Abstract
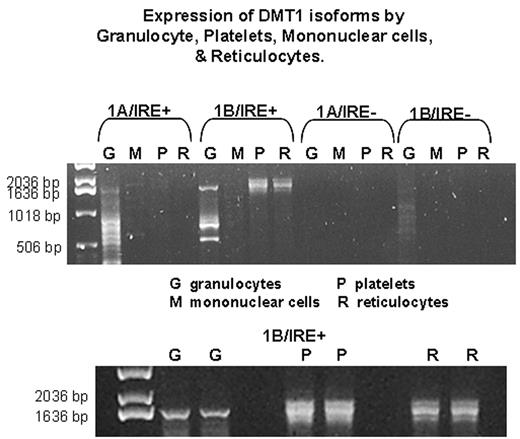
Divalent metal ion transporter 1 (DMT1) functions in iron absorption at the apical surface of the duodenal enterocyte and in iron transport out of the endosomal compartment of the erythroblast, however, DMT1 is also expressed in cells which have no apparent role in iron metabolism. At least 4 isoforms of DMT1 mRNA have been identified that contain alternative 5′ (1A, 1B) and 3′ (IRE+, IRE-) exons. Recently we reported the first human mutation of the DMT1 gene in a patient with severe hypochromic/microcytic anemia. This patient was homozygous for a mutation in the ultimate nucleotide of exon 12 which resulted in preferential skipping of exon 12 during processing of pre-mRNA. Amplification of DMT1 mRNA from peripheral blood cells of this patient revealed multiple products; hematopoietic cells from normal controls had a similar, but not identical, pattern. No such additional PCR products were seen when DMT1 mRNA from other tissues or cultured cells was evaluated. To further investigate this observation, we amplified full length (1700–1800 bp) DMT1 transcripts (1A/IRE+, 1A/IRE-, 1B/IRE+ and 1B/IRE-) from a variety of tissues types, cell lines, and from hematopoietic cells. Expression of the 4 isoforms differed between the different cell types. EBV lymphocytes expressed primarily 1B/IRE+ and 1B/IRE- isoforms. Notably, EBV lymphocytes derived from the mutant patient expressed DMT1 mRNA which was 120 bp smaller than DMT1 PCR products from controls, corresponding to skipping of exon 12. Platelets, granulocytes and reticulocytes expressed primarily the 1B/IRE+ isoform, however, unlike the other cell types examined, more than one mRNA species was present (see figure). Full length high molecular weight PCR products derived from these cells were isolated and subcloned. Plasmid DNA was isolated, subjected to digestion with EcoR1, and the DMT1 inserts were sequenced. All inserts began with exon 1B and ended with exon 16 (IRE+); however, none contained “normal” full length DMT1 sequence. The majority contained either truncated portions of exon 3 or inserted portions of intron 3. Utilization of cryptic splice sites within exon 3 or within intron 3 resulted in early stop codons. We propose that alternative splicing of DMT1 mRNA either via skipping of entire exons or via the use of cryptic splice sites may provide a manner for regulating DMT1 activity in hematopoietic cells. Further studies are underway to elucidate this possibility and to evaluate the signals for alternative splicing.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal