Abstract
A diverse population of T cells that express a broad array of T cell receptors (TCR) is required for effective immune surveillance. In some clinical settings, such as cancer, HIV, autoimmunity, transplantation and aging, the diversity of the TCR repertoire may become restricted or skewed. This repertoire skewedness may be indicative of reduced immune surveillance capabilities, leading to failure to recognize and eliminate tumor cells or virus-infected cells and reduced ability to respond to vaccines. T cell repertoire skewing is observed in many patients with autoimmune diseases, where it may correlate with the outgrowth of pathogenic T cell clones. Several clinical studies have shown a positive correlation between post-therapy normalization of TCR repertoire and improved therapeutic outcome in patients with autoimmune diseases. Therefore, therapeutic strategies that restore a healthy, diverse TCR repertoire may be of clinical benefit.
We have developed the Xcellerate™ Technology, in which T cells are activated and expanded ex vivo using microscopic paramagnetic beads conjugated with anti-CD3 and anti-CD28 mAbs (Xcyte™Dynabeads®). T cells manufactured ex-vivo using this technology - Xcellerated T Cells™ - have been administered to patients with cancer and HIV in several clinical trials. Recent data have shown that the XcellerateTechnology can return the skewed TCR repertoires of patients with HIV and several hematological malignancies to normal.
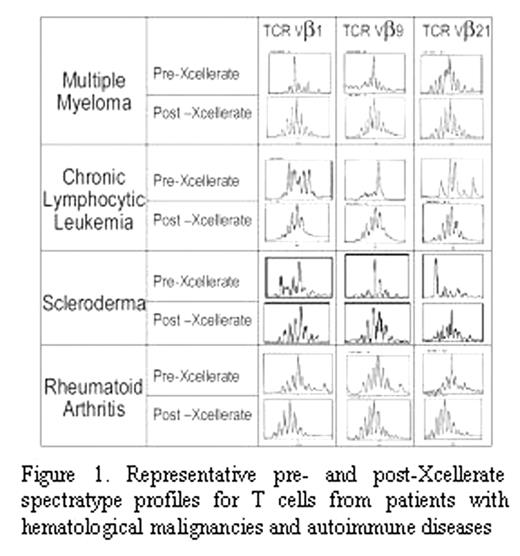
In the current study, we investigated the ability of the Xcellerate Technology to normalize the TCR repertoire from patients with hematological malignancies or autoimmune diseases. Repertoire skewedness was determined by measuring TCR variable βchain (Vβ) usage using: 1) the reverse transcriptase-PCR spectratype technique, which measures the size distribution of CDR3 regions of all 24 Vβ families and 2) flow cytometric analysis using antibodies for 23 Vβ families. We analyzed PBMC from >30 patients with hematological malignancies, including multiple myeloma (n=9) and chronic lymphocytic leukemia (n=15) as well as 12 patients with autoimmune diseases, including scleroderma (n=4), rheumatoid arthritis (n=3), psoriatic arthritis (n=1), systemic lupus erythematosus (n=3), and Crohn’s disease (n=1). Initially, the patients’ T cells demonstrated more severe TCR repertoire skewing than that observed for tissues from healthy donors (p=0.0003). There did not appear to be a correlation between type of disease or its severity and the degree of skewedness of the repertoire. The Xcellerate Technology broadened the TCR repertoire in 23 out of 24 patients with hematological malignancies and 11 out of 12 patients with autoimmune diseases (Figure 1) Stronger CD3 and CD28 engagement, which was achieved by using bead to T cell ratios greater than or equal to 3:1, was necessary to restore the skewed TCR repertoire to normal in these patients. Therefore, the Xcellerate Technology™ can be used to normalize the TCR repertoire in tissues frompatients with hematological disorders and autoimmune diseases. Planned and ongoing clinical studies will assess the ability of Xcellerated T Cells to restore TCR repertoire diversity in patients following infusion.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal