Current therapy of primary systemic (AL) amyloidosis with oral melphalan and prednisone remains unsatisfactory, with a median survival of only 13 months. Between 1996 and 2003, 93 patients with biopsy-proven AL amyloidosis were enrolled in a prospective US national cooperative group trial. Treatment schema consisted of induction therapy with pulse dexamethasone (DEX), followed by maintenance therapy with DEX and alpha interferon. Hematologic complete remissions were observed in 24% and improvement in AL amyloidosis–related organ dysfunction occurred in 45% of patients evaluable for response. Median survival of the entire cohort is 31 months, with an estimated 2-year overall survival (OS) and event-free survival (EFS) of 60% and 52%, respectively. Presence of congestive heart failure and increased level of serum β2 microglobulin (≥ 0.0035 g/L [3.5 mg/L]) were dominant predictors of adverse outcome. Estimated 2-year OS in patients who are eligible to receive transplants with this approach was 78%. These data demonstrate for the first time in the context of a US multicenter prospective clinical trial that front-line therapy with a DEX-based regimen in AL amyloidosis can lead to durable reversal of AL amyloidosis–related organ dysfunction and prolonged survival.
Introduction
Primary systemic (AL) amyloidosis, the most common systemic amyloidosis in the United States (US), is a clonal plasma cell disorder characterized by the deposition of a congophilic beta-pleated fibrillary protein containing monoclonal light-chain fragments in affected organs.1,2 The pattern of organ involvement varies among individuals and determines clinical presentation. The prognosis of AL amyloidosis is generally poor, with a median survival of 12 to 18 months. Current standard therapy with oral melphalan and prednisone (MP) yields clinical responses in only 20% to 25% of patients, which occur slowly at a median time of 12 months.3 Thus prolonged alkylator therapy is required, contributing to increased risk of myelodysplasia/leukemia (estimated actuarial risk 21% at 3.5 years).4
In 1997, Dhodapkar et al5 first described the clinical activity of high-dose dexamethasone (DEX) in AL amyloidosis, testing the hypothesis that more aggressive anti–plasma cell approaches, analogous to those in myeloma, might lead to improved outcome in this disease. Clinical activity of DEX or DEX-based regimens in AL amyloidosis has since been confirmed by other investigators as well.6-10 However, most of the current data regarding therapy for AL amyloidosis are based on single-institution studies, raising unresolved issues related to referral and patient selection bias.11,12 Therefore, the South West Oncology Group (SWOG) initiated a prospective multicenter clinical trial to evaluate the clinical efficacy of DEX/alpha interferon in patients with AL amyloidosis. In this paper, we describe the results of this first large prospective US national cooperative group effort in patients with AL amyloidosis.
Patients, materials, and methods
Study design and eligibility criteria
Study design consisted of sequential registration to induction followed by maintenance therapy. Patients with histologic diagnosis of AL amyloidosis and evidence of tissue involvement other than carpal tunnel syndrome were registered in this trial. Other eligibility criteria included SWOG performance status (PS) score of 0 to 3 and no prior therapy with DEX or alpha interferon. Patients with PS score of 4 were eligible if solely due to peripheral or autonomic neuropathy. Exclusion criteria were overt multiple myeloma, uncontrolled diabetes, active peptic ulcer disease, New York Heart Association class IV congestive heart failure (CHF), prior malignancy, and pregnant or nursing women. All diagnostic material was subjected to central pathology review. All patients signed informed consent, in accordance with the Declaration of Helsinki, approved by the local institutional review board prior to study entry.
Laboratory and clinical monitoring
Baseline evaluation prior to each registration included a complete physical examination, serum and urine immunofixation electrophoresis, serum β2 microglobulin level, assessment of liver and renal function, electrocardiogram, echocardiography, bone marrow aspiration and biopsy, abdominal fat aspirate, and skeletal survey. Other studies to assess organ involvement depended on clinical presentation. These included computed tomography (CT) or ultrasound of the abdomen in patients with liver involvement, electromyography (EMG) and nerve conduction studies in patients with symptomatic neuropathy, and 24-hour fecal fat and serum carotene levels in patients with gastrointestinal involvement. Follow-up studies for organ assessment were repeated every 4 months for the first year and yearly thereafter. Follow-up EMG was only repeated at yearly intervals.
Treatment schema
Induction therapy consisted of pulse DEX, 40 mg/d orally as a single dose on days 1 to 4, 9 to 12, and 17 to 20, every 35 days for 3 cycles. The protocol required salt restriction and peptic ulcer and antimicrobial prophylaxis throughout the duration of dexamethasone therapy. Although the protocol included guidelines (eg, trimethoprim-sulfamethoxazole; and H2 blockers/proton pump inhibitors), investigator preference for specific agents was allowed. In patients with CHF or nephrotic syndrome with edema, a small dose of loop diuretic was recommended to help offset fluid retention with DEX. Patients with known or suspected cardiac involvement were recommended to have a baseline 24-hour Holter monitor and formal cardiology evaluation. Consideration of antiarrhythmic therapy (eg, amiodarone) or placement of an automatic implantable cardioverter/defibrillator was recommended for patients with symptomatic or significant ventricular arrhythmias. These patients were also recommended for inpatient monitoring at the initiation of therapy to help minimize the potential for sudden cardiac death. For patients older than 70 years, the starting dose of DEX for the first cycle was 20 mg. Escalation of dose in cycle 2 was allowed based on tolerance. Patients achieving less than 50% reduction in monoclonal protein after the first 3 cycles received 3 additional cycles of therapy concurrent with the registration to the maintenance phase, if therapy was well tolerated with toxicity level lower than grade 3. Patients not evaluable for hematologic response received only 3 cycles of induction therapy. After the completion of induction therapy, all patients who were potentially eligible for stem cell transplantation were allowed the option of stem cell collection, at the discretion of the treating physician.
Maintenance phase was begun approximately 5 weeks from day 1 of cycle 3 of induction therapy. Maintenance therapy consisted of oral DEX at 40 mg/d for 4 days every 4 weeks, together with alpha interferon at 5 million units subcutaneously 3 times per week for the first 2 years, followed by alpha interferon alone for the next 3 years. In patients unable to tolerate interferon, DEX alone was used in the maintenance phase at 40 mg/d for 4 days every 4 weeks for 2 years, followed by 20 mg/d for 4 days every 2 months for years 3 to 5. In the event of grade 3 or persistent (> 1 month) grade 2 toxicity during any phase of therapy, the offending agent was temporarily withheld until resolution of toxicity to grade 1 or better and then resumed at 50% of the original dose.
Response assessment
Response to therapy and disease progression was assessed separately for both hematologic response and improvement in organ dysfunction, based on SWOG and Mayo Clinic criteria, respectively.13 Briefly, cardiac response required both improvement in cardiac symptoms as well as echocardiographic improvement, manifest as reduction in ventricular wall thickness by greater than 2 mm. Improvement in neuropathy required both clinical as well as EMG evidence of improvement, without concurrent use of palliative medications. A renal response occurred if there was greater than 50% reduction in 24-hour urine proteinuria in the absence of greater than 50% reduction in creatinine clearance. Hepatic response required greater than 50% reduction in elevated alkaline phosphatase level along with reduction in hepatomegaly. Improvement in performance status alone was not considered adequate for organ response, as it may also be due to improved supportive care.
Statistical analysis
Overall survival (OS) was calculated as time from registration to the date of last contact or date of death. Disease progression was assessed as previously described.5 Progression-free survival (PFS) was calculated as time from registration to the date of progression, death, or last contact. Survival curves were estimated by using the product limit method14 and compared using the log-rank test.15 Proportions were compared using the chi-square test. Univariate and multivariate analyses were done using the Cox regression model for survival16 and logistic regression for binary endpoints.17 Independently significant variables in the multivariate analyses were selected using backward selection at the .05 level with results confirmed using forward selection at the .05 level.
Potential prognostic factors were examined primarily as dichotomous groups, as the goal was to use easily interpreted groupings. Recursive partitioning methods were used to calculate the best cutpoint (based on the log-rank statistic) for continuous variables.18 Recursive partitioning methods are based on the raw data and compute cutpoints that provide the greatest separation in the outcome of interest, in this case OS. Groups defined by the optimal cutpoint were restricted to contain a minimum of 20% of the evaluable patients. Permutation-adjusted log-rank tests were performed to estimate the significance on overall survival of recursively selected cutpoints.19
Results
Accrual
Between November 1996 and March 2003, 93 patients were registered to this clinical trial. Five patients were excluded due to failure to meet eligibility requirements. One patient withdrew informed consent before receiving any protocol treatment. This analysis is restricted to the remaining 87 eligible and analyzable patients. Feasibility of national cooperative group studies in AL amyloidosis in the United States was a major objective in this trial. A total of 25 SWOG institutions and 9 Cancer and Leukemia Group B (CALGB) institutions participated in this trial, with 32 institutions contributing fewer than 5 patients, which constituted 59% of the total accrual. Therefore, these data illustrate the feasibility of a national cooperative group effort for accruing patients with this relatively uncommon disorder.
Clinical characteristics
Clinical characteristics of this cohort are shown in Table 1. Median age of all patients was 67.1 years. Most patients were previously untreated, although 14 (16%) patients had previously received oral MP. Most patients (71%) had multiorgan involvement, with at least 3-organ involvement in 36%. The most commonly involved organs were kidney (74%) and heart (50%). Of 81 patients with information regarding the date of initial pathologic diagnosis, 62 (77%) patients were diagnosed within 90 days prior to enrollment.
Patient characteristics
Factor . | % . |
|---|---|
| At least 65 years old | 53 |
| Male | 74 |
| Performance status score | |
| 0 | 30 |
| 1 | 44 |
| 2 | 18 |
| 3 | 7 |
| 4 | 1 |
| Organ involvement | |
| Cardiac | 50 |
| Renal | 74 |
| Neurologic | 36 |
| Gastrointestinal | 13 |
| Hepatic | 19 |
| Number of organs/systems involved | |
| 1 | 29 |
| 2 | 35 |
| More than 2 | 36 |
| Cardiac involvement | |
| Abnormal echocardiogram | 50 |
| Congestive heart failure | 27 |
| Laboratory features | |
| Serum creatinine level of at least 2.0 mg/dL | 28 |
| Serum B2M level of at least 3.5 mg/L | 43 |
| Serum albumin level less than 3.5 g/dL | 59 |
| Serum alkaline phosphatase level greater than ULN | 31 |
| Urine M protein level of at least 1 g/d | 39 |
Factor . | % . |
|---|---|
| At least 65 years old | 53 |
| Male | 74 |
| Performance status score | |
| 0 | 30 |
| 1 | 44 |
| 2 | 18 |
| 3 | 7 |
| 4 | 1 |
| Organ involvement | |
| Cardiac | 50 |
| Renal | 74 |
| Neurologic | 36 |
| Gastrointestinal | 13 |
| Hepatic | 19 |
| Number of organs/systems involved | |
| 1 | 29 |
| 2 | 35 |
| More than 2 | 36 |
| Cardiac involvement | |
| Abnormal echocardiogram | 50 |
| Congestive heart failure | 27 |
| Laboratory features | |
| Serum creatinine level of at least 2.0 mg/dL | 28 |
| Serum B2M level of at least 3.5 mg/L | 43 |
| Serum albumin level less than 3.5 g/dL | 59 |
| Serum alkaline phosphatase level greater than ULN | 31 |
| Urine M protein level of at least 1 g/d | 39 |
Total number of patients in the study population = 87.
ULN indicates upper limit normal.
Response to therapy
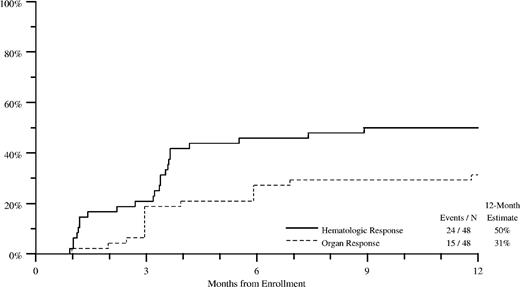
Improvement in AL amyloidosis–related organ dysfunction (termed “organ response”) was a major end point in this trial and was assessed separately for each involved organ, as in other amyloid trials. Organ response in at least one involved organ was seen in 33 (45%) of 73 patients evaluable for organ response (Table 2). Frequency of the organ response depended on the nature of the involved organ/system and was the highest for patients with renal involvement (39%) and lowest for patients with neuropathy (3%). Hematologic response to therapy (≥ partial response) occurred in 53% of 55 patients evaluable for hematologic response, with complete remissions (CRs) in 24%. Improvement in organ function correlated with hematologic response to therapy (data not shown). Figure 1 and Table 3 show the cumulative incidence of hematologic and organ response over the first year of therapy in the cohort assessable for both responses.
Hematologic and organ response to therapy
Factor . | n/N . | % . |
|---|---|---|
| Hematologic response | 29/55 | 53 |
| CR | 13/55 | 24 |
| Organ response | ||
| Any | 33/73 | 45 |
| Cardiac | 5/43 | 12 |
| Soft tissue | 3/12 | 25 |
| Hepatic | 2/16 | 13 |
| Renal | 25/64 | 39 |
| GI | 2/11 | 18 |
| Neurologic | 1/31 | 3 |
Factor . | n/N . | % . |
|---|---|---|
| Hematologic response | 29/55 | 53 |
| CR | 13/55 | 24 |
| Organ response | ||
| Any | 33/73 | 45 |
| Cardiac | 5/43 | 12 |
| Soft tissue | 3/12 | 25 |
| Hepatic | 2/16 | 13 |
| Renal | 25/64 | 39 |
| GI | 2/11 | 18 |
| Neurologic | 1/31 | 3 |
n indicates number with factor; and N, the number evaluated.
Cumulative incidence of hematologic and organ response through 12 months of therapy. Only patients with both assessable organ responses (– – –) and hematologic responses (—) are included. Nonresponders through 12 months of therapy are censored at 12 months.
Cumulative incidence of hematologic and organ response through 12 months of therapy. Only patients with both assessable organ responses (– – –) and hematologic responses (—) are included. Nonresponders through 12 months of therapy are censored at 12 months.
Cumulative incidence of hematologic and organ response through 12 months of therapy
. | Events/N . | 12-month estimate, % . |
|---|---|---|
| Hematologic response | 24/48 | 50 |
| Organ response | 15/48 | 31 |
. | Events/N . | 12-month estimate, % . |
|---|---|---|
| Hematologic response | 24/48 | 50 |
| Organ response | 15/48 | 31 |
N indicates the number evaluated.
The median time to hematologic PR or better was 103 days, and the median time to CR was 173 days. Median time to organ response was 120 days. A proportion of patients were taken off study by the treating physician to pursue other therapies such as high-dose melphalan/stem cell transplantation (n = 4), thalidomide (n = 3), or vaccines (n = 1). Data for response to these therapies are not available. Cox regression analysis was used to test for factors predictive of response. No factors predicting response were identified, although age of 65 years or older approached significance as an adverse factor (hazard ratio, 0.5; 95% CI, 0.2-1; P = .07).
Survival
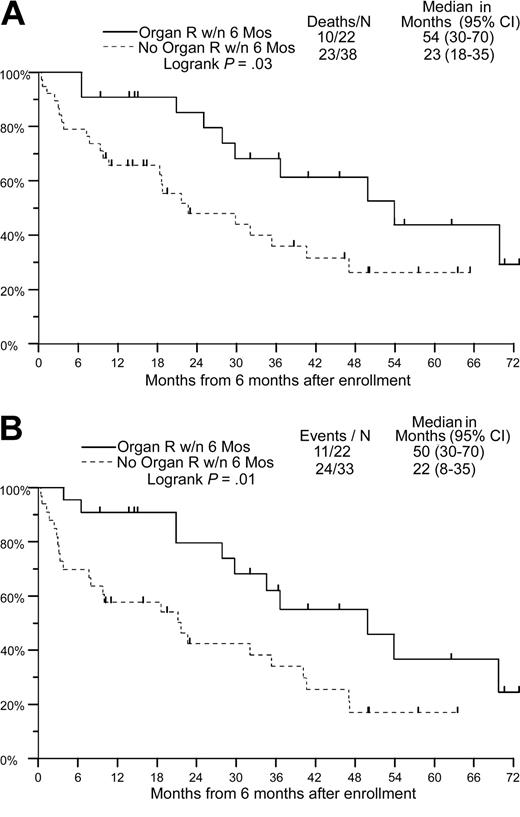
With a median follow-up of 41 months, 34 patients are alive with a median OS of 31 months (Figure 2A; Table 4). The estimated 2- and 5-year OS in the entire cohort is 60% and 28%, respectively. The median time to disease progression in the entire cohort is 27 months, with 2- and 5-year PFS of 52% and 18%, respectively (Figure 2B; Table 4). Using a 6-month landmark, median OS and PFS in patients with hematologic response were similar to that in nonresponders (median OS, 30 versus 25 months, P = .86; median PFS, 30 versus 21 months, P = .47). OS and PFS in patients with organ response using a 6-month landmark was higher in responders than in nonresponders (OS, 54 versus 23 months, P = .03; PFS, 50 versus 22 months, P = .01; Figure 3A-B; Table 5).
Overall and progression-free survival of the entire cohort. (A) Overall survival. (B) Progression-free survival.
Overall and progression-free survival of the entire cohort. (A) Overall survival. (B) Progression-free survival.
Overall and progression-free survival of the entire cohort
. | Deaths/N . | Median, mo (95% CI) . |
|---|---|---|
| Overall survival | 53/87 | 31 (16-41) |
| Progression-free survival | 62/87 | 27 (11-31) |
. | Deaths/N . | Median, mo (95% CI) . |
|---|---|---|
| Overall survival | 53/87 | 31 (16-41) |
| Progression-free survival | 62/87 | 27 (11-31) |
N indicates the number evaluated.
Overall and progression-free survival based on organ response using a 6-month landmark. Only patients with assessable organ response and time to organ response are included. (A) Overall survival. (B) Progression-free survival. — indicates organ response within 6 months; – – –, no organ response within 6 months.
Overall and progression-free survival based on organ response using a 6-month landmark. Only patients with assessable organ response and time to organ response are included. (A) Overall survival. (B) Progression-free survival. — indicates organ response within 6 months; – – –, no organ response within 6 months.
Overall and progression-free survival based on organ response using a 6-month landmark
. | Deaths/N . | Median, mo (95% CI) . | P* . |
|---|---|---|---|
| Overall survival | .03 | ||
| Organ response within 6 mos | 10/22 | 54 (30-70) | |
| No organ response within 6 mos | 23/38 | 23 (18-35) | |
| Progression-free survival | .01 | ||
| Organ response within 6 mos | 11/22 | 50 (30-70) | |
| No organ response within 6 mos | 24/33 | 22 (8-35) |
. | Deaths/N . | Median, mo (95% CI) . | P* . |
|---|---|---|---|
| Overall survival | .03 | ||
| Organ response within 6 mos | 10/22 | 54 (30-70) | |
| No organ response within 6 mos | 23/38 | 23 (18-35) | |
| Progression-free survival | .01 | ||
| Organ response within 6 mos | 11/22 | 50 (30-70) | |
| No organ response within 6 mos | 24/33 | 22 (8-35) |
N indicates the number evaluated.
P determined by log-rank test.
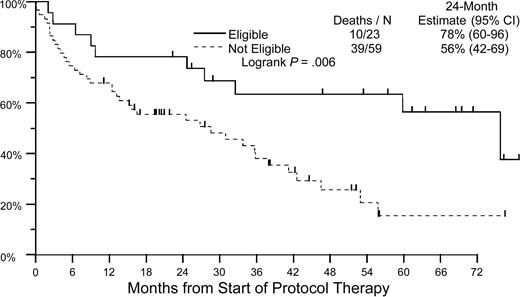
The majority of patients enrolled in this trial had advanced amyloidosis. Only 23 of these patients met the criteria generally used for eligibility for high-dose chemotherapy and stem cell transplantation in this disease (age < 70 years, without CHF, < 3-organ involvement, and serum creatinine level of < 176.8 μM [2 mg/dL]). Estimated 2-year OS in this cohort was 78% compared with 56% in patients known to be transplantation ineligible (P = .006; Figure 4; Table 6).
Overall survival by eligibility for transplantation. Patients who are eligible to receive transplants must fulfill the following criteria: younger than 70 years, without CHF, fewer than 3-organ involvement, and serum creatinine level of less than 176.8 μM (2 mg/dL). — indicates eligible; – – –, not eligible.
Overall survival by eligibility for transplantation. Patients who are eligible to receive transplants must fulfill the following criteria: younger than 70 years, without CHF, fewer than 3-organ involvement, and serum creatinine level of less than 176.8 μM (2 mg/dL). — indicates eligible; – – –, not eligible.
Overall survival by eligibility for transplantation
. | Deaths/N . | 24-month estimate, % (95% CI) . |
|---|---|---|
| Eligible | 10/23 | 78 (60-96) |
| Not eligible | 39/59 | 56 (42-69) |
. | Deaths/N . | 24-month estimate, % (95% CI) . |
|---|---|---|
| Eligible | 10/23 | 78 (60-96) |
| Not eligible | 39/59 | 56 (42-69) |
Log-rank P = .006.
N indicates the number evaluated.
Toxicities
Pulse DEX administered at full dose (40 mg/d) has an acceptable toxicity profile in patients with myeloma; however, the tolerance to this regimen in patients with AL amyloidosis seems to be lower. Major treatment-related grade 3 or greater toxicities during the induction or maintenance phase are noted in Table 7. Treatment-related mortality was observed in 6 (7%) patients. This included 4 patients with advanced cardiac amyloidosis who experienced sudden death, likely due to a cardiac event. Overall, the incidence of grade 3 or greater toxicities during the induction or maintenance phase was 51% and 67%, respectively. However, these toxicities generally occurred early during the initiation of therapy and responded well to dose reduction in most instances. Fifty-eight (67%) patients received at least 3 cycles of induction therapy. The most common toxicity during induction therapy was cardiovascular/fluid overload related to dexamethasone, which occurred in 28% of patients. The most common toxicity during the maintenance phase was neurologic in 24% of patients, which included neuropsychiatric manifestations and depression possibly related to alpha interferon. Four patients could not tolerate interferon and were maintained on dexamethasone alone. Median duration of follow-up on maintenance therapy was 214 days, and 11 patients had a follow up of longer than 2 years on maintenance therapy. In univariate analysis, patient characteristics significantly associated with higher risk of grade 4 or greater toxicity were the presence of more than 2 organs/systems involved and gastrointestinal (GI) involvement, whereas the presence of CHF and serum β2 microglobulin (B2M) level of at least 3.5 mg/L approached significance (data not shown). On multivariate analysis, only the presence of CHF (odds ratio, 5.8; P = .01) remained as a significant variable, whereas serum B2M level of at least 3.5 mg/L (odds ratio, 3.8; P = .08) approached significance.
Major treatment-related toxicities of at least grade 3 by NCI Common Toxicity Criteria
. | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|
| Induction, n = 86 | |||
| Adverse event | |||
| Cardiovascular/fluid overload | 14 | 6 | 4 |
| Hematologic | 9 | 1 | 0 |
| Gastrointestinal | 7 | 2 | 0 |
| Renal | 5 | 2 | 0 |
| Infection | 5 | 1 | 0 |
| Total no. of patients | 29 | 11 | 4 |
| % | 34 | 12 | 5 |
| Maintenance, n = 49 | |||
| Adverse Event | |||
| Neurologic | 12 | 0 | 0 |
| Renal | 9 | 1 | 0 |
| Hematologic | 6 | 2 | 0 |
| Infection | 7 | 0 | 1 |
| Cardiovascular | 6 | 1 | 1 |
| Gastrointestinal | 5 | 2 | 0 |
| Flulike symptoms | 6 | 1 | 0 |
| Total no. of patients | 25 | 6 | 2 |
| % | 51 | 12 | 4 |
. | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|
| Induction, n = 86 | |||
| Adverse event | |||
| Cardiovascular/fluid overload | 14 | 6 | 4 |
| Hematologic | 9 | 1 | 0 |
| Gastrointestinal | 7 | 2 | 0 |
| Renal | 5 | 2 | 0 |
| Infection | 5 | 1 | 0 |
| Total no. of patients | 29 | 11 | 4 |
| % | 34 | 12 | 5 |
| Maintenance, n = 49 | |||
| Adverse Event | |||
| Neurologic | 12 | 0 | 0 |
| Renal | 9 | 1 | 0 |
| Hematologic | 6 | 2 | 0 |
| Infection | 7 | 0 | 1 |
| Cardiovascular | 6 | 1 | 1 |
| Gastrointestinal | 5 | 2 | 0 |
| Flulike symptoms | 6 | 1 | 0 |
| Total no. of patients | 25 | 6 | 2 |
| % | 51 | 12 | 4 |
Prognostic factors for survival
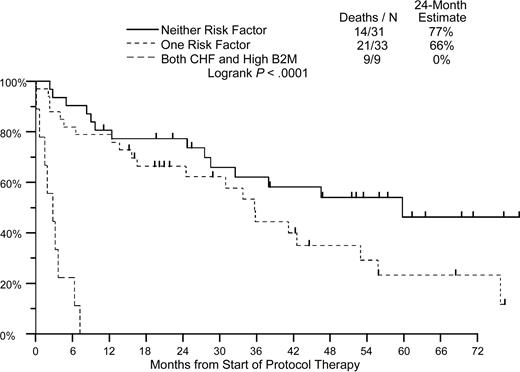
Results of univariate analysis for predictors of OS and PFS in this study are shown in Table 8. Significant univariate prognostic factors for adverse outcome at the .05 level were 65 years of age or older, performance status score of more than 1, more than 2 organs/systems involved, CHF, and neurologic involvement, whereas serum B2M and urine M levels approached significance. In the multivariate model, only the presence of congestive heart failure and serum levels of B2M were significant independent predictors of both OS and PFS (Table 9). Using these 2 variables, we developed a risk model for predicting outcome in this disease, based on the presence of none, one, or both of these risk features. Two-year overall survival in these cohorts was 77%, 66%, and 0% months, respectively (Figure 5; Table 10; P < .001).
Analysis of prognostic factors for overall and progression-free survival univariate model
. | . | Overall survival . | . | Progression-free survival . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | n/N(%) . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| At least 65 y of age | 46/87 (53) | 1.94 (1.11-3.40) | .020 | 1.73 (1.04-2.90) | .036 | ||
| Male | 64/87 (74) | 0.96 (0.52-1.81) | .911 | 1.09 (0.60-1.98) | .780 | ||
| PS greater than I | 22/83 (27) | 2.86 (1.57-5.23) | .001 | 1.97 (1.11-3.51) | .021 | ||
| Laboratory features | |||||||
| Creatinine level of at least 2.0 mg/dL | 23/83 (28) | 1.24 (0.67-2.28) | .292* | 1.10 (0.62-1.93) | .753 | ||
| Serum albumin level less than 3.5 g/dL | 48/82 (59) | 0.80 (0.45-1.41) | .84* | 0.59 (0.35-0.99) | .046 | ||
| Serum B2M level of at least 3.5 mg/L | 32/74 (43) | 2.05 (1.13-3.73) | .074* | 1.51 (0.88-2.61) | .134 | ||
| Alkaline phosphatase level greater than ULN | 25/80 (31) | 1.44 (0.80-2.60) | .220 | 1.37 (0.79-2.37) | .263 | ||
| Urine M level of at least 0.5 g/d | 20/46 (43) | 2.03 (0.94-4.38) | .073* | 1.41 (0.69-2.85) | .343 | ||
| Serum M level of at least 0.8 g/dL | 22/57 (39) | 0.69 (0.33-1.48) | .301* | 0.70 (0.35-1.41) | .323 | ||
| At least 3 organ systems involved | 31/86 (36) | 2.25 (1.28-3.96) | .005 | 1.70 (1.00-2.88) | .050 | ||
| Organ involvement | |||||||
| Cardiac | 43/86 (50) | 1.58 (0.91-2.73) | .101 | 1.34 (0.81-2.21) | .255 | ||
| Soft tissue | 12/86 (14) | 1.10 (0.51-2.33) | .812 | 1.51 (0.77-2.99) | .232 | ||
| Hepatic | 16/86 (19) | 1.25 (0.66-2.39) | .494 | 1.04 (0.56-1.92) | .909 | ||
| Renal | 64/86 (74) | 0.81 (0.45-1.46) | .484 | 0.66 (0.38-1.13) | .130 | ||
| Gastrointestinal | 11/87 (13) | 1.99 (0.89-4.48) | .095 | 1.41 (0.64-3.13) | .398 | ||
| Neurologic | 31/86 (36) | 2.63 (1.48-4.67) | .001 | 2.01 (1.18-3.42) | .010 | ||
| Cardiac involvement | |||||||
| Abnormal echocardiogram | 43/86 (50) | 1.58 (0.91-2.73) | .101 | 1.34 (0.81-2.21) | .255 | ||
| Echocardiogram LV thickness at least 16 mm | 18/43 (42) | 1.10 (0.52-2.34) | .800 | 1.00 (0.50-1.99) | .993 | ||
| CHF | 23/86 (27) | 3.01 (1.70-5.33) | < .001 | 3.03 (1.77-5.20) | < .001 | ||
. | . | Overall survival . | . | Progression-free survival . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | n/N(%) . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| At least 65 y of age | 46/87 (53) | 1.94 (1.11-3.40) | .020 | 1.73 (1.04-2.90) | .036 | ||
| Male | 64/87 (74) | 0.96 (0.52-1.81) | .911 | 1.09 (0.60-1.98) | .780 | ||
| PS greater than I | 22/83 (27) | 2.86 (1.57-5.23) | .001 | 1.97 (1.11-3.51) | .021 | ||
| Laboratory features | |||||||
| Creatinine level of at least 2.0 mg/dL | 23/83 (28) | 1.24 (0.67-2.28) | .292* | 1.10 (0.62-1.93) | .753 | ||
| Serum albumin level less than 3.5 g/dL | 48/82 (59) | 0.80 (0.45-1.41) | .84* | 0.59 (0.35-0.99) | .046 | ||
| Serum B2M level of at least 3.5 mg/L | 32/74 (43) | 2.05 (1.13-3.73) | .074* | 1.51 (0.88-2.61) | .134 | ||
| Alkaline phosphatase level greater than ULN | 25/80 (31) | 1.44 (0.80-2.60) | .220 | 1.37 (0.79-2.37) | .263 | ||
| Urine M level of at least 0.5 g/d | 20/46 (43) | 2.03 (0.94-4.38) | .073* | 1.41 (0.69-2.85) | .343 | ||
| Serum M level of at least 0.8 g/dL | 22/57 (39) | 0.69 (0.33-1.48) | .301* | 0.70 (0.35-1.41) | .323 | ||
| At least 3 organ systems involved | 31/86 (36) | 2.25 (1.28-3.96) | .005 | 1.70 (1.00-2.88) | .050 | ||
| Organ involvement | |||||||
| Cardiac | 43/86 (50) | 1.58 (0.91-2.73) | .101 | 1.34 (0.81-2.21) | .255 | ||
| Soft tissue | 12/86 (14) | 1.10 (0.51-2.33) | .812 | 1.51 (0.77-2.99) | .232 | ||
| Hepatic | 16/86 (19) | 1.25 (0.66-2.39) | .494 | 1.04 (0.56-1.92) | .909 | ||
| Renal | 64/86 (74) | 0.81 (0.45-1.46) | .484 | 0.66 (0.38-1.13) | .130 | ||
| Gastrointestinal | 11/87 (13) | 1.99 (0.89-4.48) | .095 | 1.41 (0.64-3.13) | .398 | ||
| Neurologic | 31/86 (36) | 2.63 (1.48-4.67) | .001 | 2.01 (1.18-3.42) | .010 | ||
| Cardiac involvement | |||||||
| Abnormal echocardiogram | 43/86 (50) | 1.58 (0.91-2.73) | .101 | 1.34 (0.81-2.21) | .255 | ||
| Echocardiogram LV thickness at least 16 mm | 18/43 (42) | 1.10 (0.52-2.34) | .800 | 1.00 (0.50-1.99) | .993 | ||
| CHF | 23/86 (27) | 3.01 (1.70-5.33) | < .001 | 3.03 (1.77-5.20) | < .001 | ||
n indicates number with factor; N, the number evaluated; CI, confidence interval; B2M, β2 microglobulin; ULN, upper limit normal; and LV, left ventricular.
Significance levels from a permutation-adjusted log-rank test, otherwise from a Wald test using Cox regression.
Prognostic factors for overall and progression-free survival: multivariate model
. | . | Overall survival . | . | Progression-free survival . | . | ||
|---|---|---|---|---|---|---|---|
| Factors . | n/N(%) . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| CHF | 19/73 (26) | 4.42 (2.23-8.74) | < .001 | 3.71 (1.98-6.95) | < .001 | ||
| Serum B2M level of at least 3.5 mg/L | 32/73 (44) | 2.80 (1.47-5.35) | .002 | 1.92 (1.08-3.42) | .026 | ||
. | . | Overall survival . | . | Progression-free survival . | . | ||
|---|---|---|---|---|---|---|---|
| Factors . | n/N(%) . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| CHF | 19/73 (26) | 4.42 (2.23-8.74) | < .001 | 3.71 (1.98-6.95) | < .001 | ||
| Serum B2M level of at least 3.5 mg/L | 32/73 (44) | 2.80 (1.47-5.35) | .002 | 1.92 (1.08-3.42) | .026 | ||
The estimates of relative risk reported are simultaneously adjusted for B2M level of at least 3.5 mg/L and CHF.
Prognostic factors considered but not significant after adjusting for CHF and B2M level of at least 3.5 mg/L are age of 65 years or older, sex, performance status greater than 1, serum creatinine level at least 176.8 μM (2.0 mg/dL), serum albumin level less than 35 g/L (3.5 g/dL), serum alkaline phosphatase level greater than upper limit normal, and number of organ systems involved (> 2).
Disease risk model based on the presence of congestive heart failure and serum β2 microglobulin level. High B2M is defined as serum β2 microglobulin level of at least 3.5 mg/L. — indicates neither risk factor; – – –, 1 risk factor; – –, both CHF and high B2M level.
Disease risk model based on the presence of congestive heart failure and serum β2 microglobulin level. High B2M is defined as serum β2 microglobulin level of at least 3.5 mg/L. — indicates neither risk factor; – – –, 1 risk factor; – –, both CHF and high B2M level.
Disease risk model based on the presence of congestive heart failure and serum β2 microglobulin level
. | Deaths/N . | 24-month estimate, % . |
|---|---|---|
| Neither risk factor | 14/31 | 77 |
| One risk factor | 21/33 | 66 |
| Both CHF and high B2M level | 9/9 | 0 |
. | Deaths/N . | 24-month estimate, % . |
|---|---|---|
| Neither risk factor | 14/31 | 77 |
| One risk factor | 21/33 | 66 |
| Both CHF and high B2M level | 9/9 | 0 |
Log-rank P < .0001.
N indicates the number evaluated.
Discussion
AL amyloidosis is a rapidly fatal disease with only modest impact of current therapies.1 Most of the current data regarding therapy of these patients is based on single-institution studies from major amyloid treatment centers. However, these studies also suggest an important impact of patient selection on outcome.11 Therefore referral bias depending on the expertise of the treatment center may have a major impact on the existing data. This trial represents the first large US multicenter national cooperative group effort to treat patients with AL amyloidosis. Feasibility of such collaborative efforts, as demonstrated here, should greatly enhance clinical investigation in this disease.
These data demonstrate that DEX-based regimens, as used in this trial, can lead to durable improvements in AL amyloidosis–related organ dysfunction. Prior data for clinical activity of DEX in AL amyloidosis was based on small studies and lacked information about the durability of responses.6-8 In these studies, the overall response to DEX was variable and the impact on survival difficult to assess. Differences in response rates in these studies may be due to small sample sizes; differences in patient populations or treatment regimens, including lack of maintenance phase; or alpha interferon. Alpha interferon as a single agent does not seem to have significant activity in this disease20 but the potential for synergy with DEX cannot be excluded. Recent studies have also explored the combination of DEX with vincristine/adriamycin (as in the VAD regimen)9 and with melphalan.10 However, it remains unclear whether the addition of these agents to DEX will lead to improved outcome compared with DEX alone. Nonetheless, cumulative results from these studies (including the present study) support the consideration of DEX or DEX-based regimens as one of the options for front-line therapy in AL amyloidosis. In view of the activity of combinations of DEX with thalidomide or proteasome inhibitors in myeloma, formal evaluation of these regimens in AL amyloidosis is warranted.
In this study, different organs/systems had a differing propensity to respond to this regimen. For example, organ response rates were highest among patients with renal involvement, whereas only 1 of the 31 patients with neuropathies had an objective response. Higher rates of response in neuropathies have been reported after high-dose melphalan,21 although response criteria in some of these studies did not include objective evidence of improvement on serial EMG testing, as required here. Nonetheless, pattern of organ involvement may be worthy of consideration when choosing initial therapy in patients with AL amyloidosis. Improvement in organ function, however, does not necessarily mean regression of amyloid deposits. This issue was not addressed in this trial, as follow-up biopsies of the involved organ were not performed. Improvement in organ function correlated with improved survival. Surprisingly, similar association could not be demonstrated for hematologic response, suggesting that organ improvement is a stronger predictor of survival. Hematologic response to therapy did however correlate with organ responses. Measurement of serum-free light chains was not commercially available when this study was initiated and might have improved the assessment of hematologic responses.
Both DEX and alpha interferon have an acceptable toxicity profile in patients with myeloma, but the tolerance to both these agents in patients with AL amyloidosis seems to be lower. In this study, nearly half of the patients required dose reduction early in the induction phase due to dose-limiting toxicities. This occurred in spite of using a lower starting dose (20 mg) in older patients. Intolerance to maintenance DEX-alpha interferon was also higher than would be expected and again required dose reduction, particularly for interferon. Therefore, based on these data, we recommend that the use of DEX in AL amyloidosis should be individualized, starting with a lower dose such as 20 mg. Patients with CHF appear to be at higher risk of toxicity without survival benefit and therefore should not be treated with such regimens.
Another approach with promising results in selected patients is the use of high-dose or risk-adapted melphalan with autologous stem cell transplantation (ASCT).21,22 However, the degree to which the current results with ASCT in AL amyloidosis are influenced by patient selection factors remains unclear. In this trial, the survival of the cohort eligible for ASCT is comparable to the data from single-institution ASCT trials.21 These data therefore underscore the need to compare this approach in a randomized trial to DEX-based therapies to avoid repeating the lessons learnt with ASCT in breast cancer.23 Perhaps more importantly, only a minority of patients in this trial would have met the eligibility of published transplantation trials. Therefore, there is a great need to identify newer approaches to benefit this population.
Presence of CHF and high serum B2M levels were the dominant prognostic indicators in this study. Prognostic impact of symptomatic cardiac involvement in AL is well established. Although not tested in this trial, it is likely that more objective measures of cardiac involvement, such as troponin levels or levels of brain natriuretic peptide, along with serum B2M levels, will help identify risk groups in this disease.24 Adverse impact of high serum B2M levels in AL amyloidosis was also noted earlier by the Mayo Clinic group (Gertz et al25 ). Level of serum B2M is also a dominant prognostic factor in myeloma, where it is generally considered to be indicative of high clonal tumor burden. Therefore, we find its prognostic impact in AL amyloidosis intriguing because these patients characteristically have much lower clonal burden relative to myeloma. Patients with both these risk features did very poorly in this trial and should not be treated with DEX. Unfortunately, these patients are also likely to do poorly with other approaches and may be candidates for trials testing novel agents.
In conclusion, these data provide evidence for clinical activity of a novel approach to treat patients with AL amyloidosis and consideration of DEX-based regimens as an option for front-line therapy in AL amyloidosis, particularly when more rapid response is desirable. This therapy can lead to durable improvement in AL amyloidosis–related organ dysfunction and prolonged survival. Many patients however do not tolerate full-dose DEX pulsing and it may be preferable to begin therapy at reduced doses (eg, 20 mg/d). Patients should be carefully monitored, particularly at the initiation of therapy, and those with both CHF and high B2M levels are not good candidates for this therapy. Randomized controlled studies are needed to compare this approach with others, including MP or high-dose melphalan with stem cell transplantation. Improved understanding of the molecular mechanisms of amyloidogenesis and resulting organ dysfunction is needed to allow the development of novel targeted therapies for this difficult disease.1,2
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-05-1924.
A complete list of the members of the Southwest Oncology Group who participated in this study appears in the “Appendix.”
Supported in part by the following Public Health Service (PHS) Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services (DHHS): CA38926, CA32102, CA04919, CA37981, CA67575, CA76429, CA58416, CA28862, CA35431, CA20319, CA42777, CA68183, CA35261, CA35178, CA74647, CA16385, CA13612, CA76447, CA45807, CA35176, CA58658, CA58415, CA35119, CA31946. M.V.D. is supported in part by funds from the National Institutes of Health, Damon Runyon Cancer Research Fund, and Irene Diamond Foundation.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Larissa Rios, Jeana Cromer, Mark Blitzer, Joth Jacobson, Bonnie Granados, and the operations and statistical offices of the Southwest Oncology Group for assistance with protocol management. Patients in this study were treated at the following SWOG- or CALGB-affiliated institutions: University of Arkansas, Cleveland Clinic, Montana CCOP, University of Cincinnati, Puget Sound, Scott and White Clinic, Wichita CCOP, Columbus CCOP, Grand Rapids CCOP, North Colorado Medical Center, St Luke's Roosevelt/Columbia University, University of Arizona, Breslin Cancer Center, Temple University, Central IL CCOP, Eisenhower Army Medical Center, Kansas City CCOP, Keesler USAF Medical Center, Meridia Hillcrest, University of Mississippi, LSU-Shreveport, St Joseph Mercy, Upstate Carolina, West Florida Medical Center, Duke University, University of California at San Diego, Memorial Mission Hospital, Martha Jefferson Hospital, Missouri Cancer Associates, University of Iowa, University of Vermont, and Wake Forest School of Medicine.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal