Abstract
Acquired von Willebrand (AvWD) disease is a relatively rare bleeding disorder and since the initial description, fewer than 300 cases of AvWD have been reported. The most frequently observed association is with dysproteinemias such as monoclonal gammopathy of undetermined significance (MGUS) and plasma cell proliferative disorders such as Waldenstroem macroglobulinemia and multiple myeloma. Other hematological diseases associated with AvWD have been reported including lymphoproliferative and myeloproliferative disorders. We report the case of a 59 year old male patient with an AvWD associated with a chronic lymphocytic leukemia.
In November 2000 this patient was diagnosed with a B cell chronic lymphocytic leukemia (CLL). Because of an initial Binet-Stage A no specific therapy was initiated. In October 2003 the CLL progressed to Binet-Stage B and molecular examinations revealed an unfavorable risk profile with a 11q22.3-q23.1 deletion and an unmutated IgVH gene status. Because of the presence of an HLA-identical brother the patient was enrolled into a pilot study of allogeneic stem cell transplantation following conditioning with fludarabine and cyclophosphamid in patients with high-risk chronic lymphocytic leukemia. Four cycles of fludarabine and cyclophosphamide were given prior to allogeneic stem cell transplantation and induced a partial remission.
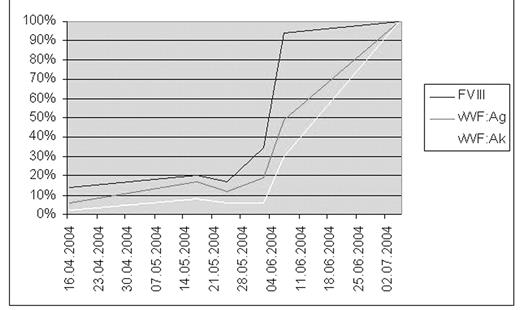
Since 2000 the patient reported a new clinical bleeding tendency, presenting mainly as frequent epistaxis. Further laboratory investigations showed a prolonged activated partial thromboplastin time (50 sec.) and a reduced plasma von Willebrand factor (vWF) antigen and vWF activity of 6% and 2%, respectively. This was accompanied by a reduced Factor VIII activity of 14% (factor IX-, XI-, XII- activity > 100%, respectively; lupus anticoagulant assay negative; immunofixation negativ). A vWF multimer analysis showed selective decrease in the high-molecular-weight multimers as seen in type II congenital vWD.
The reduced intensity conditioning (RIC) regimens consisted the following combination: Fludarabine 30mg/m² /d (=150mg/m² total dose) and Cyclophosphamide 500mg/m² /d (=2500mg/m² total dose) (day -7 through day -3), GVHD prophylaxis with Cyclosporin A (CsA) and mycophenolate mofetil (MMF).
A therapeutic trial of desmopressin (DDAVP) was insufficient and therefore a continuous infusion of the factor VIII/vWF concentrate (Haemate® P; Aventis Behring) was administered from the start of conditioning until platelet recovery. Under this regimen no bleeding complication was observed, neither spontaneously nor during intensive procedures. In accordance with previous reports of replacement therapy in AvWD we observed a shortened half-life of the vWF.
On day 30 after allogenic hematopoietic stem cell transplantation we observed for the first time a normalization of the activated partial thromboplastin time followed by a gradual increase of the vWF antigen and activity. On day 60 we were able to document the normalization of both the vWF antigen and activity and the vWF multimer analysis. This nomalisation was coincident with the establishment of a complete donor chimerism for the first time.
We therefore believe that this case shows a form of AvWD associated with chronic lymphocytic leukemia and its reversal by treatment of the underlying disease by allogeneic hematopoietic stem cell transplantation.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal