Abstract
Background The role of intensive chemotherapy in treatment of childhood MDS has not been clarified yet. We conducted a prospective trial which employed intensive AML-type chemotherapy for children with de novo MDS.
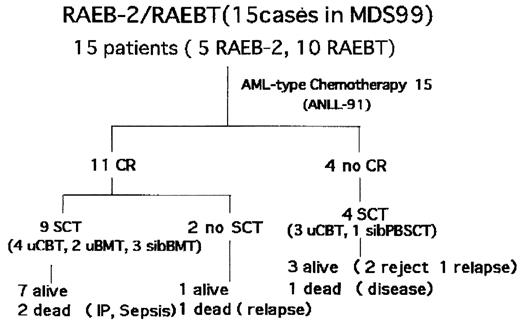
Methods Fifteen children aged 1 to 15 years who met diagnostic criteria of the FAB refractory anemia with excess of blasts (RAEB) with more than 10% of blasts in the bone marrow (BM) (RAEB-II) and RAEB in transformation (RAEBT) were prospectively enrolled in the study. Abnormal karyotype was documented in 11 patients including 3 with monosomy 7, 2 with complex karyotype, 1 with trisomy 8. Induction therapy consisted of etoposide (150mg/m2, on days 1–5), cytarabine (200mg/m2/12hr, on days 6–12), and mitoxantrone (5mg/m2, on days 6–10) in all but 1 patient, who received another regimen of AML induction therapy. The use of granulocyte-colony stimulating factor was allowed. Allogeneic stem cell transplantation (SCT) was scheduled after remission induction therapy. The median follow-up for 13 surviving patients was 685 days (58 to 1,476 days).
Findings Eleven patients (73%) including 3 with monosomy 7 achieved a complete remission after induction therapy at a median of 40 days (31 to 61 days). Remission was not obtained in the other 4 patients.
There was no significant difference in the frequency and severity of toxicity between in patients with RAEB-II/RAEBT and in those with de novo AML. Allogeneic SCT was performed in 13 patients including 9 in the remission at a median of 4 months (2 to 6 months) after the beginning of the therapy. None of nine patients who underwent SCT in the remission relapsed. Although two patients died of the complications of SCT. Overall survival for all the 15 patients at 2 years was 66.8 ± 14.1% (s.e.).
Interpretation AML-type induction therapy appears to be safe and effective for children with RAEB with more than 10% of blasts in the BM and RAEBT.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal