharose columns.
Immunophenotyping
Indirect immunofluorescence assays were performed with the panel of cell lines described above, using murine LL1 (CD74, IgG1) and 2B8 (CD20, IgG2a; ATCC) as the primary antigen-specific mAbs and fluorescein isothiocyanate (FITC)–goat antimouse (GAM) IgG (Tago, Burlingame, CA), essentially as described previously,20 and analyzed by flow cytometry using a FACScalibur (Becton Dickinson, San Jose, CA).
In vitro cell proliferation assays
Effects of mAbs on cell growth were determined by assessing [3H]-thymidine incorporation in the NHL and MM cell lines with and without the presence of a cross-linking second antibody, essentially as described by Shan et al.21 Second antibodies used for evaluating the effects of cross-linking were F(ab')2 GAM IgG Fcγ–specific or F(ab')2 goat antihuman (GAH) IgG Fcγ–specific (Jackson Laboratories, West Grove, PA). All tests were performed in triplicate. Statistical analyses were performed using the Student t test.
Analysis of apoptosis
Flow cytometric analysis of cellular DNA was performed after propidium iodide staining.21,22 Cells were placed in 24-well plates (5 × 105 cells/well) and were subsequently treated with mAbs (5 μg/mL) in the presence or absence of a second mAb (20 μg/mL) for cross-linking (F(ab')2 GAM IgG Fcγ–specific or F(ab')2 GAH IgG Fcγ-specific). After a 48-hour incubation (37°C, 5% CO2), cells were transferred to test tubes, washed with phosphate-buffered saline (PBS), and resuspended in hypotonic propidium iodide solution (50 mg/mL propidium iodide in 0.1% sodium citrate, 0.1% Triton X-100). Samples were analyzed by flow cytometry using a FACScalibur. Percentage of apoptotic cells was defined as the percentage of cells with DNA staining before G1/G0 peak (hypodiploid).
Changes in the intracellular levels of caspase-3 and -8 were analyzed using ApoAlert caspase colorimetric assay kits (Clontech, Palo Alto, CA). Raji cells at an initial density of 4 × 105 cells/mL were incubated in medium containing 5 μg/mL of hLL1 with or without a cross-linking agent (GAH IgG Fcγ-specific antibody, 20 μg/mL) under normal cell culture conditions for 24 hours. Cell lysates were prepared and measured for the intracellular caspase-3 and -8 activities according to the user's manual provided by the manufacturer.
Cytotoxicity assays
Standard chromium-51 (51Cr) release assays were performed in triplicate to measure antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), essentially as described.23 Blood specimens used in these studies were collected under a protocol approved by the Institutional Review Board. All blood donors gave voluntary written informed consent. Normal human serum complement was purchased from Quidel (San Diego, CA). For the CMC assay, 25 μL of a 1:5 dilution was added, followed by a 3-hour incubation. For ADCC, effector/target cell ratios of approximately 50:1 were used, and incubations lasted 4 hours. Percentage specific lysis was calculated according to the formula: % lysis = [51Cr release from experimental sample – spontaneous 51Cr release]/[51Cr release from maximum release – spontaneous 51Cr release] × 100.
In vivo effects of naked mAbs on SCID mice bearing disseminated tumors
For studies on the therapeutic effect of hLL1 in NHL-bearing severe combined immunodeficient (SCID) mice (female, 6-8 weeks old; Charles River Laboratories, Frederick, MD), the animals were injected intravenously with 1 × 106 Raji cells or 1 × 107 Daudi cells. For studies in an MM model, SCID mice were pretreated with 0.4 mg/mouse Fludara (Florida Infusion, Palm Harbor, FL) and 2 mg/mouse Neosar (Florida Infusion) 3 days before intravenous injection of 1 × 107 MC/CAR tumor cells. After the injections of tumor cells according to the dose schedules described, mAb administration was initiated. Mice were examined daily for signs of distress or hind leg paralysis and were weighed weekly. Paralysis of the hind legs or a weight loss of more than 25% was used as the survival end point. Animals were humanely killed at these end points. Animal studies were performed under protocols approved by the Institutional Animal Care and Use Committee. Statistical analyses were performed using the log rank test.
Results
Construction of hLL1
The gene sequences encoding the Vκ and VH of LL1 were cloned by RT-PCR and 5′-RACE, respectively. Before the process of humanization, the authenticity of the genes was confirmed by construction and expression of a chimeric LL1 IgG molecule composed of the cloned mouse V genes and human constant domain sequences. Chimeric LL1 competed with the murine LL1 for cell surface antigen binding, and the apparent avidities of these 2 antibodies were similar (data not shown). LL1 mAb was humanized by CDR grafting and transfection according to procedures similar to those used in the humanization of other murine antibodies.15-18 It should be noted that the humanized hLL1 was designed to have the same human IgG1/κ constant regions and hinge sequences as those of other humanized antibodies, including epratuzumab (anti-CD22),15 IMMU-106 (anti-CD20),18 and hRS7 (antiepithelial glycoprotein-1 mAb).17 Antigen-binding specificity and affinity of hLL1 were confirmed to be comparable to those of the parental chimeric LL1, cLL1 (not shown).
Cell surface antigen expression
CD74 and CD20 antigen expression levels were determined by flow cytometric analysis of NHL and MM cell lines and are summarized in Table 1. The NHL cell lines include Burkitt lymphoma and several non-Burkitt lymphoma lines, most of which have the Bcl-2 gene rearrangement t(14;18). In the NHL cell lines, CD74 was expressed on the cell surface of all the cell lines tested; however, intensity varied between the cell lines, and staining was generally lower than it was for CD20 levels. In contrast, CD74 was detected on a greater number of MM cell lines than CD20, with 4 of 6 CD74+ MM cell lines compared to 2 of 6 CD20+ MM cell lines in the panel evaluated.
Antigen expression: indirect flow cytometry assay
. | Positive (geometric mean FL), % . | . | . | ||
|---|---|---|---|---|---|
. | Control, Ag8 . | CD20, 2B8 . | CD74, LL1 . | ||
| NHL Burkitt | |||||
| Daudi | 2 (7) | 99 (101) | 82 (26) | ||
| Ramos | 1 (5) | 99 (64) | 89 (12) | ||
| Raji | 4 (3) | 99 (103) | 99 (64) | ||
| NHL non-Burkitt | |||||
| DoHH2 | 1 (3) | 99 (175) | 89 (17) | ||
| Karpas422 | 5 (4) | 46 (13) | 39 (12) | ||
| RL | 1 (3) | 98 (47) | 40 (8) | ||
| SU-DHL-4 | 1 (2) | 72 (11) | 84 (13) | ||
| SU-DHL-6 | 1 (2) | 99 (149) | 96 (23) | ||
| WSU-FSCCL | 2 (3) | 88 (23) | 94 (17) | ||
| MM | |||||
| ARD | 3 (3) | 4 (3) | 3 (3) | ||
| ARK | 3 (3) | 4 (3) | 3 (3) | ||
| ARH-77 | 2 (3) | 99 (71) | 93 (30) | ||
| CAG | 4 (4) | 2 (3) | 99 (48) | ||
| KMS12-PE | 1 (2) | 2 (2) | 11 (5) | ||
| MC/CAR | 2 (3) | 98 (72) | 92 (30) | ||
. | Positive (geometric mean FL), % . | . | . | ||
|---|---|---|---|---|---|
. | Control, Ag8 . | CD20, 2B8 . | CD74, LL1 . | ||
| NHL Burkitt | |||||
| Daudi | 2 (7) | 99 (101) | 82 (26) | ||
| Ramos | 1 (5) | 99 (64) | 89 (12) | ||
| Raji | 4 (3) | 99 (103) | 99 (64) | ||
| NHL non-Burkitt | |||||
| DoHH2 | 1 (3) | 99 (175) | 89 (17) | ||
| Karpas422 | 5 (4) | 46 (13) | 39 (12) | ||
| RL | 1 (3) | 98 (47) | 40 (8) | ||
| SU-DHL-4 | 1 (2) | 72 (11) | 84 (13) | ||
| SU-DHL-6 | 1 (2) | 99 (149) | 96 (23) | ||
| WSU-FSCCL | 2 (3) | 88 (23) | 94 (17) | ||
| MM | |||||
| ARD | 3 (3) | 4 (3) | 3 (3) | ||
| ARK | 3 (3) | 4 (3) | 3 (3) | ||
| ARH-77 | 2 (3) | 99 (71) | 93 (30) | ||
| CAG | 4 (4) | 2 (3) | 99 (48) | ||
| KMS12-PE | 1 (2) | 2 (2) | 11 (5) | ||
| MC/CAR | 2 (3) | 98 (72) | 92 (30) | ||
Antiproliferative effects
Antiproliferative effects of hLL1 were evaluated using [3H]-thymidine uptake assays and were compared to the activity of the anti-CD20 mAb rituximab. As shown in Figure 1A, incubation with hLL1 caused specific inhibition of proliferation in 5 of 6 NHL cell lines in the presence of a cross-linking second antibody. The sensitivity profile of the cell lines was different for hLL1 and rituximab (Figure 1B). For example, in the absence of cross-linking, no antiproliferative activity was seen with hLL1 in any cell line, whereas rituximab yielded approximately 23% inhibition of [3H]-thymidine incorporation in Daudi and Ramos cells and 88% inhibition in SU-DHL-6 cells. However, significant augmentation of the antiproliferative effects of rituximab was also observed with cross-linking. This is shown in Figure 1B, where the activity of rituximab was increased by the addition of a second antibody in 5 of 6 NHL lines and in Figure 1D in 2 of 2 CD20+ cell lines from among the 6 MM cell lines tested. Interestingly, the variation in level of inhibition was not correlated strictly to antigen density. In WSU-FSCCL, CD74 antigen density is half that of CD20; however, [3H]-thymidine incorporation was inhibited 80% by hLL1 + GAH IgG cross-linking, whereas these cells were unaffected by rituximab + GAH IgG. In addition, Raji cells expressed a higher density of CD74 than Daudi, SU-DHL-6, and WSU-FSCCL cells, but were less sensitive to inhibition of proliferation by hLL1+GAH IgG.
Effects of hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs were assessed by measuring the uptake of [3H]-thymidine. Cells were cultured with the mAbs with or without a second antibody for cross-linking to mimic the role of effector cells or cross-linking molecules present in vivo. Error bars represent standard deviations (SD). (A) NHL cell lines incubated with hLL1. (B) NHL cell lines incubated with rituximab. (C) MM cell lines incubated with hLL1. (D) MM cell lines incubated with rituximab. *Not determined.
Effects of hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs were assessed by measuring the uptake of [3H]-thymidine. Cells were cultured with the mAbs with or without a second antibody for cross-linking to mimic the role of effector cells or cross-linking molecules present in vivo. Error bars represent standard deviations (SD). (A) NHL cell lines incubated with hLL1. (B) NHL cell lines incubated with rituximab. (C) MM cell lines incubated with hLL1. (D) MM cell lines incubated with rituximab. *Not determined.
Evaluation of the antiproliferative effects of hLL1 in MM cell lines demonstrated that the effectiveness of hLL1 in the presence of GAH second antibody correlated with the presence of antigen in this cell line panel (Figure 1C-D). ARH-77, MC/CAR, CD20+, and CD74+ cell lines were sensitive to hLL1 and rituximab; CAG, KMS12-PE, CD20-, and CD74+ were only inhibited by hLL1. The 2 CD20- and CD74- cell lines, ARK and ARD, were insensitive to both mAbs.
Induction of apoptosis
Induction of apoptosis was evaluated by flow cytometry on a panel of CD74+ cell lines. Cells were cultured with the mAbs for 48 hours, with or without a second mAb for cross-linking, followed by DNA staining with propidium iodide. Cells were analyzed by flow cytometry, and positive florescence below the G1 region represented DNA fragmentation and was a measure of apoptosis. Controls included rituximab, no first mAb, and the isotype-negative control mAb labetuzumab. Representative histograms are shown in Figure 2A for the SU-DHL-6 cell line. The peak in the histogram line, corresponding to mAb + GAH IgG–treated cells in region M1, represents hypodiploid DNA and is a measure of apoptotic nuclei induced by the antibodies. hLL1 induced apoptosis, but only when the specific second antibody was added. The level of apoptosis caused by hLL1 was similar to that for rituximab, except in the WSU-FSCCL cell line, which was relatively insensitive to rituximab in this assay, consistent with the observation in the proliferation study. The percentage of apoptotic cells after a 2-day exposure to hLL1 ranged from 58% to 99% that of rituximab in 5 of the 6 NHL lines tested and was 310% of the rituximab level in WSU-FSCCL (Figure 2B).
Apoptotic effect of mAbs on CD74+ cells. Induction of apoptosis was evaluated by flow cytometry determination of hypodiploid DNA on the cell line panel. Cells were cultured with the mAbs without a second antibody, with GAM IgG, or with GAH IgG, followed by DNA staining with propidium iodide. (A) Representative histograms are shown for SU-DHL-6 cells. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified in region M1. Dashed line indicates no second antibody; solid gray line, GAM IgG; solid black line, GAH IgG. (B) Percentage apoptosis (hypodiploid DNA) induced by cross-linked mAbs is shown for a panel of cell lines. (C) Caspase-3 and -8 activities in Raji cells. Cells were cultured in the presence of hLL1 (5 μg/mL) or a GAH IgG Fcγ–specific antibody cross-linking agent (CL) (20 μg/mL), with both antibodies (LL1 [5 μg/mL] + CL [20 μg/mL]) or no antibody added (Untreated) for 24 hours. Intracellular levels of caspase-3 and -8 were measured by using ApoAlert Caspase assay kits (Clontech).
Apoptotic effect of mAbs on CD74+ cells. Induction of apoptosis was evaluated by flow cytometry determination of hypodiploid DNA on the cell line panel. Cells were cultured with the mAbs without a second antibody, with GAM IgG, or with GAH IgG, followed by DNA staining with propidium iodide. (A) Representative histograms are shown for SU-DHL-6 cells. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified in region M1. Dashed line indicates no second antibody; solid gray line, GAM IgG; solid black line, GAH IgG. (B) Percentage apoptosis (hypodiploid DNA) induced by cross-linked mAbs is shown for a panel of cell lines. (C) Caspase-3 and -8 activities in Raji cells. Cells were cultured in the presence of hLL1 (5 μg/mL) or a GAH IgG Fcγ–specific antibody cross-linking agent (CL) (20 μg/mL), with both antibodies (LL1 [5 μg/mL] + CL [20 μg/mL]) or no antibody added (Untreated) for 24 hours. Intracellular levels of caspase-3 and -8 were measured by using ApoAlert Caspase assay kits (Clontech).
To assess whether the cross-linked hLL1 activates an apoptosis pathway, the activities of key caspases were analyzed in the treated and control cells. Parallel to the antiproliferative effect seen with cross-linked hLL1, approximately 3-fold increases of caspase-3 and caspase-8 activities were detected in Raji cells treated with the cross-linked hLL1 compared with untreated controls or cells treated with hLL1 or with the cross-linking antibody alone (Figure 2C).
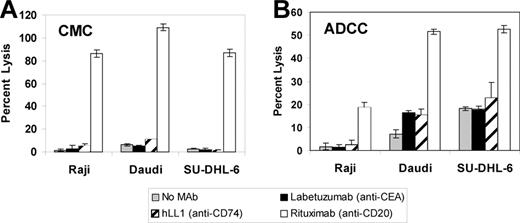
The ability of hLL1 to induce ADCC and CMC was assayed using standard 51Cr release assays. In contrast to rituximab, the anti-CD74 mAb did not induce significant levels of ADCC or CMC (Figure 3A-B). This may be related to the rapid internalization of hLL1.
ADCC and CDC.51Cr-labeled NHL cells were incubated in the absence of an mAb, with the negative control mAb labetuzumab, hLL1, or rituximab in the presence of human complement (A) or human peripheral blood mononuclear cells (B), as described in “Materials and methods.” Percentage specific lysis of 3 cell lines is shown. Error bars represent SD.
ADCC and CDC.51Cr-labeled NHL cells were incubated in the absence of an mAb, with the negative control mAb labetuzumab, hLL1, or rituximab in the presence of human complement (A) or human peripheral blood mononuclear cells (B), as described in “Materials and methods.” Percentage specific lysis of 3 cell lines is shown. Error bars represent SD.
Effects of combining anti-B cell mAbs
Given that combinations of mAbs recognizing different antigens that act by means of distinct mechanisms may enhance antitumor activity, we examined the effects of combining anti-CD74 and anti-CD20 mAbs. Antiproliferative effects of the combination of hLL1 and rituximab were tested and compared with the effects of each mAb given alone, using [3H]-thymidine uptake assays. Results of the combined mAb treatments are shown in Figure 4A-B, with and without cross-linking by GAH second antibody. In all cell lines, the effect of the combination of hLL1, rituximab, and second antibody was equal to or greater than that of the single cross-linked mAb treatments. In 4 of 6 lines (FSCCL, SU-DHL-4, DoHH2, and MC/CAR) growth inhibition of cells given the combination treatment was significantly greater than that of cross-linked rituximab alone. In the other 2 cell lines, Daudi and RL, adding hLL1 to rituximab and GAH did not cause a significant change in proliferation. The effect became more pronounced as the sensitivity to rituximab decreased, except in RL, which is relatively insensitive to hLL1. In all 6 cell lines, the antiproliferative effect of the combined antibody treatment was greater than that of cross-linked hLL1 alone. Statistical significance was reached in all cell lines except FSCCL and MC/CAR, in which the effects of hLL1 alone were most pronounced. In the absence of cross-linking Daudi was the only tested cell line inhibited by rituximab, and the effect was not enhanced by addition of hLL1. In the absence of GAH second antibody, the combined treatment was more effective than either mAb alone in the more hLL1-sensitive cell lines, FSCCL and MC/CAR; however, statistical significance was not reached (P = .07 and .09, respectively) in the comparison between hLL1 plus rituximab and hLL1 alone.
Effects of combining hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs given in combination were assessed by measuring the uptake of [3H]-thymidine in comparison with untreated control cells. Error bars represent SD. (A) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) in the presence of GAH second antibody. (B) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) without a cross-linking agent.
Effects of combining hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs given in combination were assessed by measuring the uptake of [3H]-thymidine in comparison with untreated control cells. Error bars represent SD. (A) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) in the presence of GAH second antibody. (B) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) without a cross-linking agent.
These studies were also performed using a humanized anti-CD20 mAb, IMMU-106, in place of the human–murine chimeric anti-CD20 mAb rituximab. As shown in Table 2, similar results were obtained with rituximab and IMMU-106 in the in vitro proliferation assays. For example, in the SU-DHL-4 cell line, the anti-CD20 mAbs yielded 8% to 15% inhibition of proliferation compared with untreated cells when given as single agents, and this was increased to between 60% and 65% inhibition when combined with hLL1 treatment. This observation is important because it is expected that in humans, IMMU-106 should be at least as effective as rituximab, and, because of its human framework, it may exhibit different pharmacokinetic, toxicity, and therapy profiles than the chimeric antibody.18
Effects of combining hLL1 with the chimeric anti-CD20 mAb rituximab and the humanized anti-CD20 MAb IMMU-106 on proliferation of cell lines
. | Relative proliferation, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | hLL1 . | Rituximab . | Rituximab + hLL1 . | IMMU-106 . | IMMU-106 + hLL1 . | ||||
| FSCCL | 19.7 ± 6.2 | 111.2 ± 8.0 | 12.1 ± 3.9* | 109.5 ± 0.8 | 15.6 ± 3.0† | ||||
| SU-DHL-4 | 60.8 ± 3.6 | 92.0 ± 1.1 | 40.3 ± 1.9‡ | 85.1 ± 1.4 | 34.6 ± 2.2†‡ | ||||
| RL | 86.9 ± 4.9 | 61.2 ± 7.5 | 62.7 ± 1.7‡ | 67.3 ± 6.6 | 65.9 ± 4.6‡ | ||||
| DoHH2 | 32.1 ± 3.7 | 43.9 ± 4.5 | 22.8 ± 3.9‡ | 45.1 ± 5.6 | 22.6 ± 1.1†‡ | ||||
| Daudi | 52.2 ± 9.1 | 21.1 ± 3.0 | 15.1 ± 0.4‡ | 20.0 ± 3.7 | 23.4 ± 1.6‡ | ||||
| MC/CAR | 23.9 ± 3.2 | 39.6 ± 3.6 | 19.3 ± 1.1* | 60.7 ± 5.9 | 23.5 ± 3.3† | ||||
. | Relative proliferation, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | hLL1 . | Rituximab . | Rituximab + hLL1 . | IMMU-106 . | IMMU-106 + hLL1 . | ||||
| FSCCL | 19.7 ± 6.2 | 111.2 ± 8.0 | 12.1 ± 3.9* | 109.5 ± 0.8 | 15.6 ± 3.0† | ||||
| SU-DHL-4 | 60.8 ± 3.6 | 92.0 ± 1.1 | 40.3 ± 1.9‡ | 85.1 ± 1.4 | 34.6 ± 2.2†‡ | ||||
| RL | 86.9 ± 4.9 | 61.2 ± 7.5 | 62.7 ± 1.7‡ | 67.3 ± 6.6 | 65.9 ± 4.6‡ | ||||
| DoHH2 | 32.1 ± 3.7 | 43.9 ± 4.5 | 22.8 ± 3.9‡ | 45.1 ± 5.6 | 22.6 ± 1.1†‡ | ||||
| Daudi | 52.2 ± 9.1 | 21.1 ± 3.0 | 15.1 ± 0.4‡ | 20.0 ± 3.7 | 23.4 ± 1.6‡ | ||||
| MC/CAR | 23.9 ± 3.2 | 39.6 ± 3.6 | 19.3 ± 1.1* | 60.7 ± 5.9 | 23.5 ± 3.3† | ||||
3H-thymidine uptake is shown as a percentage of the uptake in untreated control cells. Results are shown only for incubations performed in the presence of second antibody.
P < .05 compared to rituximab
Compared with IMMU-106
Compared with hLL1 by Student t test
In vivo effects of hLL1
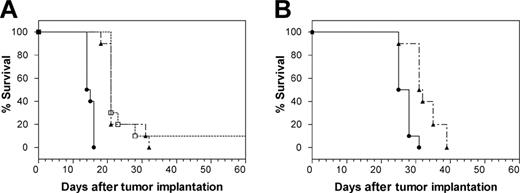
The therapeutic effects of the anti-CD74 mAb were studied in vivo in SCID mice using 2 NHL cell lines, Raji and Daudi. As shown in Figure 5A, treatment of Raji tumors with hLL1 yielded a significant survival benefit, providing a 45% increase in median survival by either of 2 treatment schedules. In one group, hLL1 was administered 5 times a week for 2 weeks, then twice weekly, at 100 μg/injection. In the second group, hLL1 was administered twice a week for 2 weeks, at 350 μg/injection. In both, hLL1 treatment was initiated 1 day after the injection of Raji cells. Control mice died of disseminated disease manifested with central nervous system paralysis, with a median survival time of 14.5 days after Raji tumor inoculation. Median survival in both treated groups was extended to 21 days, statistically significant survival extensions by log rank analysis (P < .0001). Similar therapeutic effects were observed in Daudi-bearing SCID mice given low doses (25 μg) of hLL1 twice weekly, starting 1 day after cell injection (Figure 5B). The effect of dose escalation was not evaluated. Untreated Daudi-bearing mice had a median survival time of 26.5 days after tumor inoculation compared with 31.5 days in the hLL1-treated animals (P = .0004).
Survival proportions in SCID mice bearing disseminated NHL cells. (A) mAb hLL1 was administered to Raji-bearing SCID mice at 2 dose schedules, both initiated 1 day after tumor cells were inoculated intravenously. Survival was compared with that in untreated Raji-bearing SCID mice. □, 100 μg/injection, 5 times/wk for 2 weeks, then twice weekly; ▴, 350 μg/injection, twice weekly for 2 weeks; •, untreated. (B) Survival of Daudi-bearing SCID mice given a low dose of hLL1 was compared with survival of untreated Daudi-bearing SCID mice. ▴, 25 μg twice-weekly intravenous injections, starting 1 day after injection of tumor cells; •, untreated. Each treatment group contained 10 mice.
Survival proportions in SCID mice bearing disseminated NHL cells. (A) mAb hLL1 was administered to Raji-bearing SCID mice at 2 dose schedules, both initiated 1 day after tumor cells were inoculated intravenously. Survival was compared with that in untreated Raji-bearing SCID mice. □, 100 μg/injection, 5 times/wk for 2 weeks, then twice weekly; ▴, 350 μg/injection, twice weekly for 2 weeks; •, untreated. (B) Survival of Daudi-bearing SCID mice given a low dose of hLL1 was compared with survival of untreated Daudi-bearing SCID mice. ▴, 25 μg twice-weekly intravenous injections, starting 1 day after injection of tumor cells; •, untreated. Each treatment group contained 10 mice.
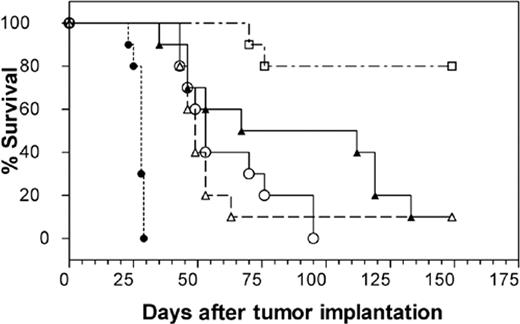
Therapeutic efficacy was found to be markedly greater in the MC/CAR MM cell line. Median survival time in untreated mice was 28 days. Figure 6 shows the results of a study comparing the efficacy of hLL1 in MC/CAR-bearing SCID mice treated by 4 dose schedules. In the experiment shown here, a single injection of hLL1 given 5 days after tumor cell injection yielded a significant survival benefit, providing an approximately 75% increase in median survival time. Survival of MC/CAR-bearing SCID mice treated with a single 350-μg dose of hLL1 was compared with that in mice treated with two 350-μg doses (once a week for 2 weeks), both initiated 5 days after tumor cells were inoculated, and also after multiple 100-μg doses of hLL1 (5 times a week for 2 weeks, then twice weekly), with treatment initiated either 1 day or 5 days after tumor cells were transplanted. Median survival times in the groups in which treatment was initiated 5 days after tumor cells were injected were 49 days, single 350-μg dose; 53 days, 2 doses of 350 μg; and 112 days, multiple 100-μg doses. All 3 survival curves were significantly different than they were for untreated mice (P < .0001), but differences between the groups did not reach statistical significance. Initiation of the multiple 100-μg dose schedule 1 day after tumor cells were inoculated yielded a median survival time of more than 150 days, with 8 of 10 mice long-term survivors (P = .0021 vs the multiple 100-μg dose schedule initiated 5 days after tumor cells were injected). Thus, the hLL1 survival benefit in myeloma-bearing SCID mice was increased by a multiple-dose schedule and early administration. No treatment-related toxicities were observed, as measured by body weight loss.
Survival proportions in SCID mice bearing disseminated MC/CAR MM cells. mAb hLL1 was administered to MC/CAR-bearing SCID mice at 4 dose schedules. Survival was compared with untreated MC/CAR-bearing SCID mice. ▵, 350 μg administered 5 days after tumor cells were grafted; ○, 350 μg/injection once a week for 2 weeks, treatment initiated 5 days after tumor cells were injected; ▴, 100 μg/injection 5 times/wk for 2 weeks, then twice weekly, treatment initiated 5 days after tumor cells were inoculated; □, 100 μg/injection 5 times/wk for 2 weeks, then twice weekly, treatment initiated 1 day after tumor cells were injected; and •, untreated control.
Survival proportions in SCID mice bearing disseminated MC/CAR MM cells. mAb hLL1 was administered to MC/CAR-bearing SCID mice at 4 dose schedules. Survival was compared with untreated MC/CAR-bearing SCID mice. ▵, 350 μg administered 5 days after tumor cells were grafted; ○, 350 μg/injection once a week for 2 weeks, treatment initiated 5 days after tumor cells were injected; ▴, 100 μg/injection 5 times/wk for 2 weeks, then twice weekly, treatment initiated 5 days after tumor cells were inoculated; □, 100 μg/injection 5 times/wk for 2 weeks, then twice weekly, treatment initiated 1 day after tumor cells were injected; and •, untreated control.
Discussion
For patients with hematologic malignancies, mAbs against lineage-specific B-cell antigens have provided clinical benefit.24-26 The chimeric anti-CD20 mAb rituximab induces responses in approximately 50% of patients with low-grade follicular lymphoma, with a median time to progression in responders of 13 months.24 Approximately 40% of initial responders respond to retreatment.25 In patients with more aggressive histologic lymphoma subtypes, rituximab therapy induces an approximately 30% overall response rate.26 However, the rituximab response rate in patients with MM is not as high as that observed for patients with follicular lymphoma. In contrast to the high prevalence of CD20 expression in NHL, only 20% of patients with MM express CD20 on bone marrow plasma cells.27 In a phase 2 clinical study of rituximab in 19 patients with MM, 1 patient had a partial response and 5 patients had stable disease; median time to treatment failure was 5.5 months. Efforts are ongoing to improve on the success of rituximab treatments for B-cell malignancies, including combining mAbs with other biologic agents such as interleukin-2 (IL-2),28 combining mAb therapy with chemo-therapy such as CHOP,29,30 the development of humanized anti-CD20 mAbs,18 and the use of mAbs that target antigens other than CD20. Other antibodies in clinical trial for hematologic malignancies include anti-CD22 (epratuzumab),31,32 anti-CD52 (alemtuzumab [Campath-1H]),33 anti-CD80 (galiximab),34 and anti-HLA-DR (apolizumab [Hu1D10]).35,36 Although these mAbs have shown evidence of antitumor activity in patients, as with rituximab, it is unlikely that these mAbs will be curative as single agents. Thus, the use of additional mAbs and of mAb combinations is important.
In this report, we evaluated the antitumor effects and mechanism of action of hLL1, a humanized anti-CD74 mAb. Because rituximab is widely used for the treatment of B-cell malignancies, the expression of antigen and antitumor effects of hLL1 were compared with those of rituximab. CD74 and CD20 were both expressed on the cell surface of all the NHL cell lines tested; however, CD74 staining intensity was generally lower than that of CD20. In contrast, CD74 was detected on a greater number of MM cell lines than CD20. We demonstrated that, as with rituximab in most human lymphoma or in MM cell lines, hLL1 alone does not show a direct cytotoxic effect in vitro. However, in the presence of an appropriate cross-linking agent, hLL1 causes inhibition of cell proliferation and induces apoptosis. Unlike rituximab, hLL1 induces little or no ADCC or CMC. This is apparently not caused by the IgG constant sequences used in hLL1 because a humanized anti-CD20 mAb, IMMU-106—which has the same IgG1 heavy chain constant regions, hinge, and Ck as hLL1—mediates ADCC and CMC as effectively as rituximab.18 The absence of ADCC and CMC may be related to the rapid internalization of hLL1. Nevertheless, in SCID mouse xenograft models of NHL and MM, treatment with unconjugated hLL1 yields significant survival benefits, providing greater than a 4.5-fold increase in median survival time in the MM model. Therapeutic efficacy was observed even with the very-low–dose regimens given in the Daudi lymphoma model. Because the antibody has little or no toxicity, we could consider using higher doses or extended dosing to perhaps improve on these results even further. Although cross-linking with GAH second antibody was necessary for hLL1 activity in vitro, both for induction of antiproliferative effects and for apoptosis, GAH was not added in the animal experiments. Thus, physiologic processes are involved in the mechanism of antitumor activity of hLL1 in vivo, and activity was observed without the need for an exogenous cross-linking agent.
The effectiveness of hLL1 in the MC/CAR model of MM was greater than that in the Raji- or Daudi-bearing mice. This effect was seen also in vitro, where the inhibitory effect of hLL1 on DNA synthesis was greatest in MC/CAR. Whether this is because of differences in growth rate, greater sensitivity of MM to the antibody in general compared with that of NHL, or other differences between the cell lines remains to be established. It is apparently not because of the density of cell surface antigen given that Raji cells express a higher level of CD74 than MC/CAR on the cell surface. In a comparison of rituximab and hLL1, we observed that the 2 mAbs differed in their antiproliferative effects on the panel of cell lines examined. The largest differences were observed in the WSU-FSCCL and SU-DHL-6 NHL cell lines and also the CAG MM cell line. SU-DHL-6 cells were more sensitive to rituximab, whereas the WSU-FSCCL and CAG cell lines were more sensitive to the antiproliferative effects of hLL1.
Differences in expression of the complement regulator CD59 have been shown to be associated with resistance to rituximab-mediated CMC in patients with progressive CD20+ MM and Waldenström macroglobulinemia despite rituximab therapy.37 MM and NHL patients also express antigens that may have a role in blocking ADCC, such as Fas ligand, MUC1, or TRAIL.37 Thus, CMC and ADCC regulators are present in various B-cell tumors and are associated with resistance to rituximab. Given that our results indicate that hLL1 activity is apparently unrelated to ADCC and CMC, it may be a useful therapy for patients who are rituximab resistant because of inhibition of the ADCC and CMC pathways.
Thus, several properties emerge that make hLL1 especially attractive as another candidate antibody for the therapy of CD74-expressing malignancies, in particular MM. First, the hLL1 mAb has the ability to affect cell proliferation and to induce apoptosis. Second, CD74 has an appropriate expression profile for a therapeutic target, with high expression on malignant cells, specifically MM, and limited expression on normal tissue. The sensitivity of the MM line in the in vivo model, along with the recent report that CD74 is expressed by most (more than 90%) MM patients compared with only approximately 20% of CD20+ MM patients,8 makes the potential application of the anti-CD74 mAb hLL1 especially promising for the treatment of MM. Third, hLL1 is a good candidate for rituximab-resistant disease, either alone or in combination with other antibodies or agents, because it apparently acts through a distinct mechanism, and the 2 mAbs exhibit different expression and sensitivity profiles on B-cell malignancies. In addition, activity can be augmented when hLL1 is given in combination with an anti-CD20 mAb, either rituximab or IMMU-106.
In addition to its potential use as an unlabeled mAb, the rapid internalization of hLL1 makes it an attractive candidate for further development as a carrier of cytotoxic agents. The rapid internalization of hLL1 can lead to accumulation of large amounts of radioactivity within the cell if the antibody is conjugated to an isotope that remains trapped inside the cell, such as a radiometal. Indeed, radiolabeled LL1 has been demonstrated to effectively and specifically kill human B-cell lymphoma lines in vitro and in animal models.38-41 Similarly, LL1 has been shown to be extremely effective for drug delivery. A doxorubicin-LL1 conjugate was found to cure SCID mice bearing advanced human B-lymphoma xenografts with a single low dose of the conjugate.42 The effective use of a doxorubicin–hLL1 conjugate was also shown recently in a MM xenograft model.43
In summary, the hLL1 mAb shows promise as another candidate antibody for the treatment of CD74-expressing malignancies, particularly MM. Application of hLL1 should be examined as single-agent therapy and as part of multiple-agent therapy. Because of its rapid internalization, it also shows considerable promise as a drug immunoconjugate or as a carrier of therapeutic radioisotopes. In light of the expression of CD74 in nonhematologic malignancies, the potential of hLL1 as therapy for these diseases also should be evaluated. The precise mechanism of apoptosis induced by hLL1 is unknown and under evaluation, but the recent identification of CD74 as the cellular receptor for the cytokine MIF suggests involvement in transmembrane signaling pathways.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-03-0890.
Supported in part by research funding from Immunomedics, Inc.
Presented in part at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Effects of hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs were assessed by measuring the uptake of [3H]-thymidine. Cells were cultured with the mAbs with or without a second antibody for cross-linking to mimic the role of effector cells or cross-linking molecules present in vivo. Error bars represent standard deviations (SD). (A) NHL cell lines incubated with hLL1. (B) NHL cell lines incubated with rituximab. (C) MM cell lines incubated with hLL1. (D) MM cell lines incubated with rituximab. *Not determined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-03-0890/5/m_zh80230469970001.jpeg?Expires=1763476966&Signature=3QEOdh2SZT7HFcdP-F38zsNvDpnz6LpYr0zy-OfIBDWuz3F0U5DrI9ffr1372y7cKtH1Iv1FNjJlMP5-wsO4J863L4nt~2fooveaK8H~viSxbdJ2xsiEnbuJilQ8vKiQVvIgRduHRscEl05Kdua93PSAaQgoKtMjoeMiAC6bE57lVlxZffDnwTHgp6TWI1YPWgyTBhKPbIB-6P~j7DqyVKd-uYyimV3e6Es0HTAIjws4fvtC9Bf2Mzpkcy8~oGAJvdzHvVue~BEf4zNGbhQx52y~2LnE-ZmdZAJv2WtWQDdZIeT1FNk4U4~q50uZvSIswNMJz1o4EZ5JBG1wAN0d5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Apoptotic effect of mAbs on CD74+ cells. Induction of apoptosis was evaluated by flow cytometry determination of hypodiploid DNA on the cell line panel. Cells were cultured with the mAbs without a second antibody, with GAM IgG, or with GAH IgG, followed by DNA staining with propidium iodide. (A) Representative histograms are shown for SU-DHL-6 cells. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified in region M1. Dashed line indicates no second antibody; solid gray line, GAM IgG; solid black line, GAH IgG. (B) Percentage apoptosis (hypodiploid DNA) induced by cross-linked mAbs is shown for a panel of cell lines. (C) Caspase-3 and -8 activities in Raji cells. Cells were cultured in the presence of hLL1 (5 μg/mL) or a GAH IgG Fcγ–specific antibody cross-linking agent (CL) (20 μg/mL), with both antibodies (LL1 [5 μg/mL] + CL [20 μg/mL]) or no antibody added (Untreated) for 24 hours. Intracellular levels of caspase-3 and -8 were measured by using ApoAlert Caspase assay kits (Clontech).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-03-0890/5/m_zh80230469970002.jpeg?Expires=1763476966&Signature=KvENa-L0xaBn-KSJaGp2BFgfcH2bcOG3inuIJ8G3jHlODNK6KvboMQlXq0JI2gulbJ~qClMkslPqimaxtsFQIdZXYrvPj838PD0Dh7ha17uQqAbMQDZUHstJXhCQrW~~LRltujtqIKUMeqVj7cPWE094dSt5G6mt30dI69RjCnlPaRlN45ja60fhDs5wtkzAB6HwDL7TfY32ixSBdUZ4UX1Sg-a77by-SwoPPsq4BoyIvmQXtZ4MFkeXWbRIabt~F7PB24hxFppe61zDkKeX1PjYC~ZHX4Wn7CdO6ExqdcT3TsmvjirEAJaSdVOP~isaC-lCeO64WI2i0SPMpUnz4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effects of combining hLL1 and rituximab on proliferation of cell lines. Antiproliferative effects of the anti–B-cell mAbs given in combination were assessed by measuring the uptake of [3H]-thymidine in comparison with untreated control cells. Error bars represent SD. (A) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) in the presence of GAH second antibody. (B) Cell lines were incubated with hLL1(▪), rituximab (▦), or both mAbs (□) without a cross-linking agent.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2004-03-0890/5/m_zh80230469970004.jpeg?Expires=1763476966&Signature=vcbtKJn7c17lnuHsdf05UdN3ir9TBH6wHUtSieev81qxuI5xfciE2e3WW82~TozGoI0xOx27CKapfyfLwUiI7A65giAtV2dxX3ohHHpGIHK9OAexTSO3zHQMIqtKIoY3Palqs1sTjxM~22NrX1DGQB35zgf1qqUDe0-lIYiTQb-QLik799XECd4yCZqUBQ1m5vgpP3MXbbx-RUzpbyIMQXRj1Kb~W75ydI6x8OTsDRHBSZeuw7zCnb7D5ONRWSDiLUwHRQZoTdFOHQiwN4ADWvWtSIBn6LXiP0w60pRnjJWjLZsA~RPvnGcjj~PaUj7a-d74lDjnEjUSe-W9UgTjIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal