Abstract
A decline in stem cell function has been suggested to contribute to vertebrate aging. Several labs have documented a reduction in transplant efficiency and skewing in lineage contribution when murine bone marrow or hematopoietic stem cells (HSC) from old donors were transplanted into young recipients. Paradoxically, evidence from several labs including ours has shown that the percentage of phenotypically defined HSC in C57Bl/6 mice increases with age (Fig. 1). Within the serum of aged animals, systemic inflammatory markers such as C reactive protein in humans and IL-1 in mice have been shown to steadily increase with age, however it is unclear if systemic inflammation plays a role in these age-related HSC phenotypes. In order to investigate what might account for these functional changes in HSC aging, we have characterized gene expression in aged HSC using Affymetrix microarrays, examining expression profiles of HSC purified from C57Bl/6 mice that are 2-, 6-, 12-, 21-months old. Using polynomial regression over the time course, we have found more than 700 genes that are 2-fold up-regulated and more than 400 genes that are down-regulated with time. We used the Gene Ontology to categorize age-regulated genes, and have identified the category of ‘inflammatory response’ to be significantly enriched in genes that are up-regulated with age. Strikingly, several NF-Kb regulated genes, previously associated in other tissues with aging and inflammation (e.g. P-selectin, Cox-2, and ICAM-1) are up-regulated in aged HSC. The expression patterns of more than 15 genes, including clusterin, serum deprivation response, and growth hormone receptor, have been validated by quantitative real-time PCR. Furthermore, expression of several surface markers, including P-selectin, a protein that plays a role in inflammation, has been validated at the protein level by flow cytometry. In order to determine if the gene expression changes observed in aged HSC is caused by intrinsic or extrinsic factors, we have transplanted HSC from 21-month-old mice into young recipients and are assessing changes in their expression profile compared to young transplanted HSC. These data demonstrate a role for inflammation in HSC-aging, and may identify genes involved in stem cell transplant efficiency, lineage specification, and the onset of organismal aging.
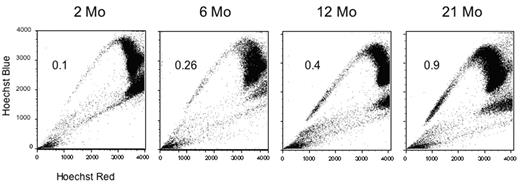
Sca-1 enriched Side Population cells at the indicated ages (in months). The percentage of cells residing within the SP increases 9-fold with age.
Sca-1 enriched Side Population cells at the indicated ages (in months). The percentage of cells residing within the SP increases 9-fold with age.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal