Abstract
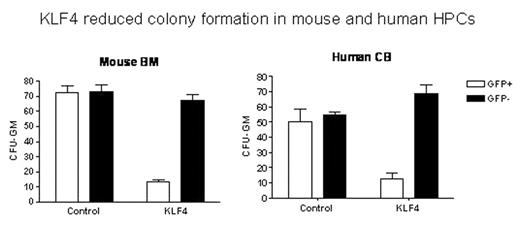
At any given time, only a very small fraction of the hematopoietic stem cells (HSCs) in an organism are actively dividing; the vast majority of these cells remain in a quiescent state. HSCs can escape this quiescent state to self-renew and give rise to progenitor cells (HPCs), which expand rapidly to form all of the mature progeny cells of the lympho-hematopoietic system. Despite the centrality of quiescence in hematopoiesis, the mechanisms by which quiescence is regulated are largely unknown. Several studies have shown that p21CIP/WAF1 is required for cell cycle arrest of HSCs, and deletion of p21 results in an increase in HSC pool size. However, little is known about the pathways upstream of p21. Recently, we identified 81 genes that were highly over-expressed by normal HSC-enriched CD34+/CD38− cells compared to HPC-enriched CD34+/CD38+ cells. One of the HSC-over-expressed genes was Krupple-Like Factor 4 (KLF4), a member of the Kruppel-like transcription factor family. KLF4 has been associated with a quiescent phenotype in T and B lymphocytes, and exogenous expression of KLF4 induces growth arrest in a colon cancer cell line. It has been shown that KLF4 is an important mediator of p21 in the G1/S cell cycle arrest following DNA damage. Thus, we hypothesized that KLF4 is an important upstream regulator of HSPC quiescence via p21. To begin to test this, we designed a dual promoter self-inactivating (SIN) lentiviral vector that expressed KLF4 under the control of the EF1α promoter and GFP under the CMV promoter. We transduced primary mouse bone marrow (BM) Lin− or human cord blood (CB) CD34+ HSPCs, and purified the resulting successfully transduced GFP+ cells by fluorescence-activated cell sorting. KLF4-transduced human or mouse HPCs displayed a major reduction in colony forming capacity, as compared to either GFP− HPCs transduced with the vector containing KLF4 and GFP or HPCs transduced with the parental control vector expressing only GFP (Fig 1 shows representative experiments). In suspension cultures, KLF4-transduced cell populations generated fewer total cells, accompanied by an increase in apoptic/necrotic cells. In the KLF4-transduced cells, a higher percent were CD34+. These results are consistent with KLF4 inhibiting HPC differentiation and inducing a p21-like pathway with cell cycle arrest and increased apoptosis. Further investigation of KLF4 cellular and molecular actions may not only lead to enhanced understanding of the molecular basis of HSPC quiescence, but also to novel methods for expansion of normal HSPCs.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal