Abstract
In ARL, rituximab may slightly improve CHOP response but is associated with greater toxicity (
Proc Am Soc Hem102:1488, 2003
). We hypothesized that the addition of rituximab to DA-EPOCH may increase fractional tumor cell kill and allow fewer treatment cycles and lower immune suppression. Patients received DA-EPOCH-R (E=etoposide 50 mg/m2/d, O=vincristine 0.4 mg/m2/d and H=doxorubicin 10 mg/m2/d all CIV d 1–4 (96 hours); C=cyclophosphamide 750 mg/m2 IV d5; P=prednisone 60 mg/m2 PO qd d1–5 and R=rituximab 375 mg/m2 IV d1 and 5) with G-CSF. Prophylactic IT MTX x 6 was administered. HAART was discontinued before and recommenced after DA-EPOCH-R. Unlike our previous study of DA-EPOCH in ARL (BLOOD101:4653, 2003
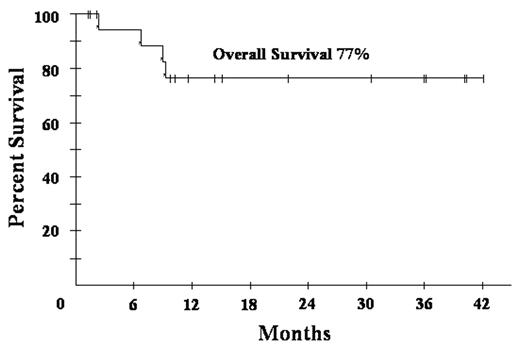
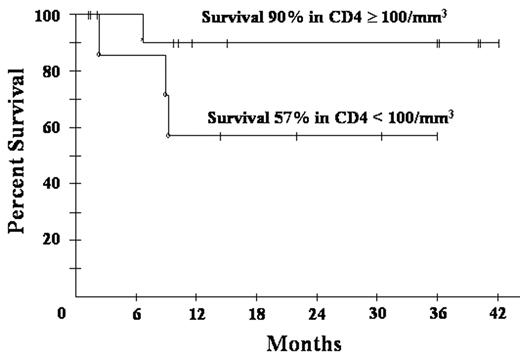
) where the dose of C was lower and based on CD4 cell count, all patients on this study received full dose C on cycle 1 with subsequent reduction if the ANC nadir was < 500/mm3 for ≥ 2 days. Patients received 1 cycle beyond CR, based on CT and PET, for a minimum of 3 cycles. Characteristics of 21 patients include median (range) age 39 (9–61) years; IPI 3 (0–4); ECOG PS 2 (1–4); CD4 212 (0–674) cells/mm3 and HIV viral load 53100 (0– 286472) RNA copies/mL. Additionally, male sex 17 (81%); LDH> nl 15 (71); stage IV 15 (71%) and histology with diffuse large B-cell 9 (90%) and Burkitt’s lymphoma 2 (10%). The 18 patients who completed treatment (2 TE; 1 NE) received a median (range) of 3 (3–5) cycles. Responses are CR/CRu 15 (83%); PR 1 (6%) and NR 2 (11%). At 19 mos median follow-up, overall PFS and OS are both 77%, and both 90% in patients with CD4 > 100 cells/mm3. Treatment outcome of DA-EPOCH-R is similar to DA-EPOCH (CR 74% and PFS 73% at 53 months) but with significantly shorter treatment (median cycles 3 vs. 6). Toxicity on 57 cycles include ANC < 500/mm3 on 27 (47%); platelets < 50,000/mm3 on 15 (26%) and; fever/neutropenia on 20 (35%) cycles. DA-EPOCH-R produced a median (range) CD4 cell decrement of 64 (−541 to + 239) cells/mm3 compared to 189 (−973 to +19) with DA-EPOCH. Hematological toxicity is higher with DA-EPOCH-R compared to DA-EPOCH with ANC < 500/mm3 47% vs 30% and fever/neutropenia 35% vs. 13%, respectively, likely due to higher C dose intensity and/or rituximab. Other toxicities are similar. Abbreviated DA-EPOCH-R is equivalent to DA-EPOCH x 6 and appears to produce less CD4 cell loss. Accrual continues.Author notes
Corresponding author
2005, The American Society of Hematology
2004

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal